To verify if the relationship between pain catastrophizing and pain worsening would be mediated by muscle weakness and disability in patients with symptomatic knee osteoarthritis.
MethodsThis was a cross-sectional study in a hospital out-patient setting. Convenience sampling was used with a total of 50 participants with symptomatic knee osteoarthritis. Pain and the activities of daily livings (ADL) were assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS) subscale. Pain catastrophizing was assessed using the Coping Strategy Questionnaire (CSQ) subscale. Muscle strength of knee extension and 30-s chair stand test (30CST) were also assessed. Path analysis was performed to test the hypothetical model. Goodness of fit of models were assessed by using statistical parameters such as the chi-square value, goodness of fit index (GFI), adjusted goodness of fit index (AGFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA).
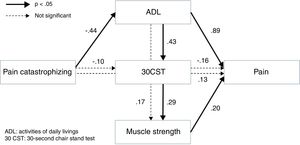
ResultsThe chi-square values were not significant (chi-square=0.283, p=0.594), and the indices of goodness of fit were high, implying a valid model (GFI=1.000; AGFI=0.997; CFI=1.000; RMSEA=0.000). Pain was influenced significantly by muscle strength and ADL; muscle strength was influenced significantly by ADL via 30CST; ADL was influenced by pain catastrophizing.
ConclusionThe relationship between pain catastrophizing with pain worsening are mediated by muscle weakness and disability.
Osteoarthritis (OA) is one of the most frequent painful musculoskeletal disorder encountered in the clinic.1 In people with knee OA, pain generally improves with exercise2,3 and/or physical therapy interventions such as hydrotherapy.4 Unfortunately, some people see little to no improvement in pain, whereas others experience chronic pain.5 Cognitive factors seem to be related to chronic pain perception.6 In particular, pain catastrophizing is associated with increasing pain.7 Pain catastrophizing is defined as to the tendency to focus on and magnify pain sensations and to feel helpless in the face of pain.8 However, the reasons for the increasing pain due to pain catastrophizing in people with knee OA have not been fully elucidated.
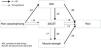
The fear-avoidance model may help explain chronic pain9–12 and describe how individuals develop chronic musculoskeletal pain as a result of fear-based avoidance behaviors.13,14 Based on this model, pain catastrophizing directly causes pain-related fear.15 People with pain-related fear tend to have a worse performance in activities of daily living,16 which results from avoidance.17 In addition, pain-related fear also leads to muscle weakness,18 which causes disability.19 Lastly, muscular weakness and low physical activity in turn is usually associated with pain worsening.20,21 These findings explain how pain catastrophizing and increased pain would be mediated by muscle weakness and disability in these patients. Pain catastrophizing influences disability and pain in individuals with knee OA.22,23 This has led to the hypothesis that pain worsening in people with knee OA is attributed to pain catastrophizing via muscle weakness and disability.
In patients with early knee OA, decreased muscle strength is associated with an increase in activity limitations.19 Avoidance of activity leads to muscle strength deterioration and consequently to more activity limitations in patients with knee and hip OA.24 However, there is no study showing that the relationship between pain catastrophizing and increased pain would be mediated by muscle weakness and disability. If this model can be shown to apply to patients with symptomatic knee OA, then cognitive intervention focused on muscle weakness and disability may be recommended for these patients. Adding cognitive intervention to exercise can improve functional status more than exercise or cognitive interventions alone.25 Combining exercise and cognitive intervention is expected to suppress pain worsening by improving muscle weakness and reducing disability in individuals with knee OA. The objective of this study was to test the hypothesis that the pain catastrophizing can influence pain intensity via muscular weakness and disability in patients with symptomatic knee OA.
MethodsDesignThis was a cross-sectional study. This study was approved by the ethics committees of the two hospitals where participants were recruited, which were the Sakamidirii Hospital and the Midorii Orthopedic, and the Oasahurusato Hospital, Hiroshima, Japan. The study was carried out in a hospital out-patient setting.
ParticipantsThe inclusion criteria were knee pain and meeting at least three out of six of the following criteria26: age >50 years, morning stiffness <30min, crepitus, bone tenderness, bone enlargement, and no palpable warmth. The exclusion criteria were as follows: (1) presence of articular rheumatism, (2) total arthroplasty, or (3) inability to complete questionnaires independently due to cognitive impairment (e.g. dementia). Convenience sampling was used. The staff of the rehabilitation departments of the two hospitals who were working as physical therapist recruited potential participants and collected data between April 2014 and February 2015. All study participants provided written informed consent. The participants did not receive any compensation for their participation.
Sample sizeAccording to Kline,27 an adequate sample size should always be ten times the number of the parameters in path analysis. As there were five parameters included in the hypothetical models (described below), at least 50 recruited subjects were needed.
