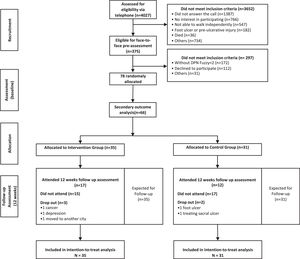
Follow-up report of secondary outcomes of a randomized, single-blinded, parallel controlled trial that investigated the benefits of a foot-ankle therapeutic exercise program on foot-ankle kinematics, plantar pressure, and lower limb kinetics during gait in individuals with diabetic neuropathy (DPN).
MethodsSixty-six participants with DPN were randomly allocated into a control group (CG; n = 31), which received usual care, and an intervention group (IG; n = 35), which received usual care plus a 12-week group-based foot-ankle exercise program. Outcomes were assessed at baseline and 12 weeks by an assessor blinded to group allocation.
ResultsThe generalized linear mixed model and intention-to-treat analysis revealed a greater hip extensor moment at push-off and greater hallux contact area in the IG than CG after 12 weeks. A within-group analysis revealed a larger arch height during stance and higher peak pressure and pressure-time integral at the central forefoot region in the IG after 12 weeks compared to baseline. There were no other significant group difference or changes over time in foot-ankle kinematics or in any other joint moment related to overall lower limb biomechanics.
ConclusionThe increases in hip moment at push-off and hallux surface contact area suggest an improvement in the propulsion phase with greater participation of the toes in foot rollover after 12 weeks of a group-based foot-ankle exercises program for people with DPN. Individual face-to-face, longer-term, and more intensive interventions may be needed to positively influence foot-ankle biomechanics and pressure parameters in other plantar areas.
Diabetic neuropathy (DPN) is one of the most common complications of diabetes mellitus.1,2 Thus, several guidelines for the prevention and treatment of complications arising from DPN have been developed, mainly focusing on therapeutic strategies for changing clinical outcomes, because they are more pragmatic for clinical practice. Among the clinical outcomes of interest, DPN symptoms, plantar sensitivity, and number of steps during daily living activities are the most relevant. These outcomes were the target of studies using different types of conservative treatment. Accordingly, we developed a large randomized controlled trial (RCT) to assess the effects of foot-ankle exercises on clinical outcomes.3 The study also included secondary biomechanical outcomes in an effort to explain the results observed in the primary clinical outcomes. The interest in understanding the effects of foot-ankle exercise on biomechanical outcomes is based on the premise that DPN compromises the quality and maintenance of daily locomotor activities.4
Gait impairments arise due to several progressive deficits in force generation and control of lower limb movements. Compared to individuals without diabetes, people with DPN have reduced ankle dorsiflexion and plantar flexion,5,6 and metatarsophalangeal7 and knee joint range of motion (ROM) during gait.5 Other relevant alterations in the lower limb kinetics and kinematics, such as reduced ankle plantar flexion at push-off,8 reduced and delayed muscle activation of the knee and ankle extensors,9–12 and reduced ankle and increased hip flexor moments,13,14 severely affect gait propulsion and efficiency. Such impairments in lower limb biomechanics can generate a cascade of functional changes that alter foot rollover and plantar pressure distribution,15–17 which in turn increase the risk of foot ulcers.18
Studies have investigated the effects of different exercise therapy strategies, including foot-related exercises, balance training, and general resistance exercises, on DPN-related clinical and functional outcomes.3,19,20 Specifically, van Netten et al.20 reviewed studies including foot-ankle exercises as the main intervention targeting modifiable-risk factors for foot ulcers, which provided the foundation for the International Working Group of the Diabetic Foot (IWGDF 2020) to include foot-related exercises as a preventive strategy in their updated guidelines. Limited controlled studies have reported the effectiveness of this type of intervention on gait kinematics, kinetics, and plantar pressure distribution21–24 In the clinical trials by Sartor et al.24 and Melai et al.,23 the intervention participants’ foot-ankle ROM did not change after 12 and 52 weeks of intervention, respectively. Sartor et al.24 attributed the negative result to the intervention duration and Melai et al.23 to the intensity and nature of the training program, indicating that these may have been inadequate to improve gait biomechanics. In contrast, Kumar et al.22 showed an increase in ankle ROM after a 12-week exercise program including general resistance, flexibility, and foot-related exercises. However, this was not a controlled study, with potential bias in the interpretation of the results.
