The cortical silent period is a transient suppression of electromyographic activity after a transcranial magnetic stimulation pulse, attributed to spinal and supraspinal inhibitory mechanisms. Electromyographic breakthrough activity has been observed in healthy adults as a result of a spinal reflex response within the cortical silent period.
ObjectivesThe objective of this case series is to report the ipsilesional and contralesional cortical silent period and the electromyographic breakthrough activity of 7 children with congenital hemiparesis.
MethodsTMS was delivered over the ipsilesional and contralesional primary motor cortices with resting motor threshold and cortical silent period measures recorded from first dorsal interosseous muscle.
ResultsSeven children (13±2 years) were included. Ipsilesional and contralesional resting motor thresholds ranged from 49 to 80% and from 38 to 63% of maximum stimulator output, respectively. Ipsilesional (n=4) and contralesional (n=7) cortical silent period duration ranged from 49 to 206ms and 81 to 150ms, respectively. Electromyographic breakthrough activity was observed ipsilesionally in 3/4 (75%) and contralesionally in 3/7 (42.8%) participants. In the 3 children with ipsilesional breakthrough activity during the cortical silent period, all testing trials showed breakthrough. Contralesional breakthrough activity was observed in only one of the analyzable trials in each of those 3 participants. The mean peak amplitude of breakthrough activity ranged from 45 to 214μV (ipsilesional) and from 23 to 93μV (contralesional).
ConclusionFurther research is warranted to understand the mechanisms and significance of electromyographic breakthrough activity within the cortical silent period in congenital hemiparesis. Understanding these mechanisms may lead to the design of tailored neuromodulation interventions for physical rehabilitation.
Trial registrationNCT02250092 (https://clinicaltrials.gov/ct2/show/NCT02250092)
The cortical silent period (CSP) is a transient suppression of electromyographic activity after the delivery of a transcranial magnetic stimulation (TMS) pulse in a voluntarily activated muscle.1,2 The CSP duration is proposed to be influenced by spinal and supraspinal mechanisms.3–5 The initial phase of the CSP is caused by an after-hyperpolarization and subsequent depression of alpha motoneurons, both occurring at the spinal level.6–8 The late phase of the CSP is attributed to supraspinal mechanisms, as a result of intracortical inhibitory neuronal activation mediated by gamma-aminobutyric acid B (GABAB).1,6,8 The CSP duration has been found to vary between 100 and 300ms in hand muscles of healthy adults when the TMS pulse is applied over the primary motor cortex.3,6 In typically developing children (ages between 4.2 and 16 years), the CSP duration has been found to vary between 1 and 207ms due to immaturity of GABAB-ergic cortical inhibitory mechanisms.9,10
The CSP duration has been proposed to be an important tool for measuring motor recovery after stroke given the mediation by spinal and supraspinal inhibitory mechanisms.11 The majority of research in adults with stroke has focused on CSP duration to study the intracortical inhibition mechanisms. In comparison to healthy controls, both prolonged ipsilesional CSP duration and shortened contralesional CSP duration have been reported.11–16 A prolonged ipsilesional CSP is associated with poor functional recovery after stroke.13 Studies have also reported a shortened ipsilesional CSP, which was found to be associated with an increased degree of spasticity, in adults in different post-stroke phases, including acute, subacute and chronic phase post-stroke.11,13 Two studies have described CSPs in children with neurologic conditions including children with spastic diplegia and children with congenital hemiparesis. In children with spastic diplegia, CSPs were found to be shorter than durations reported in typically developing children.13 In prior reports of children with hemiparesis, shorter CSP durations were found compared to healthy adults.9 More recently, CSP duration has been shown to decrease with age in healthy children and adults, reflecting a potential influence of maturation.10 Additionally, cortical excitability has been found to vary across muscle groups in healthy adult controls, with greater inhibition found over the limb muscles as compared to more proximal axial musculature.17
During the CSP, the period of electromyographic (EMG) silence is sometimes interrupted by EMG ‘breakthrough’ activity, described as small amounts of low-level EMG activity occurring in the first 50ms of the CSP.4,5 The purported origin of the EMG breakthrough activity is a spinal reflex, considering the time and potential mechanism, which indicates muscle spindle mediation of its occurrence.5 This reflex response is thought to be induced by sudden relaxation of the muscles within the silent period and a subsequent alpha motor neuron response to the activation of muscle spindles.5 Thus, the EMG breakthrough activity may be an important mechanism to evaluate spinal cord excitability in neurological populations. The EMG breakthrough activity has been reported in healthy adults.5,18,19 However, no study, to our knowledge, has reported the EMG breakthrough activity in children with congenital hemiparesis.
This case series reports ipsilesional and contralesional CSPs and EMG breakthrough activity in children with congenital hemiparesis due to perinatal stroke or periventricular leukomalacia. The report provides a basis for future studies on the underlying neurophysiological mechanisms. Further, this information could help in the design of individualized neuromodulation interventions for physical rehabilitation in children with congenital hemiparesis.
