Human Immunodeficiency Virus positive subjects present impairment in muscle function, neural activation, balance, and gait. In other populations, all of these factors have been associated with muscle strength asymmetry.
ObjectiveTo investigate the existence of muscle strength asymmetry between dominant and non-dominant lower limbs and to determine the hamstrings-to-quadriceps strength ratio in Human Immunodeficiency Virus positive subjects.
MethodsIn this cross-sectional study, 48 subjects were included (22 men and 26 women; mean age 44.6 years), all of them under highly active antiretroviral therapy. They performed isokinetic strength efforts at speeds of 60°/s and 180°/s for knee extension and flexion in concentric-concentric mode.
ResultsPeak torque was higher (p<0.01) at 60°/s for quadriceps (193, SD=57 vs. 173, SD=55% body mass) and hamstrings (97, SD=36 vs. 90, SD=37% body mass) in dominant compared to non-dominant. Similarly, peak torque was higher at 180°/s (quadriceps 128, SD=44 vs. 112, SD=42; hamstrings 64, SD=24 vs. 57, SD=26% body mass) in dominant. Average power was also higher for all muscle groups and speeds, comparing dominant with non-dominant. The hamstrings-to-quadriceps ratio at 60°/s was 0.50 for dominant and 0.52 for non-dominant, and at 180°/s, it was 0.51 for both limbs, with no significant difference between them. The percentage of subjects with strength asymmetry ranged from 46 to 58%, depending upon muscle group and speed analyzed.
ConclusionHuman Immunodeficiency Virus positive subjects present muscle strength asymmetry between lower limbs, assessed through isokinetic dynamometry.
The human immunodeficiency virus (HIV) attacks the immune system of its host and may cause acquired immunodeficiency syndrome (AIDS). However, the course of the HIV infection changed dramatically with the introduction of highly active antiretroviral therapy (HAART), which reduced patient mortality and morbidity and transitioned AIDS from an acute to a chronic disease.1 In a 2010 study, an examination of the specific causes of mortality in HIV-positive patients found that only 49.5% of deaths were AIDS-related, and the proportion of deaths classified as AIDS-related decreased with increasing duration of HAART.2 In contrast, HAART promotes several adverse effects, such as muscle atrophy, weight loss, and neurological dysfunction, which can lead to decreased muscle strength and functional capacity,3,4 and negatively influences the treatment and quality of life of HIV-positive subjects.5
Van As et al.6 studied 45 South African HIV-positive subjects and reported that 27% of the subjects presented diminished muscle power. Richert et al.7 analyzed the French Agency for AIDS and Hepatitis Research CO3 Aquitaine Cohort (n=324) and demonstrated that 50% of the HIV-positive subjects had poor performance on locomotor tests related to balance, aerobic endurance, and lower limb muscle strength, when compared to data established from the general population. In addition to the reduced muscle strength, a high percentage of HIV-positive subjects experience different types of neuropathies8 and impaired neuromuscular activation. Scott et al.9 demonstrated that decreased strength is associated with low muscle activation and not with muscle thickness in HIV-positive subjects submitted to HAART.
Muscle weakness, neurological dysfunction, and frailty index have been associated with muscle strength asymmetry10–14 in several populations including Parkinson's disease,14 aging10, multiple sclerosis,12 and traumatic brain injury.11 It has been established that force asymmetry in joints or extremities can lead to improper control of body movement and postural instability, being predictive of poorer balance and a more asymmetric gait11,15 and related to occurrence of injuries.16–18 When detected early, muscle strength asymmetry may predict locomotor impairment and frailty10; however, to the best of our knowledge, there are no studies that evaluated the occurrence of muscle strength asymmetry in HIV-positive subjects. Therefore, the aim of this study was to investigate the existence of muscle strength asymmetry between dominant and non-dominant lower limbs and to determine the hamstrings-to-quadriceps strength ratio (H:Q ratio) in HIV-positive subjects. Since HIV infection is associated with muscle weakness6 and neurological dysfunction,9 we hypothesized that this population presents some degree of muscle strength asymmetry between limbs.
MethodsResearch designA cross-sectional study was designed to measure lower limb muscle strength through isokinetic evaluation of knee extension and flexion. Each leg was tested on a dynamometer to determine muscle strength asymmetry between the D and ND lower limbs and between hamstrings and quadriceps muscles. Leg dominance was determined by asking the subjects which leg they preferred to use to kick a ball or to perform any other motor task. Procedures were conducted in two separate visits, the first to familiarize subjects with the equipment and the second to familiarize them with the testing procedures.
