To explore the relationships between clinical measures and the ability to increase walking speed in ambulatory people with chronic stroke and to identify which measures would best predict walking speed reserve.
MethodsAn exploratory, cross-sectional study was conducted with 114 individuals with chronic stroke. The outcome of interest was walking speed reserve, defined as the difference between individuals’ comfortable and maximal walking speeds. Predictors were characteristics of the participants (age, sex, time since stroke, relative lower-limb dominance) and motor impairments (tonus, strength, and motor coordination).
ResultsThe characteristics of the participants did not significantly correlate with walking speed reserve. All measures of motor impairments, i.e., tonus, strength, and motor coordination, were significantly correlated with walking speed reserve (p < 0.01), but only motor coordination was kept in the regression model. Motor coordination alone explained 35% (F = 61.5; p < 0.001) of the variance in walking speed reserve.
ConclusionsThe level of motor coordination of the paretic lower limb is associated with the walking speed reserve of individuals with stroke. Interventions aimed at improving motor coordination may have the potential to improve everyday situations that require immediate increases in walking speed.
Recent data indicate that over 30 million people in the world have survived a stroke, which is the leading cause of long-term disability worldwide.1,2 Many individuals present with residual walking disabilities after stroke, which includes decreased stride length and cadence, temporal asymmetry, and reduced walking speed.3 Reduced walking speed has been associated with restrictions in leisure activities and community ambulation.4 It is well documented that individuals after stroke have the potential to increase their comfortable walking speed after an intervention, and that benefits can be maintained one month or longer beyond the intervention period.3,5–7
Many everyday situations, such as the need to catch a bus or to pick up a phone, require immediate increases in walking speed. In addition, walking quickly may be necessary for increasing the intensity of physical activity for better health or to walk with others.8 This ability to immediately increase walking speed is known as walking speed reserve, and can be clinically calculated as the difference between maximal and comfortable walking speeds.9 After a stroke, individuals typically demonstrate increased muscle tone (e.g., spasticity and/or hypertonia),10 muscle weakness,11 deficits of motor coordination, and balance impairments.12 Intrinsic characteristics, in association with these motor impairments, may limit individuals’ ability to increase walking speed. This assumption suggests that many individuals with stroke walk at, or close to, their maximal walking speeds and may be unable to accurately respond to environmental demands.9,13 Although previous studies14,15 have shown that strength of the major lower limb muscles is a significant individual predictor of walking after stroke, factors associated with the ability to increase walking speed are still unclear.
Two recent studies9,16 examined the contribution of a single motor impairment to changes in walking speed after stroke. Hsiao et al.16 investigated the contribution of lower-limb propulsive forces, and results suggested that strength may be related to the ability to increase walking speed. More recently, Middleton et al.9 reported a strong correlation (r = 0.74) between balance impairments and walking speed reserve. No previous studies have examined the relationships between multiple personal factors (eg, age, sex, lower-limb dominance) and impairments in body structures and function (e.g., weakness, hypertonia), and walking speed reserve.
The purpose of this study was to explore the relationships between personal characteristics and clinical measures of motor impairments, and the ability to increase walking speed in ambulatory people with chronic stroke. The specific research questions were:
- 1)
What were the magnitudes and directions of the relationships between personal characteristics (age, sex, time since stroke, relative lower limb dominance) and clinical measures (tonus, strength and motor coordination), and walking speed reserve in people with stroke?
- 2)
Which measures would best predict walking speed reserve in people with stroke?
The findings may provide more precise information regarding the factors that limit the ability to increase walking speed after stroke and, therefore, help guide clinical practice by suggesting specific variables to be targeted during rehabilitation of patients with chronic stroke.
MethodsDesignThis is a cross-sectional study.
