Muscle injuries are common, and their treatment requires costs and time off. Platelet rich plasma and low level laser therapy have been shown to be affordable and easy to use. The aim of this study was to evaluate the associated effects of low level laser therapy and platelet rich plasma on the treatment of the soleus muscle injured by strain in rats.
MethodsThirty-five rats were randomly allocated into five groups: Control (C), Injury Control (IC), injury PRP (IP), injury LLLT (ILT) and injury LLLT and PRP (ILTP). The strain injury was induced in the soleus muscle and the IP group received application of platelet rich plasma immediately after the lesion, while the ILT group received low level laser therapy. After seven days, all animals were euthanized and the soleus muscle removed for further histological analysis.
ResultsThe association of both treatments (ILTP) resulted in better histological aspects than the low level laser therapy and platelet rich plasma alone, when compared with the injury group (IC). The collagen analysis exhibited a significant increase in the ILT group (2.99 SD=1.13) compared to the C (1.88 SD=0.41, p=0.012) and IP (2.04 SD=0.44, p=0.018).
ConclusionThe association of low level laser therapy with platelet rich plasma produced better results on muscle injury compared to the isolated use of these therapies. Furthermore, none of the treatments could modulate the collagen deposition in relation to injury group.
Muscle injuries are defined as any kind of damage to the muscle components and are frequent in athletes and practitioners of physical activity.1,2 The injury leads to pain, limitation of movement and disability, which, depending on the degree of injury, treatment time could be prolonged for weeks or months, increasing the costs of treatment and absenteeism from work.3–5
In this sense, the study of techniques such as low level laser therapy (LLLT) and platelet-rich plasma (PRP) have great importance to improve the quality of tissue repair and to reduce treatment time. LLLT is commonly used in rehabilitation to treat muscle injuries,6,7 acting in the metabolism of the damaged muscle and having anti-inflammatory8,9 action in addition to its regenerative effect through stimulating the formation of new muscle fibers.6,7,10 Furthermore LLLT also has a protective effect against fibrosis.6
PRP is a low cost, safe and ease to apply technique which has high regenerative capacity, making it promising for the treatment of muscle injuries.11 PRP consists of a concentration of platelets, usually extracted from autologous blood, which has high concentrations of growth factors that stimulates mitosis of fibroblasts, angiogenesis that accelerates the recruitment of satellite cells and promoting myogenesis, thus reducing recovery time in muscle injuries.12,13
Both LLLT and PRP are currently used in clinical settings for the treatment of muscle injuries.14,15 However, studies on their association in animal models were not found in the current literature. Therefore, this study analyzed the combination of both treatments in muscle injuries, hypothesizing that the already proven effects of LLLT as an anti-inflammatory and preventative of fibrosis would act together with the PRP effects on tissue repair and production of extracellular matrix, composing a new treatment with high regenerative potential. Therefore, this study aimed to analyze histologically the muscle tissue of rats after muscle injury by strain and treated with LLLT, PRP and the association of both.
MethodsAnimalsThirty-five male Wistar rats of 150 days of age and average weight of 0.486g SD=0.05 were used, provided from the Central bioterium, of the São Paulo State University (UNESP), Botucatu-SP campus (Brazil), and maintained in the Faculty of Science and Technology – FCT/UNESP, Presidente Prudente Campûs, SP, Brazil. The animals were kept in plastic boxes at a controlled temperature (22 SD=2°C) and 12-h light/dark cycle with free access to water and food (standard laboratory chow).
All procedures were previously approved by the ethics committee for animal use from FCT/UNESP, Presidente Prudente Campûs, protocol 01/2013.
Experimental groupThe animals were randomly allocated into five groups of seven animals each:
- •
Control Group (C): animals remained in the bioterium and were euthanized paired with the experimental groups.
- •
Muscle Injury Control Group (IC): animals submitted to muscle injury, remained in the bioterium and were euthanized paired with the experimental groups.
- •
Muscle Injury treated with LLLT (ILT): animals submitted to muscle injury, received laser application daily for seven days.
- •
Muscle Injury treated with PRP (IP): animals submitted to muscle injury, received PRP application immediately after the injury.
