The Post-COVID-19 Functional Status (PCFS) scale was created to assess the functional status of patients after hospital discharge due to COVID-19.
ObjectiveTo perform cross-cultural adaptation of the PCFS Scale and Manual into Brazilian Portuguese and evaluate its measurement properties in patients post-COVID-19.
MethodsFor the cross-cultural adaptation, independent translations and back-translations were performed. This was followed by a pre-test, with analysis of the Content Validity Index (CVI), and preparation of the final version, after evaluating the measurement properties. Spearman's correlation between the PCFS and the WHO Disability Classification Scheme (WHODAS 2.0) was used for convergent validity. Weighted Kappa (wκ) was used for test-retest and interobserver reliability for PCFS scores and Kappa (κ) for PCFS items. Internal consistency was assessed using Cronbach's alpha. Only patients with post-discharge COVID-19 were evaluated through video-conferencing platforms.
ResultsThe CVI was 0.75–0.83 for comprehension and 0.83–0.84 for the language of the self-administered questionnaire and the structured interview version. For measurement properties, 63 patients were evaluated, 68% male, 51.50 (12.60) years, 12.28 (7.62) days of hospitalization. For the convergent validity, a strong correlation was found (r = 0.73; p<0.01). The test-retest (wκ=0.54) and interobserver (wκ=0.43) reliability was moderate and the item-by-item analyzes ranged from fair to substantial (κ=0.25–0.66) and weak to substantial (κ=0.07–0.79). Internal consistency was excellent (0.85).
ConclusionThe final PCFS in Brazilian Portuguese showed adequate content validity, reliability, internal consistency, and convergent validity for the functional assessment of patients after hospital discharge due to COVID-19.
The 2019 coronavirus disease (COVID-19) has become a global public health emergency.1 While most affected individuals have mild symptoms, some have the severe form of the disease, requiring ventilatory support and admission to an intensive care unit.1-4 In addition to the sequelae resulting from COVID-19, hospitalization also has a significant effect on functional status that may persist for years, especially in individuals who required intensive care, exerting a negative impact on quality of life.5-7
Particularly after the acute phase of the disease, it is important to address functional status considering the long-term physical, cognitive, and psychosocial repercussions.8-10 The assessment should be conducted by a specialized multidisciplinary team8 using the biopsychosocial model, which involves body functions and structures, activities, and social participation.11 Several instruments have been used to assess the functional status of patients after COVID-19, such as the Barthel Index,10,12,13WHO Disability Assessment Schedule, and Brief Model Disability Survey.14 Despite their importance, none of these tools is specific to the functional status of patients after COVID-19.
A group from the Leiden University Medical Center adapted the Post-Venous Thromboembolism Functional Status Scale, which has good to excellent measurements properties,15,16 to specifically assess the functional status of patients in the post-acute phase of COVID-19.17 The aim was not to replace other instruments but to provide a quick, standardized tool that could help healthcare providers and researchers in clinical practice and research settings.17
The new, post-COVID-19 Functional Status (PCFS) scale, measures functionality on a scale from zero (no functional limitation) to four (severe functional limitation). If the patient dies, a grade D (death) classification is given. When answering the questions, respondents are instructed to consider their status in the previous seven days. The scale can be applied as a structured interview or can be self-administered. The respondents use the proposed flowchart or choose the option that best reflects their current condition from a table. The questions can be answered by the patient with or without assistance of a caregiver or a close family member who knows the patient's daily routine (proxy). Among the available versions, measurement properties have been evaluated for the original,18 the Turkish,19 the Chilean,20 and the Spanish versions.21 Therefore, the aim of the present study was to translate and cross-culturally adapt the PCFS into Brazilian Portuguese, and to investigate its measurement properties (i.e., reliability and convergent validity).
MethodsA methodological study22,23 conducted from July 2020 to November 2021 by researchers from the Universidade Federal de of São Carlos (UFSCar), Brazil in partnership with the Universidade Federal do Rio de Janeiro (UFRJ), Brazil. The study received formal authorization from the authors of the PCFS and was approved by the UFSCar Human Research Ethics Committee (certificate number: 37303820.0.0000.5504) in accordance with Resolution 510/2016 of the National Board of Health. A signed statement of informed consent was obtained from the participants in each phase of the study.
Translation and cross-cultural adaptationTranslation. Four qualified translators proficient in English and Portuguese produced independent versions of the Post-COVID-19 Functional Status Scale and Manual in Brazilian Portuguese.22,23 Three translated versions were made by researchers and healthcare providers from different regions of Brazil and one was made by a language teacher, naive in health sciences.23
Synthesis. The four versions were compiled, highlighting all points of divergence23 and each divergent item was analyzed and terms were selected that most reflected the Brazilian culture while maintaining the original meaning of the item, finalizing the synthesis version.