SubjectsForty-eight HIV-positive subjects (22 men and 26 women, mean age 44.6, SD=7.4 years, body mass index 26.2, SD=5.9kg/m2) were enrolled in the study. Subjects were recruited at Hospital das Clínicas da Universidade Estadual de Londrina and Centro Integrado de Doenças Infecciosas, Londrina, PR, Brazil. To be included in the study, the subjects should be aged 18–60, undergoing HAART for at least one year, not involved in any exercise program in the last six months, not taking hormones or anabolic steroids, and not presenting systemic infection (e.g., influenza, pneumonia, throat infection) within 30 days prior to the start of testing, and not having any other medical contraindication. The general characteristics and clinical parameters of the sample are presented in Table 1.
General characteristics and clinical parameters of the sample.
| Variables | HIV-positive (n=48) |
|---|---|
| Age (years) | 44.6 (7.4) |
| BMI (kg/m2) | 26.2 (5.9) |
| Time since HIV diagnosis (years) | 13.1 (5.7) |
| Time of HAART use (years) | 11.3 (5.4) |
| CD4+ lymphocytes (cells/mm3) | 693.3 (423.1) |
| CD8+ lymphocytes (cells/mm3) | 1059.3 (573.2) |
| HIV viral load | |
| Undetectable | 32 (66.7%) |
| 40–5000copies/mL | 13 (27.1%) |
| >5000copies/mL | 3 (6.2%) |
| HAART regimen composition | |
| NRTI+NNRTI | 17 (35.4%) |
| NRTI+PI | 22 (45.8%) |
| Other drug classes | 9 (18.8%) |
BMI, body mass index; HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. Data for age, BMI, time, and lymphocyte counts are presented as mean (SD).
The study was conducted at Universidade Estadual de Londrina (UEL), Londrina, PR, Brazil, and was initiated only after approval by the Human Research Ethics Committee of this university (protocol number 349512) from August 12, 2013. Subject participation was voluntary and all procedures took place after they signed an informed consent form.
ProceduresMedical recordsUsing medical records, the following data were obtained: year of HIV diagnosis; latest measures of viral load quantification and CD4+ and CD8+ lymphocyte counts; year of initiation of treatment with HAART; and actual HAART regimen composition. For HIV treatment, HAART is composed of different drug classes, and HAART regimen composition was presented as the most common classes: nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, and protease inhibitor.
Isokinetic evaluationIsokinetic evaluation was carried out at speeds of 60°/s and 180°/s for knee extension and flexion in concentric-concentric mode using a Biodex® Multi-Joint System – PRO dynamometer (Biodex Medical Systems Inc., Shirley, NY, USA). After five minutes of warming up on a stationary cycle-ergometer in a self-selected load, participants were positioned and stabilized on a dynamometer chair. Test range was determined according to range of motion of the subject that was being evaluated. Calibration and gravitational correction were also performed before the test.
Before each test speed, the subjects performed four submaximal repetitions to familiarize themselves with the movement and testing procedures. The protocol consisted of a set of three repetitions at a speed of 60°/s and a set of five repetitions at 180°/s, with a 2-min interval between sets. This procedure was performed bilaterally, and the order of which leg was to be tested first was chosen randomly. Verbal encouragement was used in order to motivate maximum effort during the test. Subjects first performed a familiarization session and then a testing session, which occurred after a minimum of 48h after the familiarization session (time range 48–72h), using identical conditions and at the same time of day. The intraclass correlation coefficient (single measurement, absolute-agreement, 2-way mixed-effects model) between the tests ranged from 0.83 to 0.97 for all variables, with no statistical differences between them, ensuring that the number of sessions were enough to determine the maximal effort across subjects. From the isokinetic evaluation, we obtained the variables peak torque (absolute values corrected by the body mass and expressed as percentage of this), average power, total work, and H:Q ratio.
H:Q ratio and side-to-side asymmetryThe H:Q ratio, which is calculated by dividing hamstrings concentric peak torque by quadriceps concentric peak torque, was provided by the equipment. Side-to-side asymmetry was calculated as the percentage difference between the peak torque of the D and ND limbs, through the formula “A%=((D−ND)/D)×100″.19 Mild asymmetry was defined as bilateral strength imbalance of more than 10%20 and marked asymmetry as imbalance of more than 25%.21
Statistical analysisGiven that sample size was not previously calculated, an a posteriori power analysis was performed using G*Power version 3.1.9.2 (Franz Faul, Universität Kiel, Germany). Considering p=0.05, the statistical power is 99% for detecting differences in peak torque for the quadriceps at 60°/s and 75% for detecting differences in peak torque for the hamstrings at the same speed.