We designed a RCT to investigate the effects of a 12-week foot-ankle therapeutic exercise program on several DPN-related clinical and biomechanical outcomes in people with DPN.25The group-based exercise program resulted in improved ankle ROM, vibration perception, fast gait speed, and quality of life compared with usual care. The outcomes support the hypothesis-driven mechanism that improved foot-ankle mobility and sensitivity leads to better physical functioning and quality of life. Thus, in this follow-up report on secondary outcomes, we report the effects of this program on the participants who were assessed for gait biomechanics, with particular interest in foot-ankle kinematics, plantar pressure, and lower limb kinetics during gait.
MethodsStudy designThis study is a follow-up report on secondary outcomes of a single-blinded and parallel-group RCT (ClinicalTrials.gov: NCT02790931). The methods of this RCT, following CONSERVE recommendations26 and have been published elsewhere,25 as have the primary outcome results.3 This report on secondary outcomes used data from the baseline and 12-week (post-intervention) assessment time points intended to explain the clinical outcomes results.
Participants and recruitmentParticipants were recruited between December 2017 and December 2019 through social media advertising and from a university tertiary hospital (Hospital das Clínicas FMUSP), local population campaign (diabetes diagnosis and detection), and primary care center, in São Paulo. Eligibility criteria included: age 18–75 years, type 1 or 2 diabetes with moderate DPN as diagnosed by a fuzzy decision support system,12 ability to walk independently, and access to electronic devices with internet. The exclusion criteria were: presence of an active ulcer; history of a surgical procedure at the hip, knee, or foot-ankle; current use of lower-limb orthosis or indication of lower limb arthroplasty throughout the intervention period; diagnosis of neurological disease; dementia or inability to give consistent information; undergoing any physical therapy care during the study; and major vascular complications or severe retinopathy. Participants signed an informed consent form approved by the Ethics Committee of the School of Medicine of the University of São Paulo (24/03/2016, protocol No. 1.464.870).
Randomization and blindingThe participants were randomly allocated to the intervention (IG) or control (CG) groups (random blocks) using Clinstat software (University of York, York, UK). Researchers involved in the allocation were blind to the group codes and block sizes. All baseline and follow-up assessments were performed by physical therapists blinded to the treatment allocation. The trial statistician was blinded to treatment allocation until the main analysis had been completed.
Treatment armsCG participants received usual care as recommended by the health care team and by the IWGDF guidelines,27 including: inspect your feet daily, wear socks without elastic and seams, cut your nails properly, avoid cutting corns or blisters without supervision, avoid walking barefoot or wearing shoes without socks or slippers, and seek medical attention whenever you identify foot problems. IG participants underwent a 12-week group-based foot-ankle exercise program in addition to usual care. The foot-ankle exercise program was performed twice/week in groups of five to eight participants under in-person supervision by a physical therapist and twice/week at home through the Sistema de Orientação ao Pé diabético - Diabetic Foot Guidance System (SOPeD, www.soped.com.br). Both protocols (SOPeD and group-based exercises) consisted of the same exercise modules: warm-up exercises, intrinsic foot muscle strengthening, extrinsic foot-ankle muscle strengthening, and functional exercises. The intensity of the exercises could be increased by the physical therapist if the participant was able to perform the exercise correctly, which ranged from one to three sets of five to 40 repetitions. The intensity of the exercises performed at home could be increased through an algorithm from the SOPeD that adjusted the training volume based on the perceived exertion reported via a visual analogue scale. The complete exercise program is published elsewhere.25 The groups were treated equally except for the group-based foot-ankle exercises program.
Outcomes and follow-up assessmentsThe secondary outcomes were foot-ankle kinematics, plantar pressure, and lower limb kinetics during gait assessed at baseline and after 12 weeks (Fig. 1).
The participants walked three times barefoot over the emed-q pressure platform (novel, Munich, Germany) at a self-selected speed to collect plantar pressure at 100 Hz. Peak pressure (kPa), contact area (cm2), and pressure-time integral (kPa*s) were analyzed in seven areas (rearfoot, midfoot, lateral forefoot, central metatarsal, medial forefoot, hallux, and toes). The average of three trials was used for statistical analysis.