MethodsParticipantsA total of seven children (4 females and 3 males) with congenital hemiparesis (mean age=13 years; age range=10–16 years) included in this study were enrolled in a transcranial direct current stimulation/constraint induced movement therapy study (ClinicalTrial.gov NCT02250092).19 This report details baseline motor threshold and CSP duration. Informed consent and assent were obtained from all legal guardians and the children for study participation and for publication in this case series. The study was approved by the University of Minnesota, Minneapolis, MN, USA, Institutional Review Board (IRB#: 1408M53169).
The inclusion criteria for this study, involving cortical excitability and silent period testing, were: (1) hemispheric stroke or periventricular leukomalacia confirmed by magnetic resonance imaging (MRI), (2) clinical signs of hemiparesis, (3) active range of motion at the metacarpophalangeal joint ≥10 degrees to establish a muscle contraction during CSP assessment, and (4) an elicitable motor evoked potential (MEP) from the contralesional hemisphere during resting motor threshold (RMT) assessment. The exclusion criteria were: (1) metabolic disorders, (2) neoplasm, (3) epilepsy, (4) disorders of cellular migration and proliferation, (5) acquired traumatic brain injury, (6) pregnancy, (7) indwelling metal or incompatible medical devices, (8) evidence of skin disease or skin abnormalities, (9) botulinum toxin within six months or phenol block within twelve-months preceding the study, (10) seizure activity within the last 2 years.
The Assisting Hand Assessment (AHA) measures how effectively children with cerebral palsy use their affected hand in bimanual performance. The raw scale ranges from 22 points (hand is not used at all) to 88 points (hand is used effectively).20 The Manual Ability Classification System (MACS) is a 5-point classification system which describes a child's ability to use both hands for functional, daily activities.21 A MACS classification rating of 1 or 2 indicates that the child is independent with most activities and does not require assistance in order to be independent. A MACS classification rating of 3–5 indicates the child is dependent for most daily activities and requires assistance in order to participate in the daily task. The majority of children included in our study had a high level of independence in daily activities according to their scores in these two scales.
Transcranial magnetic stimulation assessmentParticipants were seated in a reclining chair for the TMS assessment. For EMG recordings, surface Ag–AgCl bipolar electrodes were placed over the first dorsal interosseous (FDI) muscles on the more-affected and less-affected hands, using a muscle belly-tendon montage with a reference electrode placed over the wrist. The EMG signals from the target muscles were acquired with a sampling rate of 6.4kHz using a bandpass filter width of 20–2000Hz (Cadwell Laboratories, Kennewick, Washington). For TMS assessment, a Magstim 200 magnetic stimulator (Magstim Company Ltd., Dyfed, UK) with a 70-mm figure-of-eight coil was used. The coil was positioned to elicit a posterior to anterior cortical current and with the handle positioned 45° posterolaterally over the approximate location of the primary motor cortex. The single-pulse magnetic stimuli were delivered at approximately 0.1Hz, with an upper limit intensity of 85% of maximal stimulator output (MSO). A stereotactic neuronavigational tracking system (Brainsight, Montreal, Canada) was used to guide the TMS coil position over the primary motor cortex for individual participants, using 1mm3 resolution 3T T1-weighted MRI images obtained from a Siemens Trio Scanner with a 32-element head coil.22 Assessment commenced on the non-lesioned hemisphere and then proceeded to the lesioned hemisphere. To familiarize the participant with TMS, stimulator intensity started at 50% MSO and then increased by increments of 5%.
TMS testing included assessment of the RMT and the CSP. The RMT was defined as the minimum machine intensity required to elicit a MEP with a peak-to-peak amplitude ≥50μV, in at least 3 of 5 trials with the target muscle at rest. This location was identified as the motor hotspot. A Motion-Lab electromyography system (Y03-2, Motion Lab Systems, Inc., Baton Rouge, LA, USA) with a custom written LabView program (v2012, National Instruments, Austin, TX, USA) was used to visualize the MEP responses. To assess the CSP, 10 TMS pulses at 120% of RMT were delivered to the motor hotspot of the lesioned and non-lesioned hemispheres. TMS pulses were delivered during an attempted 20% maximal voluntary contraction (MVC) of the FDI muscle with an interval of approximately 10seconds between pulses. To assist children in maintaining 20% of MVC as closely as possible, the EMG signal was displayed on a computer screen in front of the participants with a colored trace representing the target muscle contraction level. Considering children's tolerance to brain stimulation, CSPs were not assessed in children with RMT exceeding the limits of 85% of the maximum stimulator output (MSO) as established for our safety protocol. CSP trials were considered valid when participants were able to maintain a muscle contraction when the TMS pulse was delivered. Some participants had difficulty in maintaining muscle contraction and not all provided 10 valid CSP trials. Participants were included for analysis if they had at least 5 analyzable CSP trials.