Descriptive data was presented as mean (SD) for age, body mass index, time, and lymphocyte counts and as absolute and relative frequencies for HIV viral load, HAART regimen composition, and absence or presence of asymmetry. Data normality was verified using the Shapiro–Wilk test, and the variables average power and total work were shown not to have normal distribution. When comparing differences between the D and ND legs, the paired Student t-test and Wilcoxon test were performed for parametric and non-parametric variables, respectively. The 95% confidence intervals were also calculated to provide precision of statistical estimates. Significance was set in p<0.05, and all statistical tests were performed in Statistical Package for the Social Sciences version 22.0 (SPSS, Chicago, IL, USA).
ResultsComparison of isokinetic strength variables between D and ND limbs, in different test speeds, are shown in Table 2. We observed higher values for D limb in variables peak torque and average power for quadriceps and hamstrings, at speeds of 60 and 180°/s, when compared to ND limb. For the variable total work, there were higher values for D for quadriceps at 60 and 180°/s and for hamstrings at 180°/s. The H:Q ratio ranged from 0.50 to 0.52, with no significant difference between D and ND limbs for any speed analyzed.
Mean and standard deviation (SD) of isokinetic strength variables and comparison between dominant and non-dominant limbs at different test speeds.
| Variables | Speed | D | ND | 95% CI | p |
|---|---|---|---|---|---|
| Quadriceps | |||||
| Peak torque (% BM) | 60°/s | 193.4 (56.7) | 172.9 (54.9) | 12.5;28.7 | <0.01 |
| 180°/s | 128.3 (44.4) | 112.4 (41.6) | 9.8;21;9 | <0.01 | |
| Average power (W) | 60°/s | 77.8 (30.9) | 69.0 (29.3) | 5.1;12.5 | <0.01 |
| 180°/s | 88.0 (43.7) | 75.1 (42.5) | 7.0;18.9 | <0.01 | |
| Total work (J) | 60°/s | 358.1 (130.8) | 319.7 (123.4) | 21.6;55.2 | <0.01 |
| 180°/s | 357.2 (154.9) | 302.1 (148.6) | 33.1;77.1 | <0.01 | |
| Hamstrings | |||||
| Peak torque (% BM) | 60°/s | 96.9 (35.9) | 90.3 (37.1) | 1.7;11.5 | <0.01 |
| 180°/s | 64.1 (23.9) | 56.9 (26.0) | 2.7;11.6 | <0.01 | |
| Average power (W) | 60°/s | 40.4 (19.9) | 36.8 (17.9) | 0.8;6.5 | 0.02 |
| 180°/s | 38.2 (21.5) | 32.9 (26.4) | 0.6;10.1 | 0.01 | |
| Total work (J) | 60°/s | 186.1 (83.9) | 172.9 (82.8) | -1.6;27.8 | 0.28 |
| 180°/s | 156.7 (77.1) | 134.9 (93.3) | 4.5;39.1 | 0.01 | |
| H:Q ratio | 60°/s | 0.50 (0.10) | 0.52 (0.11) | -0.46;0.13 | 0.27 |
| 180°/s | 0.51 (0.13) | 0.51 (0.14) | -0.32;0.37 | 0.89 | |
D, dominant limb; ND, non-dominant limb; CI, confidence interval; (%BM), absolute values of peak torque corrected by body mass and expressed as a percentage of this; H:Q ratio, hamstrings-to-quadriceps ratio. Data are presented as mean (SD). The variables peak torque and H:Q ratio were analyzed using a paired t-test, and the variables average power and total work were analyzed using a Wilcoxon test.
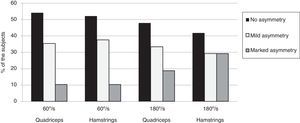
Fig. 1 shows the relative frequency of subjects with asymmetry in quadriceps and hamstrings peak torque at 60°/s and 180°/s. At 60°/s, the percentage of subjects with asymmetry was approximately 46% in quadriceps and 48% in hamstrings. The speed of 180°/s demonstrated that the percentage of subjects with asymmetry was approximately 52% in quadriceps and 58% in hamstrings.
DiscussionThe main findings of our study were that the values observed in isokinetic strength variables of lower limbs of HIV-positive subjects are higher for D compared to ND limbs. There was a significant proportion of subjects with between-limbs strength asymmetry (difference ≥10%) ranging from 45.8% in quadriceps at 60°/s to 58.4% in hamstrings at 180°/s. According to our knowledge, this is the first study that assessed lower limb strength asymmetry in HIV-positive subjects, although these differences have also been observed in other populations, such as older adults10 and subjects with multiple sclerosis12 and traumatic brain injury.11
It is difficult to compare studies that assess lower limb strength asymmetry because of the differences in testing protocols, parameters, strength indices, and populations. Lanshammar and Ribom22 tested muscle asymmetry in healthy females using peak torque obtained from concentric movements of knee extension and flexion and found a significant difference of 5.3% for quadriceps muscle strength and 8.6% for hamstrings, when comparing D and ND limbs. Siqueira et al.23 reported small differences for their control group, composed of healthy subjects. Our study demonstrated variations ranging from 10.6 to 12.4% for quadriceps and 6.8 to 11.2% for hamstrings when tested at 60°/s and 180°/s speeds, respectively. These asymmetry percentages are higher than those reported in healthy subjects are.