Foot-ankle kinematics were evaluated by eight cameras at 100 Hz (Vicon® VERO; Vicon Motion Systems, Oxford, UK) and 42 markers (9.5-mm diameter) placed over the lower limbs. Plug-In Gait and Oxford Foot28 setup protocols were used. Ground reaction forces were acquired by a force plate (OR-6–1000; AMTI, Watertown, MA, USA) at 1 kHz. Force and kinematic data acquisition were synchronized and sampled by an A/D board (Control Box LOCK; Vicon; 192 kHz, 24 bits). Participants walked barefoot at a self-selected speed on a walkway, and three valid steps (stance phase) of the left side were acquired. Foot-ankle, knee, and hip kinematics and kinetics in the sagittal plane during stance phase of gait were computed with the open-source Python package pyCGM2 (www.pycgm2.github.io) replicating the Vicon Plug-In Gait protocol and Plug-In of Oxford Foot Model.
Sample size and statistical analysisSample size calculation was based on primary outcomes of the RCT25 resulting in 78 participants, and data from 66 participants (for plantar pressure) and 52 participants (for kinematics) were included in this report on secondary outcomes. There were no differences in clinical characteristics between the original 78 participants and the 52 or 66 participants in this secondary analysis (p values for the demographic and clinical characteristics ranging from 0.266 to 0.984).
Statistical analyses were performed using SPSS v.23.0 (IBM, Armonk, New York, USA) at a 5% significance level. All analyses used the full set of randomly assigned participants under the intention-to-treat assumption who had their gait biomechanics assessed. Due to technical (marker set and data processing) problems at baseline, plantar pressure data (excluded: CG=8, IG=4; included CG=31, IG=35) and kinematic data (excluded: CG=18, IG=8; included CG=21, IG=31) were missing for some participants.
Originally, we planned to use ANOVAs; however, due to the missing data for the follow-up visits (due to the COVID-19 pandemic and acquisition data problems), a generalized linear mixed model (GLMM) was adopted instead.26 This model is more robust for long prospective datasets because it considers not only fixed but also random effects. We included participants and time of assessment as random effects. We assumed that the missing data were completely at random because there was no pattern to patients’ absences in the follow-up visits. Different from imputation methods, GLMM does not artificially impute any data but allows to check the robustness and validity of the model by analyzing the normality of the residues and adherence indexes. Q-Q graphs were plotted to verify the adequacy (normality) of each model. GLMM was then used for univariate analyses considering the following as factors: groups (CG and IG), time of assessment (baseline and 12 weeks), and interaction effect (time by group), which was our secondary outcome comparison.
ResultsBaseline data (groups were similar, with no significant differences) of key outcomes across both arms are described in Table 1.
Clinical, demographic, and anthropometric data at baseline.
Data are mean ± standard deviation or frequency (percentage). Abbreviation: MNSI – Michigan Neuropathy Screening Instrument.
In the IG, 32 participants (91.4%, 3 dropouts) completed the 12-week exercise program (Fig. 1). The dropout rate at 12 weeks was 6.4% in the CG (2/31). Reasons for dropout in both groups are described in Fig. 1.
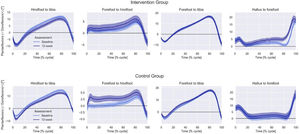
After 12 weeks, hip extensor moment at push-off was significantly greater in the IG than CG, although groups were significantly different at baseline (p = 0.02) (Table 2). Regarding within-group comparisons, the IG showed increased maximum (within-group mean difference = 1.01, 95%CI: 0.95, 1.07) and minimum arch height during stance after 12 weeks compared to baseline (within-group mean difference = 1.02, 95%CI: 0.96, 1.08). There were no other differences between groups after 12 weeks for the foot-ankle kinematics or lower limb kinetics (Fig. 2).