- •
Muscle Injury treated with LLLT and PRP (ILTP): animals submitted to muscle injury, application of both protocols mentioned above.
The animals of the IP and ILTP groups underwent cardiac puncture for preparation of PRP and were then submitted to the muscle injury protocol. The other groups were submitted to the injury protocol immediately after confirmation of anesthesia. The C group was not submitted to any procedure.
After the lesion protocol, the PRP was immediately applied with the animals still under anesthesia, as well as the first LLLT session. The details of each protocol are described below.
Platelet-rich plasma preparationBlood collection was performed through cardiac puncture in the animals of the IP and ILTP groups. The animals were submitted to anesthesia by intraperitoneal administration of ketamine (70mg/kg) and xylazine (15mg/kg),7 and after confirmation of anesthesia a cardiac puncture was performed using a 0.2mL disposable syringe containing sodium citrate at 10%, 4mL of blood was obtained from each animal. Immediately after the puncture, saline solution was injected to restore blood volume.
The collected blood was centrifuged at 200g for 15min, splitting the sample into three parts: red bottom fraction, composed primarily of red blood cells; intermediate yellow-straw fraction (buffy coat), with the serum component; and the top fraction, composed of the blood plasma.
The top fractions were pipetted, including the buffy coat and the pipetted contents were centrifuged again at 500g for 10min. Next, 0.2mL of the bottom content PRP was pipetted.16
Platelet countingBlood and PRP samples were analyzed in the laboratory of the Veterinary Hospital of the Universidade do Oeste Paulista (UNOESTE) by means of an automatic blood cell analyzer (pocH 100iy Diff, Sysmex®). The analysis was performed on two blood samples and three PRP samples, for confirmation of platelets.17
Muscle injury protocolIn the IP and ILTP groups the muscle injury was performed immediately after cardiac puncture, avoiding the application of new doses of anesthesia. In the IC and ILT groups, the animals received an intraperitoneal injection of xylazine and ketamine, as described above. After confirmation of anesthesia, each animal was placed on the damage inductor equipment, in a supine position, with the hip in slight flexion, knee extension, and ankle in plantar flexion, the right leg attached to the machine with adhesive tape (duct tape). After positioning in the equipment, two electrodes were placed on the paw of the animal, on the calcaneal tendon and popliteal fossa, respectively. Electrical stimulation was applied suddenly to the positioned animal until full contraction of the lower limb in plantarflexion. Immediately afterwards, the equipment was fired, which promoted abrupt dorsiflexion of the lower limb of the animal while it was stimulated; the electrical current was stopped immediately after dorsiflexion. The dorsiflexion stimulation caused by the equipment and interruption of the current, in total, took an average of 2s to complete. This procedure was repeated until totaling 10 series, with a 30s interval between applications. In each series 2.25J was released, totaling 22.5J of energy applied to the muscle injury. This protocol was adapted from Pachioni et al.18
Platelet-rich plasma applicationIn animals of the IP and ILTP groups, 100μL of PRP was injected using a sterile syringe. The syringe with the needle was placed on the injured limb in the distal third of the tibia to be applied in the belly of the soleus muscle. The application of PRP was performed within 6h of the muscle injury protocol and withdrawal of blood.11
Low level laser therapy parameters and protocolDiode laser equipment was used (Coherent, Laser Cube), previously calibrated, with a wavelength of 637nm (visible red), output power of 25mW, beam 1mm in diameter, 3.18W/cm2 power density, and 10s of application, totalizing an energy per point of 0.25J.
The laser was applied to a single point, perpendicular to the muscle lesioned region, for 10s. The LLLT started on the day of injury and was conducted for seven consecutive days with 24h intervals between applications. This protocol was adapted from Iyomasa et al.19 and Luo et al.6 The dose was 31.85J/cm2, and the total dosage after seven applications was 222.95J/cm2.
Collection and sample preparationEuthanasia was performed 24h after the final laser therapy session paired with the other groups, totaling seven days between muscle injury and euthanasia. The animals were euthanized with an overdose of xylazine and ketamine, following ethical principles in animal research. The soleus muscle was removed surgically. For all analyses the muscle belly was used since it is the most damaged location as seen in the Pachioni et al.18 study.