Safety measures for TMS assessment included systematic questions related to potential adverse event and vital sign assessment (blood pressure and heart rate) before and after all TMS evaluation sessions.23,24 The risk mitigation protocols for this study are described by Gillick et al. (2018).25
Data processing and analysisTo analyze CSP duration, EMG activity was full-wave rectified and smoothed using a 10-ms moving standard deviation (SD) window with subsequent baseline activity threshold set at 100% of the average of the smoothed pre-stimulus EMG activity (−100 to −5ms). The EMG signal was processed using MatLab software (The MathWorks, Inc, MatLab® R2015a). The CSP onset was defined as the end of the MEP, where the MEP curve declined below the baseline threshold. The CSP offset was defined as the return of the EMG activity above the baseline threshold. The CSP duration was then measured from the designated CSP onset to the CSP offset (CSP offset – CSP onset). CSP duration was averaged for each muscle. A random selection of 20% of CSP traces was used to test the inter-rater reliability in three independent and trained raters using the intraclass correlation coefficient (ICC3,1).26 The ICC was considered poor when <0.20, fair from 0.21 to 0.40, moderate from 0.41 to 0.60, good from 0.61 to 0.80, and very good from 0.81 to 1.00.26 The CSP duration measurements from the main rater were used for analysis.
Based on previous reports,5 the breakthrough activity was defined as an EMG burst occurring between the CSP onset and offset, within a latency around 60–100ms. The latency of the EMG breakthrough activity was measured from the stimulus artifact to the first inflection of the breakthrough EMG burst that exceeded the baseline threshold. The EMG breakthrough amplitude was defined as the peak amplitude of the rectified and smoothed EMG burst activity. The same rater, chosen for CSP duration measurements, determined the EMG breakthrough duration and amplitude. All data are reported descriptively.
ResultsParticipants characteristicsIndividual participant's data are reported in Tables 1 and 2. Six of the seven participants (85.7%) had left hemisphere imaging confirmed lesions. Lesion location included the cortical and subcortical areas (n=5, 71.4%) or in subcortical areas only (n=2, 28.6%). (Table 1). Ipsilesional and contralesional RMTs were obtained for all 7 participants. The mean ipsilesional RMT was 63% of MSO (range 49–80% of MSO). The mean contralesional RMT was 49% of MSO (range=38–63% of MSO) (Table 2).
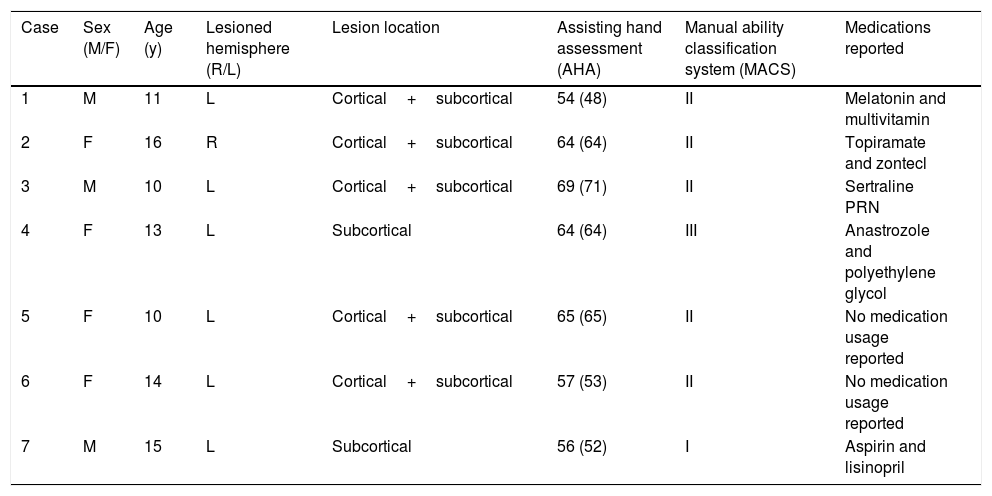
Demographic and clinical characteristics of the participants.
| Case | Sex (M/F) | Age (y) | Lesioned hemisphere (R/L) | Lesion location | Assisting hand assessment (AHA) | Manual ability classification system (MACS) | Medications reported |
|---|---|---|---|---|---|---|---|
| 1 | M | 11 | L | Cortical+subcortical | 54 (48) | II | Melatonin and multivitamin |
| 2 | F | 16 | R | Cortical+subcortical | 64 (64) | II | Topiramate and zontecl |
| 3 | M | 10 | L | Cortical+subcortical | 69 (71) | II | Sertraline PRN |
| 4 | F | 13 | L | Subcortical | 64 (64) | III | Anastrozole and polyethylene glycol |
| 5 | F | 10 | L | Cortical+subcortical | 65 (65) | II | No medication usage reported |
| 6 | F | 14 | L | Cortical+subcortical | 57 (53) | II | No medication usage reported |
| 7 | M | 15 | L | Subcortical | 56 (52) | I | Aspirin and lisinopril |
AHA score are sum score (scaled score); M, male; F, female; R, right; L, left; NR, not reported; y, years. The lesion location was determined by a neurologist using neuroimaging.