ParticipantsCommunity-dwelling people with stroke, who were living in a metropolitan city, were recruited by means of social media advertisements and referrals from clinicians working in public hospitals and rehabilitation centers, from March 2013 to December 2014. People were included if they were ≥20 years of age and at least six months after the onset of the stroke; had weakness and/or increased tonus of the ankle plantar flexor muscles, as determined by 15% strength differences between the paretic and non-paretic limbs17 and/or scores different from zero on the Modified Ashworth Scale;18 and had no cognitive impairments, as determined by the following education-adjusted cut-off scores on the Mini-mental state examination (0–30 points): 18/19 for the individuals with illiteracy and 24/25 for those with basic education.19
ProceduresBefore data collection, eligible participants were informed about the objectives of the study and provided written consent, based upon previous approval from the Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil ethical review board (#CAAE 06609312.0.0000.5149). Demographics and clinical data were obtained by interviews, followed by clinical measurements of motor impairments and walking speeds.
Outcome measuresPotential predictors of walking speed reserve included characteristics of the participants (age, sex, time since stroke, and relative lower-limb dominance) and motor impairments (tonus, strength, and motor coordination). The dominant lower-limb was determined by asking the participants which leg they would have used prior to the stroke for kicking a ball.20 Relative lower-limb dominance was categorized as dominant if the paretic side was the dominant lower limb or non-dominant if the non-paretic side was the dominant lower limb.21 Clinical measures of motor impairments (tonus, strength, and motor coordination) were collected during a single-testing session in a laboratory setting. Data were obtained by two well-trained physical therapists who have 10 years of clinical and research experience, submitted to a week-training to ensure consistency of measurements.
Tonus of the paretic ankle plantar flexors was measured using the Modified Ashworth Scale18 and reported on a 6-point Likert scale, that ranges from zero (no increase in muscle tone) to four (paretic limb rigid in flexion or extension). Measurements were taken with the participants lying in the supine position, with their lower limb completely relaxed. This scale is widely used clinically and has shown adequate reliability (kappa = .84 for interrater and .83 for intrarater comparisons) for individuals with stroke.18
Maximal isometric strength of the paretic hip flexors, knee flexors, and knee extensors was measured using a hand-held dynamometer (Microfet 2 M T, Hoggan Health Industries, West Jordan, UT) and normalized to body mass. The strength measures of the three muscle groups were summed and reported, in N/kg. All strength measurements were taken with the participants lying in the supine position, with the lower limb to be tested placed on a stool at 90° of hip and knee flexion. The participants were instructed to push as hard as they could against the dynamometer for three to four seconds.22 Hand-held dynamometry has shown adequate psychometric properties when used to measure strength in individuals with stroke.23
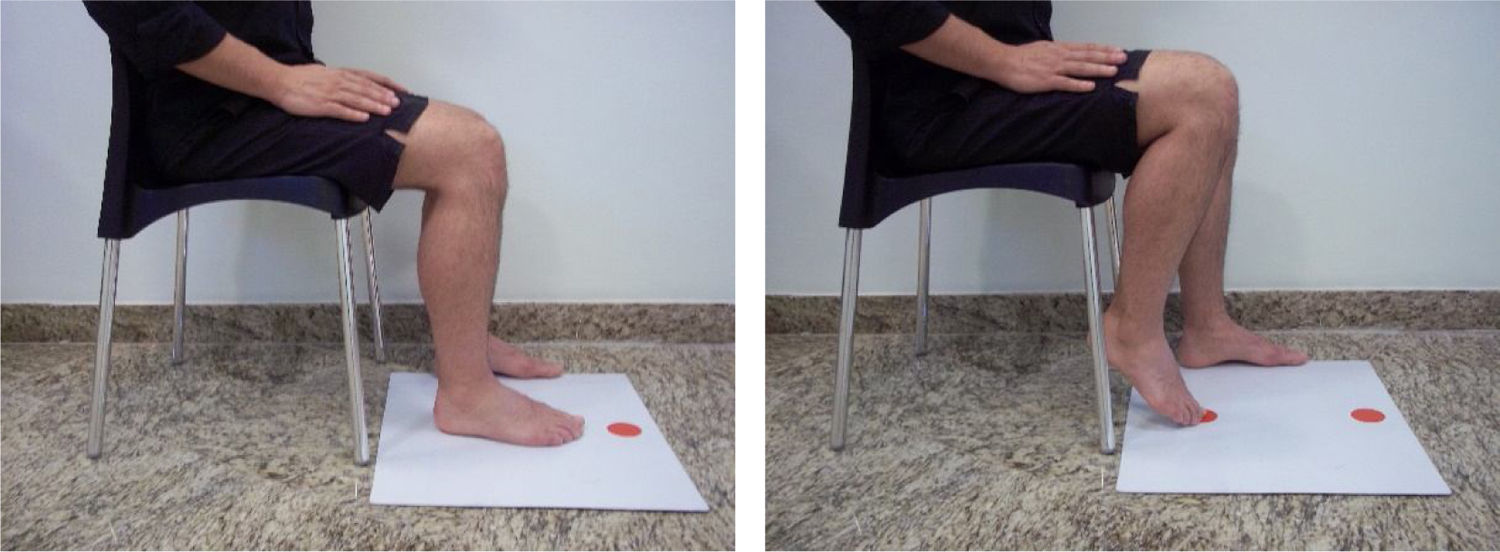