Foot-ankle kinematics and hip, knee, and ankle joint moments during gait for each group (control and intervention) at two assessments (baseline and 12-week).
| Intervention Group (n = 31) | Control Group (n = 21) | Mean difference between groups at 12-week | |
|---|---|---|---|
| ANKLE Kinematics and Kinetics | |||
| Ankle ROM (degree) | |||
| Baseline | 23.79 ± 1.69 | 23.05 ± 1.84 | |
| 12-week | 23.07 ± 1.80 | 22.31 ± 1.94 | 0.76 (−4.48, 5.99) |
| Ankle dorsiflexion at heel strike (degree) | |||
| Baseline | −0.43 ± 1.14 | −0.39 ± 1.31 | |
| 12-week | 1.24 ± 1.25 | 0.68 ± 1.52 | 0.56 (−3.44, 4.56) |
| Ankle plantar flexion at push-off (degree) | |||
| Baseline | −4.79 ± 1.39 | −3.84 ± 1.63 | |
| 12-week | −3.24 ± 1.66 | −2.66 ± 2.03 | −0.58 (−5.91, 4.76) |
| Ankle flexor moment at heel strike (Nm/BM*height) | |||
| Baseline | −0.03 ± 0.04 | −0.04 ± 0.04 | |
| 12-week | −0.04 ± 0.4 | −0.07 ± 0.04 | 0.27 (−0.01, 0.07) |
| Ankle extensor moment at push-off (Nm/BM*height) | |||
| Baseline | 1.29 ± 0.05 | 1.14 ± 0.06 | |
| 12-week | 1.19 ± 0.08 | 1.25 ± 0.08 | −0.11 (−0.32, 0.09) |
| Ankle peak eccentric power at the push-off (W/BM*height) | |||
| Baseline | 2.64 ± 0.16 | 2.34 ± 0.17 | |
| 12-week | 2.57 ± 0.26 | 2.49 ± 0.26 | 0.08 (−0.67, 0.83) |
| KNEE AND HIP kinetics | |||
| Hip joint moment at heel strike (Nm/BM*height) | |||
| Baseline | 0.25 ± 0.12 | 0.09 ± 0.13 | |
| 12-week | 0.20 ± 0.12 | 0.31 ± 0.13 | −0.11 (−0.47, 0.25) |
| Hip joint moment at push-off (Nm/BM*height) | |||
| Baseline | −0.26 ± 0.07# | −0.01 ± 0.08# | |
| 12-week | −0.38 ± 0.15* | −0.17 ± 0.16* | −0.21 (−0.67, −0.14) |
| Knee joint moment at heel strike (Nm/BM*height) | |||
| Baseline | 0.31 ± 0.10# | 0.50 ± 0.10# | |
| 12-week | 0.40 ± 0.10 | 0.34 ± 0.10 | 0.06 (−0.11, 0.24) |
| Knee joint moment at push-off (Nm/BM*height) | |||
| Baseline | 0.11 ± 0.02# | 0.05 ± 0.02# | |
| 12-week | 0.08 ± 0.04 | 0.03 ± 0.04 | 0.05 (−0.07, 0.17) |
| OXFORD FOOT MODEL kinematics | |||
| Hindfoot to tibia ROM (degree) | |||
| Baseline | 21.57 ± 0.88 | 21.81 ± 1.05 | |
| 12-week | 21.91 ± 0.77 | 22.95 ± 1.01 | −1.04 (−3.62, 1.55) |
| Hindfoot to tibia peak angle (degree) | |||
| Baseline | 13.02 ± 0.94 | 14.53 ± 1.13 | |
| 12-week | 10.90 ± 1.06 | 13.18 ± 1.43 | −2.28 (−5.93, 1.38) |
| Forefoot to hindfoot ROM (degree) | |||
| Baseline | 10.07 ± 0.69 | 10.70 ± 0.83 | |
| 12-week | 11.11 ± 0.83 | 10.35 ± 1.16 | 0.76 (−2.09, 3.61) |
| Forefoot to hindfoot peak angle (degree) | |||
| Baseline | 4.92 ± 0.79 | 6.19 ± 0.96 | |
| 12-week | 6.13 ± 1.00 | 6.53 ± 1.46 | −0.39 (−3.95, 3.16) |
| Forefoot to tibia ROM (degree) | |||
| Baseline | 27.02 ± 0.91 | 26.22 ± 1.10 | |
| 12-week | 27.72 ± 1.22 | 27.51 ± 1.71 | 0.21 (−4.10, 4.52) |
| Forefoot to tibia peak angle (degree) | |||
| Baseline | 16.78 ± 0.74 | 18.21 ± 0.90 | |
| 12-week | 16.78 ± 0.88 | 19.26 ± 1.24 | −2.48 (−5.62, 0.65) |
| Hallux to forefoot ROM (degree) | |||
| Baseline | 20.14 ± 1.27 | 22.33 ± 1.51 | |
| 12-week | 21.69 ± 1.30 | 21.42 ± 1.84 | 0.27 (−4.36, 4.90) |
| Hallux to forefoot peak angle (degree) | |||
| Baseline | 17.67 ± 1.68 | 18.90 ± 1.99 | |
| 12-week | 21.43 ± 1.81 | 17.41 ± 2.47 | 4.02 (−2.20, 10.24) |
| Maximum arch height (% foot length) | |||
| Baseline | 10.23 ± 0.32a | 10.84 ± 0.39 | |
| 12-week | 11.24 ± 0.48a | 11.62 ± 0.67 | −0.38 (−2.08, 1.32) |
| Minimum arch height (% foot length) | |||
| Baseline | 7.78 ± 0.34b | 8.79 ± 0.41 | |
| 12-week | 8.80 ± 0.48b | 9.21 ± 0.67 | −0.41 (−2.09, 1.27) |
Data are mean ± standard error or mean difference (95% confidence interval). BM – body mass. ROM - range of motion.