Individual and group data of ipsilesional and contralesional resting motor threshold, cortical silent periods and EMG breakthrough activity during the CSP.
| Case | Ipsilesional hemisphere | Contralesional hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| RMT %MSO | CSP ms | EMG breakthrough | RMT %MSO | CSP ms | EMG breakthrough | |||
| Latency ms | Amplitude (μV) | Latency ms | Amplitude (μV) | |||||
| 1 | 70 | UD | UD | UD | 55 | 150 (29) | 89 (NA) | 24 (NA) |
| 2 | 55 | 134 (25) | 57 (3) | 42 (5) | 38 | 142 (31) | UD | UD |
| 3 | 71 | 129 (27) | 62 (7) | 118 (45) | 63 | 125 (15) | UD | UD |
| 4 | 65 | UD | UD | UD | 57 | 121 (22) | 30 (NA) | 68 (NA) |
| 5 | 80 | UD | UD | UD | 45 | 81 (35) | 54 (NA) | 93 (NA) |
| 6 | 49 | 206 (16) | 65 (7) | 214 (130) | 43 | 95 (41) | UD | UD |
| 7 | 50 | 49 (18) | UD | UD | 45 | 96 (30) | UD | UD |
| Mean (SD; range) | 63 (12; 49–80) | 130 (55; 49–206) | 62 (3; 57–65) | 125 (70; 45–214) | 49 (6; 38–63) | 115 (26; 81–150) | 58 (24; 30–89) | 61 (28; 23–93) |
Individual data are mean (standard deviation) and group data are mean (standard deviation; range). CSP, cortical silent period; EMG, electromyography; ms, milliseconds; MSO, maximal stimulator output; NA, not applicable; RMT, resting motor threshold; SD, standard deviation; UD, undetectable; μV, microvolts. Standard deviation was not calculated for contralesional EMG breakthrough activity because contralesional EMG breakthrough activity was evident in only 1 cortical silent period (CSP) trial.