Motor coordination of the paretic lower limb was measured using the Lower Extremity Motor Coordination test (LEMOCOT) and reported as number of touched targets in 20 s. The LEMOCOT has excellent psychometric properties, when performed by people with stroke, and has been shown to be the most suitable lower limb motor coordination test for this population.24 Participants performed the LEMOCOT three times with their paretic lower limb,24,25 and the average score of the 3 trials was recorded for further analyses. Participants sat on a height adjustable chair with their feet resting flat on a thin rigid foam, heels on the proximal target, and knees at 90° of flexion. Then, after a familiarization trial, they were instructed to alternately touch the proximal and distal targets placed 30 cm apart with their big toe for 20 s (Fig. 1). They were instructed to go as fast as they could while maintaining the accuracy and quality of the movement during the test. The LEMOCOT evaluates the spatial and temporal domains of motor coordination of the knee and ankle joints.22,26
The primary outcome of interest was walking speed reserve, defined as the difference between the individuals’ comfortable and maximal walking speeds, reported in m/s. Walking speed was measured using the 10 m Walk Test, which has demonstrated appropriate psychometric properties when used to measure walking ability in individuals with stroke.27 First, the participants were instructed to walk at their most “comfortable speed” along a 14-meter hallway, using their usual assistive devices or orthoses. Next, they were instructed to walk according to the following command: “walk as fast as possible and safely, but without running, to reach a bus, which is about to pull out”.28 One familiarization trial was performed followed by a registered trial, which was used for analyses. The time to cover the central 10 m was recorded with a digital stopwatch.28
Sample sizeBased on the assumption that about 15 participants per planned predictor is needed for an equation that will cross-validate with little loss in predictive power,29 a minimum of 105 participants would be required. Efforts were made to include participants with diverse levels of walking ability,30 that would be representative of individuals with stroke, who are housebound, limited-community ambulators, or unlimited-community ambulators.
Statistical analysesData were tested for normality (Kolmogorov–Smirnov) and descriptive data calculated. Pearson correlation coefficients or Chi-Square tests were used to explore the relationships between the predictors and walking speed reserve. The strength of the relationships was classified as low (r < 0.30), moderate (0.30 < r < 0.50), and high (>0.50).31 Step-wise multiple linear regression analysis was used to identify which measures, i.e., age, sex, time since stroke, relative lower limb dominance, tonus, strength, and motor coordination, would significantly explain the ability to increase walking speed, i.e., walking speed reserve. Step-wise multiple regression analysis uses specific criteria to retain or to eliminate variables to maximize prediction accuracy with the smallest number of predictors. This approach is useful to identify variables that make the most valuable contribution to a given relationship, therefore, building a model with fewer variables.32 All analyses were performed with the SPSS statistical software 23.0 for Windows, with a significance level of 0.05.