Interaction effect p = 0.049, difference between intervention and control group at 12-week (post hoc p = 0.037).
time effect p = 0.04, difference between baseline and 12-week in the intervention group (post hoc p = 0.039).
After 12 weeks, hallux surface contact area was significantly increased in the IG compared to baseline (within-group mean difference = 0.5, 95%CI: 0.33, 0.68) (Table 3). The CG showed increased heel peak pressure after 12 weeks compared to baseline (within-group mean difference = 53.4, 95%CI: 38.6 to 68.3). Regarding within-group comparisons, the IG showed increased peak pressure (within-group mean difference= 88.4, 95%CI: 68.9, 107.9) and pressure-time integral at the central forefoot after 12 weeks compared to baseline (within-group mean difference = 36.6, 95%CI: 27.8, 45.4).
Plantar pressure variables during gait for each group (control and intervention) at baseline and 12-week.
| Region of Interest | Variable | Intervention Group (n = 31) | Control Group (n = 21) | Between-group SE difference at 12-week |
|---|---|---|---|---|
| HEEL | Contact Area [cm2] | |||
| Baseline | 38.5 ± 1.1 | 39.9 ± 1.5 | ||
| 12-week | 37.9 ± 1.5 | 41.1 ± 1.5 | −3.2 (−7.42, 0.96) | |
| Peak [kPa] | ||||
| Baseline | 383.5 ± 29.2 | 371.9 ± 31.0)& | ||
| 12-week | 451.6 ± 43.3$ | 425.3 ± 32.4&,$ | 26.3 (8.3, 1.27) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 104.9 ± 6.7 | 95.7 ± 6.5 | ||
| 12-week | 110.7 ± 8.8 | 109.2 ± 12.5 | 1.5 (−3.6, 6.6) | |
| MIDFOOT | Contact Area [cm2] | |||
| Baseline | 12.2 ± 0.7 | 13.4 ± 0.6 | ||
| 12-week | 13.0 ± 0.6 | 14.1 ± 0.7 | −2.48 (−5.62, 0.65) | |
| Peak [kPa] | ||||
| Baseline | 239.8 ± 23.5 | 112.6 ± 11.0 | ||
| 12-week | 320.9 ± 38.1 | 122.6 ± 8.5 | 198.4 (−73.6, 470.4) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 80.2 ± 7.3 | 37.1 ± 5.1 | ||
| 12-week | 90.8 ± 9.3 | 36.5 ± 2.5 | 54.4 (−10.2, 118.9) | |
| LATERAL FOREFOOT | Contact Area [cm2] | |||
| Baseline | 10.7 ± 0.6 | 12.2 ± 0.4 | ||
| 12-week | 10.4 ± 0.7 | 11.6 ± 0.4 | 0.76 (−2.09, 3.61) | |
| Peak [kPa] | ||||
| Baseline | 476.3 ± 52.8 | 474.5 ± 58.4 | ||
| 12-week | 483.2 ± 61.7 | 484.5 ± 53.4 | −1.3 (−28.3, 25.7) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 167.8 ± 18.8 | 159.6 ± 21.0 | ||
| 12-week | 175.9 ± 23.6 | 136.1 ± 14.1 | 39.9 (−14.10, 93.8) | |
| CENTRAL FOREFOOT | Contact Area [cm2] | |||
| Baseline | 30.2 ± 0.5 | 31.0 ± 0.4 | ||
| 12-week | 31.1 ± 0.8 | 31.4 ± 0.4 | −0.4 (−4.2, 3.5) | |
| Peak [kPa] | ||||
| Baseline | 558.4 ± 37.7a | 612.6 ± 39.5 | −34.0 (−166.5, 98.6) | |
| 12-week | 646.9 ± 45.2a | 680.8 ± 50.3 | ||
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 201.1 ± 12.4b | 210.1 ± 14.3 | ||