Determination of CSP duration revealed very good inter-rater reliability between three raters (ICC=0.93; 95% confidence interval=0.88–0.96).
Ipsilesional CSPs were detected in participants 2, 3, 6 and 7. The mean ipsilateral CSP duration for these 4 children was 130ms (range=49–206ms) (Fig. 1A). Ipsilesional CSP was undetectable in 3 of 7 participants (42.8%). Missing data from ipsilesional data collection was attributed to participant 5 who had an RMT exceeding the established tolerability limits of 85% MSO in this study and no analyzable CSP for participants 1 and 4 (Fig. 2A and B). Contralesional CSPs were found in all participants (n=7, 100%). The mean CSP duration was 115ms (range=81–150ms) (Fig. 1B).
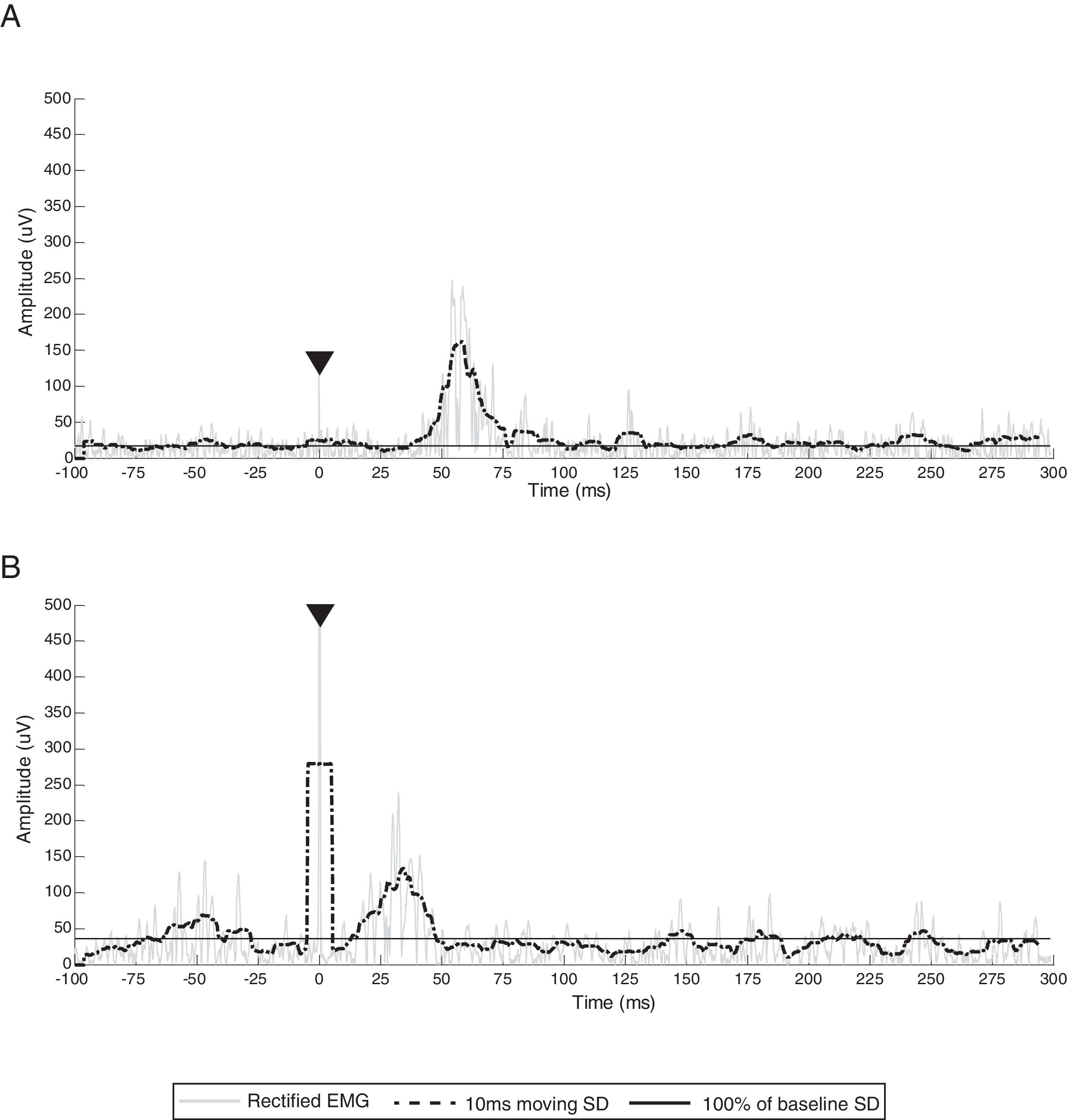
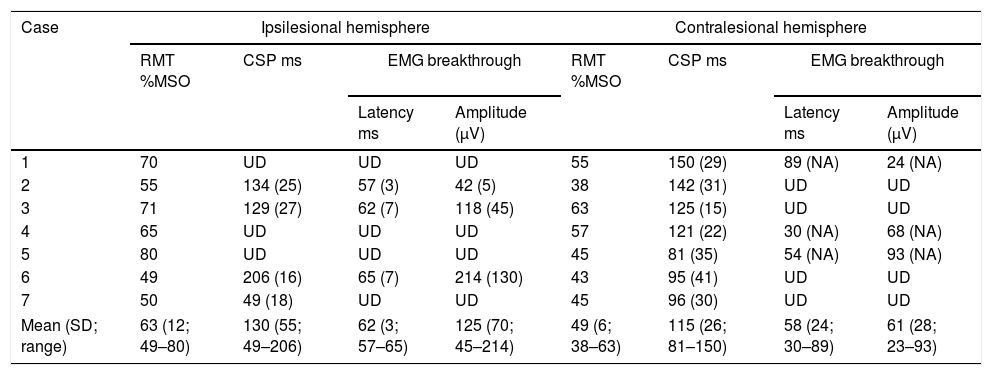
Sample EMG traces from participants who did not present with an analyzable CSP. (A) Ipsilesional EMG traces from participant 1 and (B) ipsilesional EMG traces from participant 4. Gray line represents rectified EMG; black dotted line represents 10ms moving SD EMG activity; black full line represents baseline threshold; EMG, electromyography; SD, standard deviation.