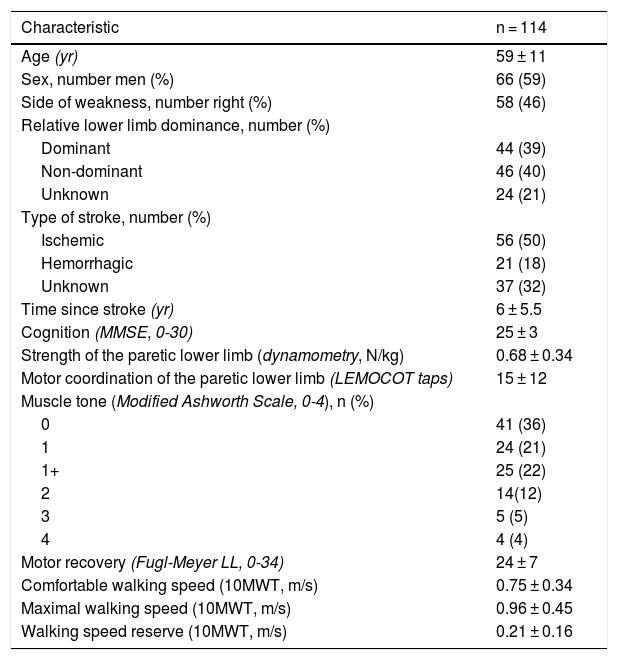
ResultsCharacteristics of the participantsOf 535 potential participants, 170 agreed to participate and were physically screened, of which, 114 met the inclusion criteria. Thus, 114 individuals, 66 men, with a mean ± standard deviationage of 59 ± 11 years and a mean time since the onset of the stroke of 6 ± 5.5 years were evaluated. Fifty-six participants had an ischemic stroke, 21 had a hemorrhagic stroke, and 37 did not know the type of stroke. The mean walking speed reserve was 0.21 ± 0.16 m/s, ranging from 0 to 0.7 m/s. Twenty-four (21%) participants were classified as housebound (walking speed <0.4 m/s), 39 (34%) as limited-community ambulators (walking speeds between 0.4 and 0.8 m/s), and 51 (45%) as unlimited-community ambulators (walking speed >0.8 m/s). The characteristics of the participants are summarized in Table 1.
Characteristics of the participants.
| Characteristic | n = 114 |
|---|---|
| Age (yr) | 59 ± 11 |
| Sex, number men (%) | 66 (59) |
| Side of weakness, number right (%) | 58 (46) |
| Relative lower limb dominance, number (%) | |
| Dominant | 44 (39) |
| Non-dominant | 46 (40) |
| Unknown | 24 (21) |
| Type of stroke, number (%) | |
| Ischemic | 56 (50) |
| Hemorrhagic | 21 (18) |
| Unknown | 37 (32) |
| Time since stroke (yr) | 6 ± 5.5 |
| Cognition (MMSE, 0-30) | 25 ± 3 |
| Strength of the paretic lower limb (dynamometry, N/kg) | 0.68 ± 0.34 |
| Motor coordination of the paretic lower limb (LEMOCOT taps) | 15 ± 12 |
| Muscle tone (Modified Ashworth Scale, 0-4), n (%) | |
| 0 | 41 (36) |
| 1 | 24 (21) |
| 1+ | 25 (22) |
| 2 | 14(12) |
| 3 | 5 (5) |
| 4 | 4 (4) |
| Motor recovery (Fugl-Meyer LL, 0-34) | 24 ± 7 |
| Comfortable walking speed (10MWT, m/s) | 0.75 ± 0.34 |
| Maximal walking speed (10MWT, m/s) | 0.96 ± 0.45 |
| Walking speed reserve (10MWT, m/s) | 0.21 ± 0.16 |
MMSE, mini-mental state examination; LL, lower limb; LEMOCOT, lower extremity motor coordination test; 10MWT, 10-meter walk test. Data are mean ± standard deviation, unless indicated.