Back-translation. Two foreign professionals whose native language is English and who are fluent in Portuguese performed two independent back-translations of the synthesis version. These versions were sent for approval to the original authors of the scale, who verified the compatibility of the content between the original and back-translated versions.
Expert committee review. A review committee was assembled that included all translators and three health professionals with academic and research methodology experience as well as experience with provision of care to patients with COVID-19 (hospital [including intensive care], clinic, or telemonitoring). All versions were carefully analyzed and a prefinal version of the PCFS was developed.
Test of the prefinal version. The Brazilian-Portuguese version of the PCFS scale was made available on the Google Forms platform and the link was sent to health professionals and to patients with confirmed diagnosis of COVID-19 by Reverse transcription polymerase chain reaction (RT-PCR), with varying levels of severity. Responses were accepted from November 2020 to January 2021. For each item on the PCFS, participants were asked about their general understanding and their perception about the language used on a five-option Likert scale: 1) Fully agree; 2) Partially agree; 3) Neutral; 4) Partially disagree; 5) Fully disagree. For each item, the participants could describe their concerns and give suggestions to improve the writing of the PCFS scale. The items were analyzed using the content validity index (CVI) considering that there was no need to make any changes to the item if CVI≥0.8. Items with CVI<0.8 were reviewed and altered by the committee, as recommended by six or more committee judges.24 The review committee analyzed the responses and made all necessary adaptations.23 After that, the PCFS Brazilian-Portuguese version was administered by health professionals to patients post-COVID-19 to verify if there would be any more concerns about the scale, thereafter the review committee made final adaptations, reaching the final version of the PCFS scale.
Participants and proceduresTo be included participants had to be over 18 years old, with a diagnosis of COVID-19 confirmed by RT-PCR which required hospitalization (including those who required semi-intensive care or intensive care), discharged from hospital between four to 10 days, able to understand the purpose of the study, and agree to participate voluntarily by signing the statement of informed consent. Patients who scored less than 20 on the Mini-Mental State Examination25 and did not have a caregiver to sign the informed consent form and answer the scale for them were not included in the study. Patients who reported difficulties in using a video-conferencing platform were also excluded.
Data were collected from March to September 2021. A minimum sample of 50 patients was needed at the end of the recruitment to complete all phases of the study.26 All assessments were conducted remotely with a structured interview using video-conferencing platforms, such as WhatsApp or Google Meet.
Assessments were performed on three occasions, two occasions by the same examiner (Examiner 1) and one occasion by another independent examiner (Examiner 2). The first evaluation was made between four to ten days after hospital discharge by Examiner 1. This first evaluation consisted of patient history questions and functional assessment by administering the PCFS, followed immediately by the WHO Disability Assessment Schedule (WHODAS 2.0).27 After a period of four to seven days, Examiner 1 administered the PCFS for the second time to determine test-retest reliability. Then, Examiner 2 administered the PCFS 24 to 48 h later to determine inter-rater reliability.28 In all three assessments, the patients were instructed to consider only the previous seven days.
The WHODAS 2.0 questionnaire was used as a reference for the assessment of functional status27 in the analysis of convergent validity. WHODAS 2.0 is a generic instrument developed by the World Health Organization (WHO) that enables the assessment of the impact of any health condition on functionality.27 Therefore, it can be administered to patients post-COVID-19 to assess functionality and identify disabilities and limitations related to activities of daily living.
InstrumentsThe following data were collected for the characterization of the sample: age, sex, schooling, profession, health history, smoking habit, and data on hospitalization (length of stay, need for intensive or semi-intensive care, need for invasive ventilatory support, etc.). Comorbidities were evaluated using the Charlson Comorbidity Index.29 Dyspnea was assessed using the modified Medical Research Council (mMRC) scale.30
The PCFS was administered during a structured interview via a video-conference. The responses (yes or no) to each question were recorded and each patient was classified based on the greatest degree of limitation (0 to 4). As all patients were interviewed directly, the first question was skipped, because no patient would receive grade D (death).