| 12-week | 237.8 ± 23.5b | 224.2 ± 20.5 | 13.6 (−47.6, 74.8) | |
| MEDIAL FOREFOOT | Contact Area [cm2] | |||
| Baseline | 13.2 ± 0.7 | 13.9 ± 0.8 | ||
| 12-week | 13.3 ± 0.8 | 14.8 ± 0.8 | −1.5 (−3.8, 0.8) | |
| Peak [kPa] | ||||
| Baseline | 384.7 ± 33.1 | 364.0 ± 41.9 | ||
| 12-week | 354.6 ± 36.4 | 359.7 ± 29.0 | −5.1 (−20.5, 10.3) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 138.8 ± 14.9 | 125.0 ± 14.9 | ||
| 12-week | 118.3 ± 11.2 | 122.3 ± 12.0 | −4.0 (−9.4, 1.5) | |
| HALLUX | Contact Area [cm2] | |||
| Baseline | 9.0 ± 0.4* | 9.8 ± 0.4 | ||
| 12-week | 9.5 ± 0.4*,$ | 10.2 ± 0.5$ | −0.7 (−0.9, −0.5) | |
| Peak [kPa] | ||||
| Baseline | 412.0 ± 41.4 | 380.0 ± 43.0 | ||
| 12-week | 400.6 ± 39.9 | 349.7 ± 40.1 | 51.0 (−59.9, 161.9) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 124.4 ± 15.6 | 111.2 ± 16.0 | ||
| 12-week | 113.6 ± 11.8 | 103.9 ± 15.6 | 9.6 (−28.81, 48.1) | |
| TOES | Contact Area [cm2] | |||
| Baseline | 12.7 ± 0.6 | 13.9 ± 0.7 | ||
| 12-week | 12.5 ± 0.7 | 13.4 ± 0.8 | −0.9 (−3.0, 1.1.) | |
| Peak [kPa] | ||||
| Baseline | 278.5 ± 24.0 | 261.0 ± 21.0 | ||
| 12-week | 320.1 ± 28.2 | 266.7 ± 24.9 | 53.4 (−20.3, 127.2) | |
| Pressure-time Integrals [(kPa)*s] | ||||
| Baseline | 86.6 ± 6.9 | 84.0. ± 7.2 | ||
| 12-week | 99.4 ± 11.2 | 80.7 ± 6.2 | 18.7 (−6.5, 43.9) |
Data are mean ± standard error or mean difference (95% confidence interval).
interaction effect p = 0.034, difference between baseline and 12-week in the intervention group (post hoc p = 0.005). Within-group (control) mean difference (CI 95%) 0.5 (0.3 to 0.7).
interaction effect p = 0.038, difference between baseline and 12-week in the control group (post hoc p = 0.013). Within-group (control) mean difference (CI 95%) 53.4 (38.6 to 68.3).
time effect p<0.001, difference between baseline and 12-week in the intervention group (post hoc p = 0.001). Within-group (intervention) mean difference (CI 95%) 88.4 (68.9 to 107.9).
Overall, the intervention slightly changed the propulsion mechanism of gait to a more physiological process, supported by some improvement in plantar pressure distribution, hip extensor moment, and plantar arch height. After 12 weeks, the IG showed a greater hip extensor moment at push-off and greater hallux contact surface area than the CG. In the within-group analysis, there was a larger plantar arch height during stance and higher peak pressure and pressure-time integral at the central forefoot at 12 weeks in the IG compared to baseline. However, the program did not result in significant changes in the foot-ankle dynamic ROM or in any other joint moment to positively impact overall lower limb biomechanics and plantar pressure in other plantar areas.