Histological analysisThe samples were submitted to the freezing method for non-fixed tissues and stored at −75°C in an ultra-low temperature freezer, Coldlab CL580-80V. Cuts measuring 5μm in thickness were made in a cryostat microtome at −20°C, placed on slides and stained with hematoxylin-eosin (HE) for analysis of the structure of the muscles, and Picrosirius red for collagen analysis.20,21
The images of the HE slides were performed using an optical microscope Nikon Eclipse 50i, attached to an Infinity 1 camera. Next, the images were analyzed using the NIS-Elements D3 software. The qualitative analysis was based on the morphology of the muscle fibers, based on features such as the shape (polygonal, rounded, angled), connective tissue (endomysium and the perimysium), and inflammatory infiltrate fibers in a state of necrosis and phagocytosis. The semi-quantitative analysis of morphological characteristics was performed by the frequency of occurrence in each animal, and classified per intensity: absent (0), mild (1), moderate (2) and intense (3). This method was adapted from De Souza et al.22
The Picrosirius stained slides were photographed by a polarized light microscope Leica DM 4000B coupled to a DFC500 camera, Leica, belonging to the Faculdade de Odontologia, UNESP, Araçatuba Campûs, using a 10× objective. After, using ImageJ software,21 the mean gray value representing the collagen intensity in the image was measured.
Statistical analysisFor the semi-quantitative analysis of morphological characteristics, a frequency table was set up with the groups and the intensity of the variables.
For collagen analysis, the Shapiro–Wilk test was performed to verify data normality. After confirmation of normal distribution, the analysis of variance ANOVA One-Way test was performed with Tukey post hoc. All analyses were performed using SPSS software v.22; the significance level was 5%.
ResultsThe PRP platelet counts averaged 4998.676×103platelets/μL of blood, a number four times larger than that found in the blood of the animal (1068×103platelets/μL of blood).
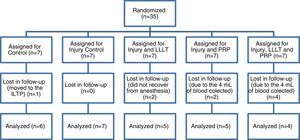
There was a sample loss of eight animals in the study (22.9%), mainly due to the autologous PRP protocol, removing 4mL of blood. As the animals were very young (150 days), drawing blood and anesthesia may have been very harmful to them (Fig. 1).
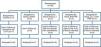
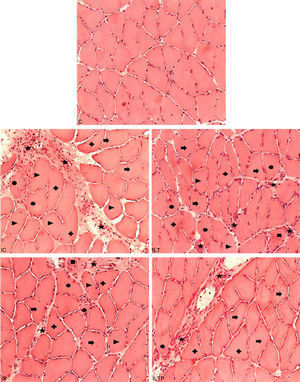
Histological analysis through the hematoxylin and eosin methodThe C group presented normal morphology, with polygonal fibers, peripheral nuclei and no presence of inflammatory infiltrate (Fig. 2). The endomysium and perimysium showed no alterations (Fig. 2). There were angular and rounded cells in some animals (Table 1).
Frequency values of morphological characteristics.
| Groups | Intensity | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Polymorphic | ||||
| C | 100% | 0% | 0% | 0% |
| IC | 0% | 0% | 0% | 100% |
| ILT | 0% | 0% | 0% | 100% |
| IP | 0% | 0% | 20% | 80% |
| ILTP | 0% | 0% | 25% | 75% |
| Rounded | ||||
| C | 83.30% | 16.70% | 0% | 0% |
| IC | 0% | 0% | 57.10% | 42.90% |
| ILT | 0% | 40% | 40% | 20% |
| IP | 0% | 60% | 40% | 0% |
| ILTP | 25% | 75% | 0% | 0% |
| Angled | ||||
| C | 50% | 50% | 0% | 0% |
| IC | 0% | 0% | 14.30% | 85.70% |
| ILT | 0% | 60% | 20% | 20% |
| IP | 0% | 60% | 40% | 0% |
| ILTP | 0% | 100% | 0% | 0% |
| Inflammatory infiltrate | ||||
| C | 100% | 0% | 0% | 0% |
| IC | 0% | 0% | 14.30% | 85.70% |
| ILT | 0% | 20% | 20% | 60% |
| IP | 0% | 20% | 60% | 20% |
| ILTP | 0% | 0% | 75% | 25% |
| Phagocytosis | ||||
| C | 100% | 0% | 0% | 0% |
| IC | 0% | 0% | 14.30% | 85.70% |
| ILT | 0% | 20% | 20% | 60% |
| IP | 0% | 0% | 20% | 80% |
| ILTP | 0% | 0% | 50% | 50% |
| Necrosis | ||||
| C | 100% | 0% | 0% | 0% |
| IC | 0% | 42.90% | 28.60% | 28.60% |
| ILT | 0% | 80% | 20% | 0% |
| IP | 0% | 80% | 0% | 20% |
| ILTP | 0% | 75% | 0% | 25% |
0, absent; 1, mild; 2, moderate; 3, intense; C, Control Group; IC, Injury Control; ILT, Injury LLLT; IP, Injury PRP; ILTP, Injury LLLT and PRP.