The PCFS grades patients based on their functional abilities after recovering from COVID-19. A grade of 0 means no functional limitations are present. Grades 1 and 2 indicate that the individual can perform their normal daily activities, including sports and social activities, but with some limitations. Grade 1 is for patients with symptoms that do not prohibit normal activities, while grade 2 is for patients who can perform normal activities but at a lower intensity and with mild limitations in social participation. Grade 3 indicates moderate functional limitations that require modifying normal activities and may require assistance with activities of daily living such as household chores, community mobility, and shopping. Grade 4 is for patients with severe functional limitations who need assistance with daily living activities, including routine hygiene and mobility. These patients likely have restricted participation in social roles. Finally, grade 5 (D) indicates death.17
The full (36-item) structured interview version of WHODAS 2.0 was used and scores were calculated based on the “item response theory”, considering multiple levels of difficulty for each item.27 The total score is given as a percentage, with higher percentages denoting greater functional impairment.28,27,31 WHODAS 2.0 has six domains on functionality: cognition, mobility, self-care, interpersonal relationships, life activities, and participation.27 The PCFS scale also has items that belong to these six domains. WHODAS 2.0 Brazilian Portuguese version has been validated and has good reliability and good internal consistency.27 Although WHODAS 2.0 addresses difficulties faced in the previous 30 days,27 the patients in the present study were asked to only consider the previous seven days so that the two instruments (PCFS scale and WHODAS 2.0) were addressing the same time period. The scoring and classification of the PCFS scale and WHODAS 2.0 used for the correlation analysis are displayed in Table 1.
Statistical analysisAll analyses were performed with the Statistical Package for the Social Sciences (SPSS, version 22.0). The data were expressed as frequency (percentage), mean (standard deviation) and median [minimum and maximum values). Data distribution was analyzed using the Kolmogorov–Smirnov test. The content validity index (CVI) was used at the pre-test, considering CVI≥0.8 (full agreement rate higher than 80%). To assess convergent validity23 Spearman's correlation coefficients were calculated because the variables had non-normal distribution. Associations were considered significant when p<0.05, assuming for interpretation of values: null 0.00, weak |0.10–0.30|; moderate |0.30–0.50|, and strong |>0.50|, as proposed by Cohen32,33 for interpretation. Internal consistency of the questionnaire was assessed using Cronbach's alpha (Cα) coefficient.
Both Kappa (κ) and weighted Kappa (wκ) tests were used for test-retest reliability and inter-rater reliability due to the nature of the data (i.e., nominal data). The values proposed by Landis and Koch34 were used to interpret the results: values from 0.0 to 0.2 = weak reliability; from 0.21 to 0.40 = fair reliability; from 0.41 to 0.60 = moderate reliability; from 0.61 to 0.80=good reliability; and from 0.81 to 1.00 = almost perfect reliability.
ResultsPre-testing phase and final PCFS Brazilian-Portuguese versionDuring the pre-testing period, the Brazilian-Portuguese version of the PFCS was administered online to 33 patients from five different states in Brazil with different levels of education and professions, who had a confirmed diagnosis of COVID-19 by RT-PCR, with varying levels of severity. Of these, five participants required hospitalization. Our analysis showed a CVI of 0.75 for general understanding and a CVI of 0.84 for perception about the language used. Comments and suggestions were mainly related to long sentences that compromised the understanding of the items. All sentences were revised to be more concise, using better punctuation, and altering the vocabulary to improve the readability of the PCFS to a broader population (e.g., replacing the word “assistance” with “help”).
The Brazilian-Portuguese version of the PFCS also was administered to 57 health professionals from 11 different states in Brazil. Our analysis showed a CVI of 0.83 for general understanding and a CVI of 0.84 for perception about the language used. Only seven items among the 22 (five explanatory statements and 17 questions) had a CVI less than 0.8 and these were carefully revised. The changes involved being more concise and making better use of punctuation as well as standardization of the explanations, using action verbs to make the sentences more emphatic and meaningful. Thus, the coherence of the whole PCFS was addressed rather than merely considering the individual items.
Three health professionals administered the PCFS Brazilian-Portuguese version to 30 patients post-COVID-19 (confirmed diagnosis by RT-PCR), and beyond the scale grade, all difficulties and/or questions during the assessment that regards the understanding and/or the language of the scale were sent to the review committee. Both the health professionals and patients had concerns regarding the answer to Item 5.3 (“Can you no longer take good care of loved ones as before?”), which was the only item on the scale in which the “yes” and “no” answers had a double meaning for Brazilians. Some patients answered “yes” agreeing with the statement (indicating limitation), whereas others answered “yes” disagreeing with the statement (absence of limitation), i.e., “yes, I can take good care of loved ones.” The same occurred with the answer “no.” Therefore, for better consistency of the entire PCFS scale and assertiveness of the interview, the sentence on this item was rewritten to “Do you have difficulties taking as good care of loved ones as you did before?”, giving the “yes” and “no” answers more assertive meaning. The explanation provided in parentheses did not need to be changed.