Many interventions aim to improve the propulsion mechanism in patients with gait disorders,21,24,29,30 because improved gait propulsion helps to increase gait speed, which is an important feature that represents independence and autonomy for keeping the functional status and performance of daily locomotor activities. In addition, gait speed is a strong predictor of mortality.31 The primary outcome of this RCT, gait speed, indeed increased after the exercise program,3 suggesting that the increase in the hip extensor moment could be somehow linked to changes in the gait propulsion mechanism, although there was no correlation between hip extensor moment and self- (R = 0.317, p = 0.151) and fast- gait speed (R = 0.198, p = 0.375).
Another biomechanical parameter that usually contributes to improvement in gait propulsion mechanism is ankle extensor moment. However, we did not observe a significant effect of the intervention on this parameter. Instead, the improvement in the propulsion mechanism was generated almost exclusively by greater hip extensor moment at push-off. A possible reason for this increase in hip extensor moment was that the program included bodyweight support training and gait training, which have been shown to improve the propulsive mechanism.32,33
The intervention resulted in an increased hallux contact surface area in the IG compared to CG and an increased peak pressure and pressure time-integral at the central forefoot compared to baseline. The CG showed increased peak pressure at the heel after 12 weeks. Although it cannot be stated that this increase in peak pressure in the heel means a worse plantar pressure distribution, this result was only observed in the control participants which suggests a potential increase in peak pressure at the heel during the natural history of DPN. In addition, higher peak pressures at the heel are resultant from a foot strike and a lower limb swing patterns that are commonly observed in people with DPN. 34 This finding is related to an abnormal foot rollover observed in these patients, resulting from a less efficient participation of the toes in the propulsion mechanism.34 According to Giacomozzi et al.,35 people with DPN have a smaller contact area under the forefoot and toes compared to people without DPN, showing that foot function is compromised resulting in inadequate weightbearing distribution during gait. We showed that a 12-week foot-ankle exercise program was effective to increase the hallux and central forefoot participation in the foot rollover process, which may represent better foot functioning during gait. In addition, the larger arch height during stance may also represent changes in the foot stiffness during gait. Such a change in the plantar arch height may be associated with a greater contribution of the intrinsic foot muscles to the stiffness of the plantar arch.
After 12 weeks, the IG showed an increase in the pressure variables at the central forefoot. Anteriorization of the load distribution appears to be a consequence of a greater contribution of the hallux and metatarso-phalangeal joints to the foot rollover. Load and pressure increase at the central forefoot are most likely correlated to the increase in walking speed in the IG. In addition, the improvement in plantar arch height during stance could also have changed the role of the forefoot during propulsion and then increase its vertical loading. Usually, attention is given to peak pressure and pressure-time integral reduction as a target to reduce the risk of ulceration, but these variables should be analyzed together with several other risk factors.36 Ulcer prevention in DPN should not aim exclusively at reducing pressures over the forefoot, mostly because this is not an optimal variable for describing changes during the whole process of foot rollover. It only correlates to the vertical loading acting on the forefoot during a very short time of the stance phase. That said, attention must be given to keeping peak plantar pressures below the proper risk threshold.37
Some studies have shown that individuals with DPN have limited ankle ROM during gait5 and during passive ROM assessment,6 which can affect foot rollover during locomotor tasks. Although this foot-specific exercise protocol was not capable of improving ankle mobility in gait/dynamic activities, there was an improvement in the plantar arch height during stance, which could have influenced the plantar pressure distribution and, consequently, foot rollover process.
No significant changes were found for any other lower limb kinetic or kinematic variables. Some hypotheses may explain this lack of differences found. The first hypothesis is related to the duration of the program (12 weeks) and/or the training intensity, which might not have been intense enough to change gait mechanics at the knee, hip, and ankle levels. The foot-ankle exercise program focused mainly on the foot joints, and although we incorporated functional exercises in the program to target the knee and hip muscles during locomotor tasks, this might not have been enough to alter hip and knee biomechanics. To make our study replicable, the program progressed in intensity (number of repetitions, sets, and resistance) following the individual tolerance to exercise and need to gain muscle strength and joint mobility. However, an insufficient workload among some participants may have influenced the absence of effects in the kinematic and kinetic variables. We opted to be consistent with the protocol previously described25 and not to change how we progressed in intensity and training volume.