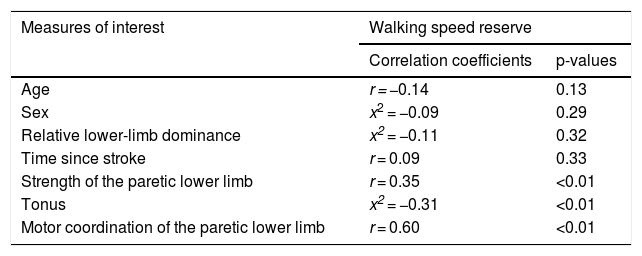
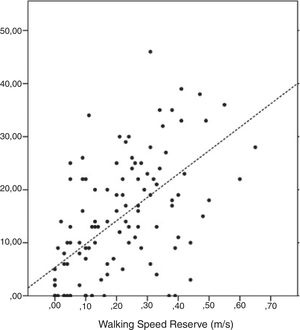
Table 2 provides the correlation coefficients between all selected measures and walking speed reserve. No significant associations were found between the characteristics of the participants and walking speed reserve; p > 0.13). All clinical measures of motor impairments were significantly correlated with walking speed reserve (p < 0.01). The correlation with tonus (Modified Asworth scores) was negative and of moderate magnitude (r = −0.31); the correlation with strength was positive and of moderate magnitude (r = 0.35); and the correlation with motor coordination (LEMOCOT scores) was positive and of high magnitude (r = 0.60) (Fig. 2).
Association between the selected measures and walking speed reserve.
| Measures of interest | Walking speed reserve | |
|---|---|---|
| Correlation coefficients | p-values | |
| Age | r = −0.14 | 0.13 |
| Sex | x2 = −0.09 | 0.29 |
| Relative lower-limb dominance | x2 = −0.11 | 0.32 |
| Time since stroke | r = 0.09 | 0.33 |
| Strength of the paretic lower limb | r = 0.35 | <0.01 |
| Tonus | x2 = −0.31 | <0.01 |
| Motor coordination of the paretic lower limb | r = 0.60 | <0.01 |
r, Pearson correlation coefficient; x2, Chi-square.
All significantly correlated variables i.e., tonus, strength, and motor coordination, were included in the regression analyses as potential predictors of walking speed reserve. However, only motor coordination reached significance (p < 0.05) and, consequently, was kept in the model (Table 3). Motor coordination alone explained 35% (F = 61.5; p < 0.001) of the variance in walking speed reserve, and the emerged regression equation was: y = 0.094 + 0.008 * LEMOCOT score.
Results of the regression analyses regarding the potential predictors of walking speed reserve (n = 114).
| Model | B (95% CI) | R | Adjusted R2 | SEE |
|---|---|---|---|---|
| Walking speed reserve | ||||
| Constant | 0.096 (0.055, 0.133) | – | – | – |
| LEMOCOT scores | 0.008 (0.006, 0.010) | 0.60 | 0.35 | 0.13 |
B, regression coefficients; CI, confidence interval; R2, coefficient of determination; SEE, standard error of the estimate; LEMOCOT, lower extremity motor coordination test.
This study explored the relationships between personal characteristics and clinical measures of motor impairments, and the ability to increase walking speed i.e., walking speed reserve, in ambulatory people with chronic stroke. Moreover, this study also planned to identify which of the measures would significantly predict walking speed reserve. The results revealed that all measures of motor impairments, i.e., tonus, strength, and motor coordination, were significantly correlated with walking speed reserve. However, only motor coordination was kept in the regression analysis and explained 35% of the variance in walking speed reserve.