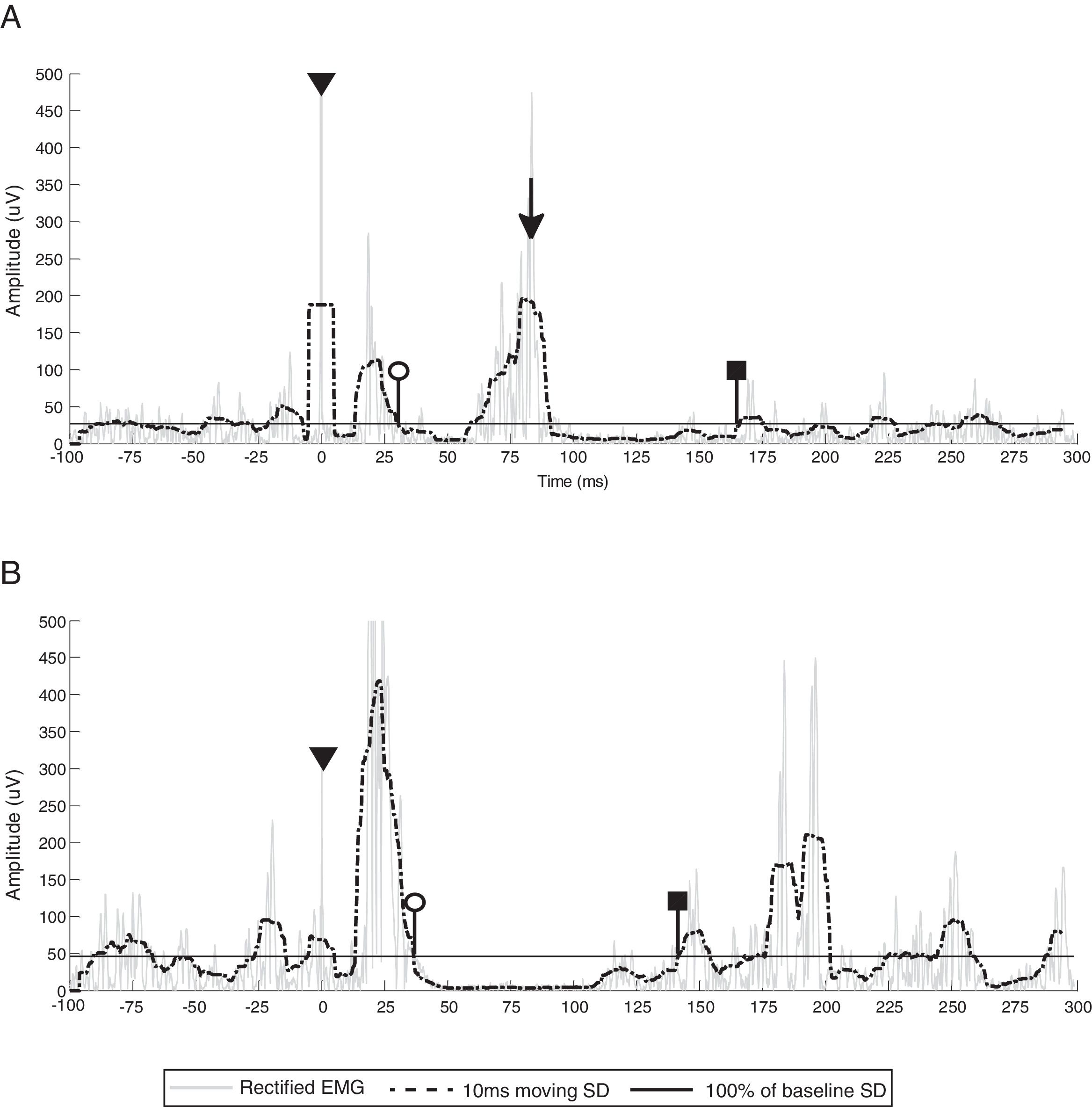
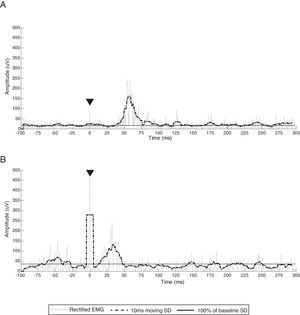
CSP duration from participant 3, representing common CSP characteristics of all participants (stimulus artifact, CSP onset, CSP offset). White circles indicate CSP onset (end of the MEP), black squares indicate CSP offset (return of EMG activity that exceeds baseline threshold) and triangles denote stimulus artifact. (A) Ipsilesional CSP and EMG burst activity (black arrow). (B) Contralesional CSP. Gray line represents rectified EMG; black dotted line represents 10ms moving SD EMG activity; black full line represents 100% baseline SD EMG activity; EMG, electromyography; SD, standard deviation.
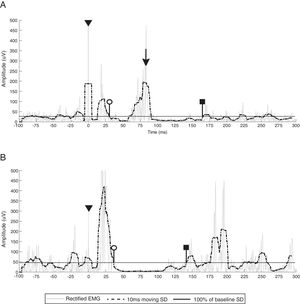
Of the 4 participants with an ipsilesional CSP, 3 participants displayed EMG breakthrough activity (75.0%) in all CSP trials (participants 2, 3 and 6). The mean EMG breakthrough onset latency and duration was 62ms (range=57–65ms) and 43ms (range=28–56ms), respectively. There was high between-participant variability in the amplitude of the EMG breakthrough activity (mean=125μV; range=42–214μV), ranging from 428–1676% of the pre-stimulus EMG activity (Fig. 3A–C).