After the changes were made, the final PCFS Brazilian-Portuguese version was finished and administered by health professionals as an online structured interview via video-conferencing platform to patients post-COVID-19, after hospital discharge. The final Brazilian-Portuguese version of the PCFS is available in the Appendix and the full version of the Brazilian-Portuguese manual available in the Supplementary material. No item was excluded from either explanatory sentences or questions. The general understanding of the scale was also evaluated by asking about the need to read the manual before or after applying the PCFS scale; only 15.8% of the participants declared needing to consult the manual for better understanding. With regards to relevance, the health professionals were asked if they would use the PCFS in their clinical practice and/or scientific activities as an important patient assessment tool; 80.7% answered “yes”, 17.5% answered “maybe”, and only 1.8% answered “no.”
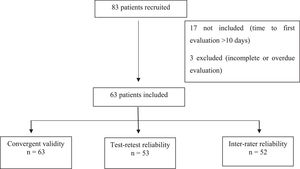
Measurement propertiesEighty-three patients discharged after being hospitalized due to COVID-19 were recruited. Seventeen patients were excluded because they had already been discharged from hospital for more than ten days and three patients were not included in the analyses for not completing all assessments or for not meeting the recommended period between assessments (i.e., more than 48 h between assessments by Examiners 1 and 2 or more than seven days between first and second assessments by Examiner 1). Thus, 63 patients were included for the analysis of convergent validity, 53 of whom were also involved in the analysis of test-retest reliability and 52 were involved in the analysis of inter-rater reliability (Fig. 1).
The sample was composed of 20 women and 43 men after hospital discharge from COVID-19, mean age of 51.50 (12.60) years old, body mass index (BMI) 31.63 (5.86) kg/m², 65% non-smokers and 35% former smokers, mMRC 2 [0–4], 12.28 (7.62) days of hospitalization, with 68.25% requiring intensive care and 50.8% requiring invasive mechanical ventilation for an average of 7.34 (5.81) days (Table 2).
Characteristics of the participants.
Legend: Data expressed as mean (standard deviation), median [minimum-maximum] and n (percentage). BMI, body mass index; MMSE, Mini Mental State Examination; mMRC, modified Medical Research Council scale; OIT, orotracheal intubation; IMV, invasive mechanical ventilation; PCFS, Post-COVID-19 Functional Status scale; WHODAS 2.0, World Health Organization Disability Assessment Schedule.
For the convergent validity, a strong correlation was found between the PCFS and WHODAS (rs=0.73; p<0.01). For test-retest reliability, absolute agreement was found in 50.9% of the cases (27 patients) and moderate agreement (wκ=0.54) was found with an error of one degree up or down in 22 cases (41.5%) and two degrees in four cases (7.5%) (Table 3). For inter-rater reliability, absolute agreement between the different examiners was found in 50% of cases and moderate agreement (wκ=0.43) was found with an error of one degree in 22 cases (42.3%) and two degrees in one case (1.9%) (Table 3). When considering the PCFS Scale item by item, we obtained a fair to substantial (k = 0.25–0.66) test-retest reliability with 64.2%–98.1% agreement, and a weak to substantial (k = 0.07–0.79) inter-rater reliability, with 44.3%−98% agreement (Table 4). Internal consistency of the questionnaire was rated as excellent (Cα=0.85).
Test-retest and inter-rater reliability for PCFS scale in grades, sum of affirmative answers, and total percentage of affirmative answers.
Legend: M1, first assessment; M2, second assessment.
Test-retest and inter-rater reliability for PCFS scales questions, percentage of agreement, and kappa value item by item.
The present study performed the cross-cultural adaptation of the PCFS scale and demonstrated moderate to excellent test-retest and inter-rater reliability as well as a strong correlation between the PCFS scale and WHODAS 2.0 questionnaire. Thus, the Brazilian Portuguese PCFS version has adequate content and convergent validity for the assessment of functional status in patients who were hospitalized due to COVID-19.
The sample size used in this study is considered reasonable according to COSMIN (2019). Moreover, choosing a convenience sample composed only of patients discharged after being hospitalized due to COVID-19 enabled us to establish a common baseline and ensure a confirmed diagnosis of the disease. Despite the relatively small sample size, the results of the present study and other validation studies17,18,20 indicate that the PCFS scale may be considered a useful screening tool for the functional assessment of post-COVID-19.
The high prevalence of patients with grade 4 (44.4%) in the PCFS was expected because our sample consisted of individuals who required hospitalization, including intensive care, with a more severe clinical and functional impairment. Studies conducted by Machado et al.,18 Çalik Kütükcü et al.,19 Moreno-Torres & Ventura-Alfaro21 included patients who were hospitalized (representing 5%, 60%, and 100% of the samples, respectively), but the authors excluded patients who had required intensive care.