The mean walking speed reserve found in the present study was 0.21 m/s, which is consistent with previous studies that measured walking speed reserve in people with stroke (range: 0.2–0.3 m/s).13,16 The results indicated that the ability to increase walking speed is considerably reduced in people with stroke, compared with healthy middle aged people (40–60 years old; mean walking speed reserve: 0.7 m/s).33 The correlation analyses indicated that age, sex, time since stroke, and relative lower limb dominance did not correlate with walking speed reserve. In contrast, all measures of motor impairments were significantly correlated with walking speed reserve. Therefore, the results reinforce the need to focus on evaluation and rehabilitation of motor impairments.34,35
There has been some investigation on the relationships between measures of strength and walking speed reserve after stroke, without including measures of muscle tone or motor coordination. Jonkers et al.13 demonstrated, in a very small group of slow walkers with stroke (n = 6), that the ability to increase walking speed was limited by impaired hip and ankle power generation. More recently, Hsiao et al.16 reported that, after gait training, the paretic lower limb increased its contribution during speed modulation, suggesting that strength could be related to the ability to increase walking speed. Although our results confirmed that both muscle strength and tonus were associated with walking speed reserve, the correlations were of low-to-moderate magnitudes and could not predict speed modulation. The lack of measurement of strength of the muscles of the ankle joint may have underestimated the relative contribution of strength on the ability to increase walking speed, and future studies may wish to re-examine these relationships.
A recent study9 reported a strong correlation (r = 0.74) between balance impairments and walking speed reserve, but the investigation did not include any other variables that could be related to walking. Both motor coordination and balance are influenced by multiple structural components, e.g., tonus, strength, joint integrity, which seem to be functionally linked to behave as a single task-specific unit.36 For that reason, when individuals have the minimum physical requirements to perform a task, their ability to coordinate the biological structures plays a larger role in explaining activity adjustments, such as the ability to increase walking speed. For instance, poor motor coordination between trunk and pelvis has been associated with the inability to adjust speed of walking in people with stroke,12 and low back pain.37 The existing evidence suggests that both motor coordination and balance may be more representative of the ability to perform or make adjustments during complex tasks, such as walking. Future studies should include both measures of balance and motor coordination impairments, to examine their relative contributions to walking speed reserve. As our data only explained 35% of the variance, the 65% of unexplained variance may be related to non-motor factors, such as fatigue, pain, visual impairments, or psychological factors, such as poor self-efficacy.38–40 A recent study demonstrated that absence of depression, urinary incontinence, and prior history of stroke were associated with the attainment of community ambulation after stroke.40
The two major strengths of the present study are the large sample, representative of the three levels of walking ability, and the inclusion of multiple measures, with the aim to predict the ability to momentarily increase walking speed after stroke. Also, the measurements of motor coordination were obtained using a simple, quick and low-cost test. However, this study was not without limitations. Although the sample was broad and drawn from various settings, it remains a sample of convenience that may not, therefore, be fully representative of the stroke population. Because the recruitment was conducted on a volunteer basis, those who agreed to participate, may differ from those who did not. In addition, measures of strength of all major muscle groups of the lower limb and balance were not obtained.
There are implications from the findings of this study for clinicians involved in the area of stroke rehabilitation. First, if time allows only one measurement of walking performance to be collected, then a quick measurement of motor coordination would best predict individuals’ abilities to momentarily increase walking speed. Consistent with the definition of motor coordination as the ability to perform a motor task in an accurate, rapid, and controlled manner,26,41 the LEMOCOT was found to be the most suitable test to examine lower limb motor coordination, with appropriate levels of reliability, validity, standard error of measurement and clinical utility.24,42 Second, exercises that require simultaneous temporal and spatial accuracies, aimed at improving lower limb motor coordination, should be performed in addition to walking practice, which may help improving the ability to momentarily increase walking speed in individuals with chronic stroke. Further research is warranted to prospectively examine whether practice of lower limb motor coordination tasks would improve walking ability after stroke.
ConclusionsThe findings of the present study showed that tonus, strength, and motor coordination of the paretic lower limb were significantly correlated with the ability to quickly and temporarily increase walking speed in ambulatory people with chronic stroke. However, motor coordination was the only measure that explained the variance in walking speed reserve. Caution should be taken when interpreting the results, since measures of balance and strength of the muscles of the ankle joint were not included as potential predictors of walking speed reserve.
Conflicts of interestThe authors declare no conflicts of interest.
Brazilian Government Funding Agencies (CNPq and FAPEMIG).