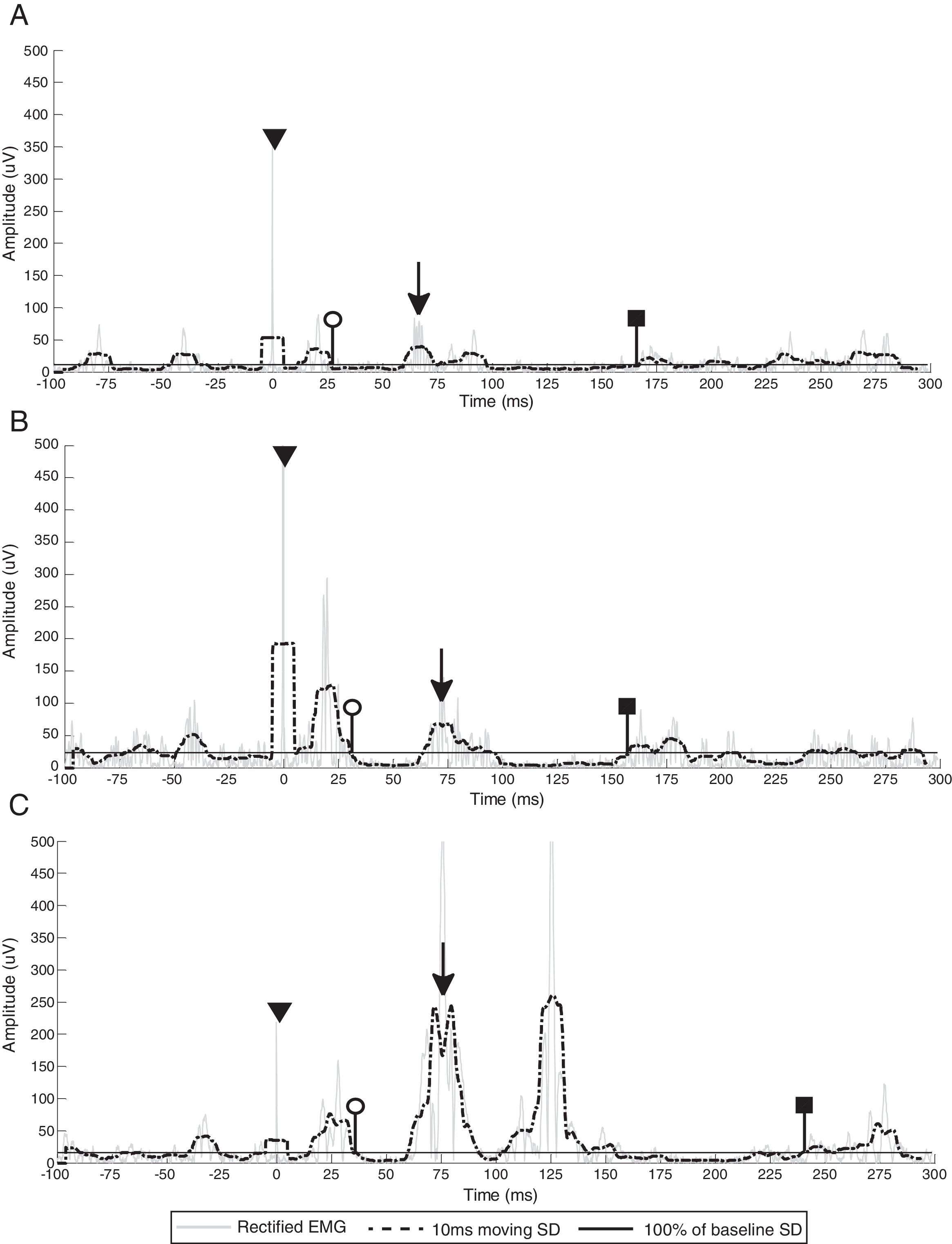
EMG breakthrough activity (marked with black arrows) during the ipsilesional CSP of participants 1 (A), 3 (B) and 6 (C). White circles indicate CSP onset (end of the MEP), black squares indicate CSP offset (return of EMG activity that exceeds the baseline threshold) and triangles denote stimulus artifact. Gray line represents rectified EMG; black dotted line represents 10ms moving SD EMG activity; black full line represents the baseline threshold; EMG, electromyography; SD, standard deviation.
Of the 7 participants with a contralesional CSP, 3 participants showed EMG breakthrough activity (42.8%) (participants 1, 4 and 5) during contralesional CSP. However, this was not consistently observed and evident in only 1 CSP trial for each of the 3 participants. The mean latency of EMG breakthrough activity was 58ms (range=30–89ms) and the mean amplitude was 62μV (range=24–93μV) (Table 2).
DiscussionThis case series describes the CSP and the presence of EMG breakthrough activity in 7 pediatric participants with congenital hemiparesis. The case series demonstrates a measurable ipsilesional CSP which included high-amplitude intra-CSP EMG breakthrough activity in 3 of the 4 participants who present with a ipsilesional CSP. The observed breakthrough activity was also noted during the contralesional CSP in 3 of 7 participants. However, the ipsilesional EMG breakthrough was only noted in a small proportion of participants (3 of 7) and the contralesional EMG breakthrough was observed during only one analyzable contralesional CSP in each participant. EMG breakthrough has been reported in healthy adults as low amplitude EMG activity (8–15% of the pre-stimulus activity) within a latency of 67–100ms after the TMS pulse.6,8 Although the latency of the EMG breakthrough activity in our series of children was consistent with previous studies, the amplitude of the breakthrough during the ipsilesional CSP was higher (428–1676% of pre-stimulus EMG activity) compared to previous studies of healthy adults.