Another reason for the lack of significant group differences is the type of intervention. We used a group approach to gain motivational feedback among participants and to optimize the program by treating more participants at the same time. However, this approach might be less effective for modifying musculoskeletal deficits and gait alterations due to DPN, as was observed in the intervention study by Melai et al.,23 which also used a group-based exercise program. While Kumar et al.22 found that individualized 12-week sessions were effective for changing gait biomechanics, it was not observed by Sartor et al.24 Thus, there is still room for debate and more RCTs should be conducted to assess if foot-related exercises could favorably change gait biomechanics.
Although there has been increasing number of trials investigating the effects of foot-related exercises in people with diabetes and DPN over the past decade,20 there are still a limited number of studies that include lower limb biomechanics during gait as outcomes. In the study by Sartor et al.,24 total ankle ROM during gait and ankle plantar flexion at propulsion was worsened among control participants after 12 weeks, and no effect was found on the ankle ROM among intervention-group participants. In the study by Melai et al.,23 no changes were observed in the lower limb biomechanics during gait after a 52-week foot-related exercise program, which the authors attributed to the low intensity of the training.
In summary, there is still little evidence that participation in a 12-week group-based foot-ankle exercise program could change lower limb joint kinematics and kinetics. Nonetheless, foot-ankle exercise may help to improve DPN-related aspects (RCT outcomes: gait speed, vibration perception, ankle ROM, and quality of life) in individuals with DPN. In addition to general aerobic exercises for glycemic control, specific exercises targeting foot-ankle and gait impairments are not yet widely recommended in clinical practice, and the only treatments available for DPN are for pain relief.38 However, only a small percentage of individuals with DPN have painful neuropathy, while deficits in musculoskeletal structure and function affect almost all individuals with diabetes. Thus, this intervention can be a useful supplementary treatment strategy especially for individuals with DPN without pain symptoms but with musculoskeletal deficits.
The strengths of this study are the use of RCT with rigorous methodology, a highly adherent population, a small dropout rate, a robust statistical model, and a larger sample size than other similar studies.24,39,40 A limitation of this study is that the CG interacted with the physical therapist and received feedback only at baseline and the 12-week assessment, whereas the IG had weekly interactions with the physical therapist. This difference might have led to a degree of dissatisfaction toward the study, which may have led to the greater loss to follow-up assessments among the CG. Furthermore, patients were not blinded to the treatment, and other usual parameters related to the clinical control of diabetes, such as glycated hemoglobin and glycemia, were not assessed and might have influenced the investigated biomechanical outcomes. Other aspects of the training, such as nocebo or placebo effects, duration, and intensity graduation, could have also obscured the effects. Regarding gait assessment and plantar pressure measurement, we performed habituation trials for each patient before data collection to avoid retroactive effects, such as step shortening or cadence modifications, however we cannot have absolute confidence that gait was not changed due to gait analysis, but if this was the case, the effects were similar in both groups and times of assessment.
ConclusionWe demonstrated that a 12-week foot-ankle exercise program for individuals with DPN resulted in a significantly greater hip extension moment at push-off, improved plantar arch height, and changes in plantar pressure distribution during gait compared to the changes following the standard care. Individual face-to-face, longer-duration, and more intensive exercise interventions may be needed to significantly affect foot-ankle biomechanics and pressure parameters in these individuals.
The authors would like to thank Full Professor Ana Claudia Latronico Xavier for supporting us with a database of diabetic patients at Hospital das Clínicas, Faculty of Medicine, University of São Paulo; and Professor Marcos Duarte from Universidade Federal do ABC for all support with the Phython program for data analysis. Monteiro was supported by Sao Paulo Research Foundation [FAPESP 2017/17848–3] and the work was supported by the National Council for Scientific and Technological Development, Brazil (CNPq) [28/2018 FOCA Trial 407252/2018–5]. Ferreira and Cruvinel were supported by FAPESP [2019/02522–0; 2021/00807–8]; Silva and Verissimo were supported by the Agency Coordination of Improvement of Higher Education Personnel (CAPES, financial Code 001). Sacco is supported as a fellow researcher 1B in CNPq, Brazil (Process 302558/2022-5). The funders had no role in the design, execution, interpretation, or writing of the study and did not have any authority over any study activity or in the decision to submit the report for publication.