In addition to a bilateral asymmetry between D and ND limbs, our sample showed H:Q ratio values between 0.50 and 0.52. This variable has been well investigated in athletes and amateur sports players, and for a speed of 60°/s, it has been suggested that values lower than 0.6 indicate asymmetry between quadriceps and hamstrings, which could predispose the subject to injuries and strains.24 No differences between D and ND were observed but if we take into consideration the reference value of 0.6, our results would indicate poor performance of hamstrings and imbalance between agonist and antagonist muscles.
Previous studies with other populations demonstrated an association between muscle strength asymmetry and impaired balance or gait. Drijkoningen et al.11 demonstrated that increased asymmetry in muscle strength between D and ND limbs was associated with poorer balance control and a more variable and asymmetric gait. It is believed that, over time, an impaired balance and gait could lead to muscle imbalance, probably because the subject exerts more strength in one of the limbs.25,26 Furthermore, step length (the distance from contact of one foot to contact of the opposite foot) could differ between legs as a consequence of impaired gait and strength asymmetry, worsening this condition over time.25 Even though muscle strength asymmetry has not been investigated in HIV-positive subjects, previous studies detected impaired balance and gait in this population.27,28 Indeed, it is reasonable to say that increased strength asymmetry may be associated with impaired gait and balance in HIV-positive subjects, as demonstrated in Parkinson's disease,14 aging,10 multiple sclerosis,12 and others. In these other diseases and conditions, muscle strength asymmetry and impaired balance and gait may increase the susceptibility to falls, which is a relevant risk factor for injury in these individuals.10,14 Studies have demonstrated that HIV-positive subjects are also susceptive to impaired locomotor function due to HAART toxicity, which is inherent to a phenomenon known as premature or accelerated aging.29,30 Smith et al.30 described the elevated prevalence of sarcopenia and metabolic diseases in HIV-patients, both characteristics of aging. Indeed, HIV-patients experience some locomotor impairment especially due the adverse effects of antiretroviral drugs. Thus, muscle strength and asymmetry evaluation must be considered in this population.
Despite the presence of locomotor dysfunction, the causative phenomenon is poorly known in HIV-infected patients. The presence of neuropathies, neurological dysfunction, and central and peripheral impairments are the major reasons for muscle strength asymmetry in people with multiple sclerosis12,13 and traumatic brain injury.11 In these populations, previous authors also found an association between strength asymmetry and physical dysfunction, postural control and gait. In HIV-positive subjects, changes in central nervous system due to the action of HIV and HAART are well documented,31,32 demonstrating a high prevalence of neuropathies of up to approximately 50% of these subjects.33 Although HIV does not infect motor neurons, it can affect the central nervous system by infecting glial cells, which may lead to neurological damage by inflammation, neurotoxic action of viral proteins, or both mechanisms.34 Previous authors also demonstrated that neural impairment can lead to impairment in muscle action and mobility in HIV-positive subjects, which is associated with impaired balance and gait in these individuals.28
This study has some limitations that must be considered. The absence of an HIV-uninfected control group appears to be the main limitation of our study. Moreover, the prevalence of neuropathy, neural mechanisms during testing, balance, gait, and motor control were not determined. Future studies should consider these variables so that locomotor dysfunction in HIV-positive subjects can be better characterized.
In summary, our data demonstrates that HIV-positive subjects present elevated muscle strength asymmetry between lower limbs, assessed through isokinetic dynamometry. Neural impairment and changes in balance and gait may have played a role in the differences found and should be investigated further. Given the association between muscle strength asymmetry and impaired balance and gait, efforts should be made to correct such imbalances when diagnosed in HIV-positive subjects. Specific training on the isokinetic dynamometer and other forms of exercise performed bilaterally appear to be effective, with positive results in different populations.35,36 Thus, the practitioner or clinician should be aware of the methods available and choose the most appropriate for the patient.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank all of the staff at Hospital das Clínicas for helping in the recruitment of the sample and providing medical records and all of the subjects of the study. This study was funded by Brazilian grants from Secretaria da Ciência, Tecnologia e Ensino Superior do Paraná (Seti).