The IC group presented intense polymorphic fibers in all animals, fibers in the process of phagocytosis, as well angular and rounded fibers with a moderate to intense presence in all animals (Table 1). The epimysium and perimysium showed a great extent of structural disarrangement followed by intense inflammatory infiltrate in most animals (Fig. 2).
The ILT group presented intense polymorphic fibers in all animals; rounded and angular fibers had similar occurrences with the majority being mild or moderate (Table 1). Inflammatory infiltrate, as well as intense phagocytose fibers, appeared in 60% of animals. Moreover, this feature looked better than in the IC group, presenting less extensive inflammatory process and better distribution on the muscle (Fig. 2).
The IP group presented an intense presence of polymorphic fibers in 80% of the animals and mild presence in the other 20%. Intense inflammatory infiltrate was present in 20% of the animals with moderate in 60% and mild in 20%.
Table 1 demonstrates that in the group with both treatments (ILTP), intense polymorphic fibers were found in 75% of the animals and moderate in 25%. Rounded and angular fibers presented mild (75% and 100%) or absent frequency (25%). In addition, the ILTP group presented lesser amount of inflammatory cells and smaller injured area resulting in a lower structural disorder compared to the other groups (Fig. 2).
Histomorphometric analysis of collagenThe Picrosirius stained slides were analyzed by mean gray values that represents the sum of collagen deposition density, divided for the area of image.
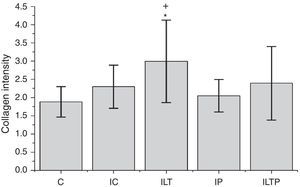
The ILT (2.99 SD=1.13) showed significantly more collagen compared to the C (1.88 SD=0.41, p=0.012) and IP (2.04 SD=0.44, p=0.018) (Fig. 3).
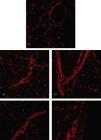
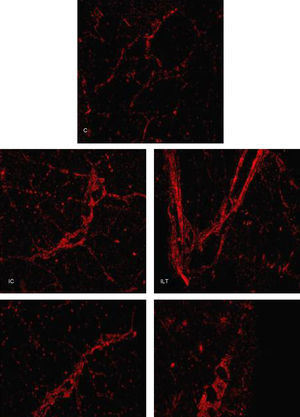
Samples that represent the distribution and organization of the collagen are presented in Fig. 4. Organization and even distribution of collagen is observed across the entire image in the control group (C), different from that found in the groups that suffered muscle injury (IC, ILT, IP, and ILTP), where the collagen is increased and concentrated at the injury sites (Fig. 4).
DiscussionMuscle injury promotes intense inflammatory infiltrate in the acute phase,6 with necrotic fibers and the presence of rounded, angled, polymorphic and atrophic cells.20,23,24 As time progresses the regeneration process becomes predominant and the presence of abnormal cells and inflammatory infiltrates decreases. New muscle fibers are generated as well as connective tissue deposition, which can lead to uncontrolled fibrosis scarring.1 These aspects were found in the groups studied (except the C group), and seven days post strain injury, muscles were in both the destruction and repair phases.