The EMG breakthrough activity observed during the CSP is purported to be mediated by spinal reflex mechanisms. During the CSP, a marked drop in muscle force due to TMS stimulus leads to muscle lengthening which increases muscle spindle firing and ultimately triggers spinal alpha motor neurons thereby generating the EMG breakthrough.27,28 This explanation is supported by previous studies that reported a decrease in the EMG breakthrough amplitude during muscle shortening contractions and joint immobilization, conditions that prevent muscle lengthening during the CSP.5,28 In contrast, an increased incidence and amplitude of ipsilesional EMG breakthrough response could reflect a facilitated spinal reflex attributed to changes in spinal circuit excitability in children with congenital hemiparesis. Increased fusimotor drive has been implicated as a reduction in presynaptic inhibition on the sensory component of Ia afferents, group Ib facilitation (instead of inhibition), group II facilitation, and reduced reciprocal inhibition. One of the most widely accepted factors that could increase the spinal reflex is the decreased presynaptic inhibition of Ia afferents.29,30 Muscle spindle Ia afferents are responsible for monitoring the velocity of muscle stretch changes. When repetitively activated at low frequencies, the Ia afferents generate presynaptic inhibition that could contribute to exacerbated reflex responses.27,29,30 Studies have reported a decreased presynaptic inhibition of Ia afferents in congenital hemiparesis which could be associated with spasticity.9,31,32
Because the present case series describes only seven participants, future studies are needed to address the relationship between EMG breakthrough activity and spasticity. The EMG breakthrough activity could be used as a measurement of spinal excitability in neurological conditions featured with spasticity. It therefore may also be important clinically due to the potential associations with motor function performance.
Regarding the CSP duration, we found a large variability in CSP duration (ipsilesional CSP duration range=49–206ms and contralesional CSP duration range=81–115ms). Comparison to previous reports is limited by methodology for calculating onset and offset and equipment set-up. Previous reports in typically developing children showed variable CSP durations. In 40 children ages 8–16 years, Moll et al.33 reported CSP durations ranging between ‘1 and 207ms’, depending on stimulus intensity. Garvey et al.34 reported CSP durations ranging between 3.5–205.0ms in 27 typically developing children ages 6.4–13.9, and Heinen et al.31 reported a mean duration of 140.0±30.2ms in 7 typically-developing children with ages 4.2–5.7 years. The large variability in CSP duration and differences from our findings could be a result variability in testing protocols (e.g. stimulus intensity, TMS machine used), study design (e.g. age range of participants), and analysis methodology (e.g. calculating the onset and offset). Since the age ranges of our participants differed from previous studies, no conclusions regarding the prolonged and/or shortened CSP duration based on clinical condition can be made. Mackey et al.9 previously reported in children with congenital hemiparesis mean ipsilesional and contralesional CSP duration of 86.0±46.8 and 141.0±56.3ms, respectively. Differences in these values and the high variability in ipsilesional CSP duration could reflect the clinical heterogeneity of congenital hemiparesis and/or could be related to the differences in brain lesion type and locality as reported in adult stroke.11 Larger studies evaluating CSP duration in children with congenital hemiparesis are indicated to explore this relationship further.
Further, no analyzable ipsilesional CSP was able to be elicited in participants 1 and 4 who presented with an ipsilesional RMT. This continuous EMG activity, where typically a CSP would be present, could originate from contralesional structures contributing to muscle contraction yet the muscle activity is unaffected by the TMS stimulus5,35,36 It is also worth noting that at the time of the study, participants 1 and 2 were taking centrally-acting medications that increase the activity of GABA-B (melatonin and topiramate) which may have had the inhibitory potential to prolong the CSP duration (Table 1). However, no such influence was observed as each of these participants displayed an unchanged CSP duration.
ConclusionThis case series describes the CSP duration in children with congenital hemiparesis and provides new information regarding the presence of the EMG breakthrough activity during the CSP. Assessing CSP duration and EMG breakthrough activity in future neuromodulation studies may be useful to build an understanding of central and spinal inhibitory mechanisms and spinal reflex responses in children with congenital hemiparesis. An understanding of these inhibitory mechanisms may then lead to the design of tailored neuromodulation interventions for physical rehabilitation in this population.
FundingThis work was supported by the following funding agencies: São Paulo Research Foundation (Process no. 2015/16744-4); NIH/NICHD 5 K01-HD078484-02, Cerebral Palsy Foundation, Foundation for Physical Therapy, UMN Marie Louise Wales Fellowship, Minnesota's Discovery, Research, and Innovation Economy Fellowship.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank all the participants and families who participated in our study. We thank Dr. Mo Chen for his technical support with data collection and analysis.