In the study by Moreno-Torres & Ventura-Alfaro,21 a strong correlation (r = 0.83) was found between the PCFS and WHODAS 2.0 (12-item version), which is similar to the result in the present study (r = 0.73) using the 36-item version of WHODAS 2.0. As COVID-19 is a systemic disease with different repercussions and varied causes of subsequent limitations, the correlation found between the PCFS and WHODAS 2.0 may be due to the nature of the instrument itself, which assesses functional impairment in all its aspects irrespective of the cause of limitation.
Regarding correlations with other assessment tools, Machado et al18 found weak to strong correlations (r = 0.23–0.66) between the PCFS and subitems on a quality-of-life questionnaire. Çalik Kütükcü et al.19 found no significant correlation with the Barthel Index, but the authors found a weak correlation (r = 0.31) with the London Chest Activity of Daily Living scale and a moderate correlation (r = 0.53) with the mMRC scale.
The study by Moreno-Torres & Ventura-Alfaro21 was the first to report sensitivity (86.2%) and specificity (96.3%), evaluating participants longitudinally (i.e., at discharge from hospital, four and eight weeks after discharge, and six months after discharge) with the PCFS being administered as a structured interview or self-reported by the patient. In the present study, the PCFS was administered using a structured interview via video-conferencing to minimize the interference of the time effect and the occurrence of missing data.
Regarding the other measurement properties, internal consistency was rated as excellent (Cα=0.85), which is consistent with data reported in other language versions that had Cα=0.82119 and Cα=0.84.21 Similar to our findings, the inter-rater reliability was also reported to be excellent (ICC=0.82; 95% CI: 0.73–0.88) for the Turkish version of the scale19 and Lorca et al.20 found high agreement (r = 0.93) for test-retest reliability. Both test-retest and inter-rater reliability for PCFS were moderate in the present study using the weighted Kappa statistic (wκ=0.54 and 0.43, respectively). For item-by-item reliability, the present study found a fair to substantial (k = 0.25–0.66) test-retest reliability and a weak to substantial (k = 0.07–0.79) inter-rater reliability, but this difference in reliability between questions may not show up in the overall score, as many questions have the same score.
Weighted Kappa and Kappa analysis were used because the PCFS scale uses a categorical scale, with nominal rather than continuous variables. By having few questions and few grades, the scale enables a quick and easy functional assessment. However, it is noteworthy that some patients scored the maximum degree of limitation due to a single impairment, despite not having other functional limitations. Thus, different patients with different limitations can have the same score on the PCFS scale (e.g., a bedridden patient can have the same score as a patient who only requires assistance for local travel). It makes sense that some items can generate more important functional repercussions than others, but having few grades to score may overestimate or underestimate functional status in some cases. Thus, alternative ways of scoring the PCFS scale should be proposed in future studies.
Moreover, due to the few grading levels, the scale may not be particularly discriminative for evaluating changes in functional status over time. This aspect should be investigated in future prospective studies with longitudinal follow-up. Moreover, further studies on other measurement properties, as responsiveness, are also needed.26,34 Future studies that assess functional status using the PCFS in larger samples of the Brazilian population, including individuals with mild to severe impairments, are needed.
The PCFS items focuses on limitations regarding activities of daily living, varying from selfcare limitations, as using the bathroom, to instrumental activities, such as grocery shopping. The assessment refers to average status in the previous seven days (or the same day if administered at discharge). The scale can be administered at discharge from hospital, in the first weeks after discharge to assess recovery, and six months after discharge to assess the persistence of functional limitations. The structured interview format is more recommended for clinical trials. The PCFS is an ordinal scale ranging from 0 to 4 (plus a D classification for death) and the score is based on the worst functional status indicated by the patient (considering the greatest limitation experienced). It is intended to be easy to administer and reproducible, contributing another form of assessment for patients following hospitalization for COVID-19.
ConclusionThe PCFS scale translated and cross-culturally adapted to Brazilian Portuguese presented adequate reliability, internal consistency, and convergent validity for the functional assessment of patients following discharge from hospital for COVID-19.
Academic Excellence Program (PROEX) from the Coordination for the Advancement of Higher Education Personnel (CAPES) - doctoral scholarship; Graduate Program in Physical Therapy at the Federal University of São Carlos - translation of documents into English.
Team of the Laboratory of Spirometry and Respiratory Physical Therapy (LEFiR) at UFSCar; Team of the University Hospital of São Carlos (HU-UFSCar) and Santa Casa de São Carlos Hospital.