In the group submitted to the combination of both treatments, characteristics of stress on muscle tissue had lower intensity than the other experimental groups, especially when observing the rounded and angled fibers which most were classified as mild. According to Valsoni et al.,25 polygonal fibers represent healthy muscle; when the injury occurs these fibers present a rounded, angled and polymorphic shapes. These characteristics could be due to the structural disorder caused by injury, low input of nutrients, oxidative process or the aggression caused by lymphocytes.1
LLLT promotes angiogenesis and has anti-inflammatory properties,26–28 while PRP contains Platelet-derived growth factor (PDGF), Transforming growth factor (TGF-β1), and Vascular endothelial growth factor (VEGF), responsible for attracting cells to the injury site and promoting tissue regeneration.29,30 These factors probably reduced the damage in cells adjacent to the injury site, resulting in a lower incidence of fibers affected by the injury. In the ILTP group the association of these properties was clearly observed, resulting in a lower intensity of polymorphic, rounded and angular fibers (Table 1 and Fig. 2). As a secondary effect of fewer injured fibers, necrosis and phagocytosis was lower in the three treated groups compared to the injured group (IC).
The ILTP group presented no reduction in the intensity of the inflammatory process compared to the treatments applied separately. Although the effects of both therapies were not cumulative in this group, it presented characteristics of both treatments and showed better regeneration. Besides, ILTP presented lesser muscle structural disarrangement with a perimysium/endomysium less thickened, and the fibers that were not directly injured showed better organization with a healthy shape.
Regarding collagen, the current literature differs regarding the action of laser therapy in relation to collagen. Assis et al.15 and Luo et al.6 observed a reduction in collagen after seven days of injury in the group treated with LLLT in relation to the control group. However, Alves et al.27 and De Souza et al.22 observed an increase in collagen in the group treated with LLLT compared to the control group. The present research showed a significant increase in the amount of collagen in the ILT group compared to the C and IP groups. LLLT inhibits TGF-β, which transforms satellite cells into fibroblasts instead of myoblasts, thus promoting a decrease in collagen at the injury site.8
The formation of new fibers is more intense with seven days after the injury,22,26,27 and excess collagen begins the formation of fibrosis 14 days after injury.6,22 As our study only evaluated the injury up to seven days, fibrosis was not observed, and there was no formation of new fibers seven days post injury.
In relation to PRP, as it contains a large amount of TGF-β, which is responsible for stimulating the synthesis of collagen, it could lead to formation of dense scar tissue, and, associated with the inflammatory process, subsequently lead to formation of fibrosis,30 which did not occur in this study, showing that the application of PRP did not exacerbate the production of collagen at the site of the injury.
The ILTP group did not present statistical difference regarding the amount of collagen compared to the other groups, demonstrating that the PRP inhibited the effect of LLLT, since there was no increase in relation to C group, or reduction in relation to ILT group. Thus, it was not possible to explain how one treatment influenced the other; however, it is noted that both influenced the collagen.
During the repair phase, a scar of connective tissue fills the space of the injury while new fibers are formed and muscle structure becomes organized.2 Although scar formation is important for the regeneration of muscle tissue, the fact that LLLT increased this process within seven days cannot be concluded as a positive effect as it was not followed by full restoration of the injured muscle tissue; in the same way that the fact that PRP did not promote an increase in collagen cannot be considered positive or negative.
The limitations of this study include the large volume of blood punctured for PRP preparation, which could impair the animal. Also, animal weight can interfere in the intensity of the injury due to variation in the size and weight of each animal, even with the use of a properly regulated device. Finally, we had a mean lost to follow up of 22.9% which may have influenced in our results.
We suggest future studies complement the present results with deeper analyses such as markers of injury proteins, proteins associated with the synthesis of new fibers, analysis of blood vessels, cellular oxidation and an extended study time in addition to human studies, where medical and clinical tests could prove the positive histological effects seen in this study.
ConclusionWe conclude that the association of LLLT with PRP presented better results in the regeneration of muscle tissue than the use of the individual therapies. Moreover, the treatments were not capable to modulate collagen production with seven days post injury in relation to injury group.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful by supported grants for São Paulo Research Foundation (FAPESP), protocol no. 2014/10086-2.