MeasuresPain catastrophizingPain catastrophizing was assessed using the Coping Strategy Questionnaire (CSQ) subscale developed by Rosenstiel and Keefe.28 The CSQ has been commonly used as an evaluation tool in knee OA research.7 The CSQ is comprised of six subscales evaluating cognitive strategies related to pain (ignoring the pain, reinterpretation of the pain, diverting attention, coping self-statements, catastrophizing, and praying/hoping) and two subscales evaluating behavioral strategies (increasing activity levels and increasing pain behaviors). Of these, subscale for measuring catastrophizing was used in this study. The Japanese version of the CSQ by Ootake and Shimai29 that has its reliability validated was used. The pain catastrophizing subscale consists of two items measured with a numerical rating scale ranging from 0 (never do that) to 6 (always do that) to indicate how frequently these strategies are used to cope with pain.
Muscle strengthMaximal voluntary isometric strength of the bilateral leg during knee extension was measured using a hand-held dynamometer (MT-100, SAKAI Medical Company, Japan). For knee extension, subjects sat in an armless chair and flexed the knee 90°. One end of the strap loop was placed on the shank just above the right lateral malleolus and the other end around the rear leg of the chair. The sole of the foot was about 1cm above the floor. Participants were encouraged to extend their knee joint as much as possible. The same measurement was performed for their bilateral knee joint. The muscle strength recorded was divided by the body weight. Hand-held dynamometry has good to excellent reliability and is valid for most measures of isometric lower limb strength.30
The 30-s chair stand testThe 30-s chair stand test (30CST) was measured to quantify the extent of disability, which result is mainly influenced by impairment of muscle functions in the lower extremity. In the International Classification of Functioning, disability and health (ICF),31 functional ability is included in category of “activity”. Negative condition of the activity referred to an activity limitation that is equivalent to disability in the International Classification of Impairment, Disabilities, and Handicap (ICIDH) model. Accordingly, functional limitation was addressed as a disability (activity limitation) in this study. 30CST was developed originally by Jones et al.32 The participant was seated in the middle of the chair, back straight; feet approximately shoulder width apart and placed on the floor at an angle slightly back from the knees, with one foot slightly in front of the other to help maintain balance. Arms were crossed at the wrists and held against the chest. At the signal “go,” the participant rose to a full stand (body erect and straight) and then returned back to the initial seated position. The participant was encouraged to complete as many full stands as possible within 30s. The participant was instructed to fully sit between each stand. The score was the total number of stands within 30s (more than halfway up at the end of 30s counted as a full stand). Incorrectly executed stands were not counted. Excellent test-retest reliability of 30CST has been confirmed in people with hip and knee OA.33 Criterion validity of 30CST was demonstrated by using the data of the leg press performance32 and the hip and knee isokinetic strength.34
ADLAnother measure used to quantify the extent of disability apart from 30CST was ADL assessment using the ADL subscale of the questionnaire Knee Injury and Osteoarthritis Outcome Score (KOOS).35 The KOOS was developed to assess the patient's opinion on their knee and its associated problems. It holds 42 items in five separately scored subscales: “symptoms”, “pain”, “ADL”, “sport and recreation”, and “quality of life”. A Likert scale was used and items had five possible answer options scored from 0 (no problems) to 4 (extreme problems). According to the KOOS scoring instructions, the KOOS ADL score was transformed to a 0–100 scale, with zero representing extreme knee problems and 100 representing no knee problems. Excellent test-retest reliability of KOOS ADL has been confirmed in people with knee OA.36 The Japanese version of the KOOS by Nakamura et al. that was determined to be both reliable and validity37 was used.
PainPain intensity was assessed using the KOOS pain subscale. Similar to the KOOS ADL score, the Japanese version of the KOOS37 was used. Nine items of the Japanese version for scoring pain were the same as the original version. The KOOS pain score was transformed to a 0–100 scale.
BlindingIn order to reduce the measurement bias, the first author who ideated the study hypothesis did not engage in the recruitment of study subjects and data collection. Hospital staff who collected the data and participants were not informed of the study hypothesis until study completion.
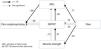
AnalysisQuantitative variables in this study were included in the hypothetical fear-avoidance model as follows: Pain would be influenced by muscle strength, 30CST, and the ADL; muscle strength would be influenced by the ADL via 30CST because muscle weakness are caused by decrease in activity in general; muscle strength, 30CST, and the ADL were influenced by pain catastrophizing. The fitness of the hypothetical model was compared with the alternative model that reverses these causal relations.
The model was tested using the following procedure: first, in order to minimize the variables included in the path analysis, the principal component analysis was performed and the muscle strength data was summarized into one variable. Next, to determine which relationships between variables should be assumed, correlation analysis was performed. Relationships with a p-value ≤0.10 were left in the hypothetical model. Finally, a path analysis was performed to confirm the fitness of both the hypothetical and alternative model. The goodness of fit of the models was assessed by using statistical parameters such as the chi-square value, goodness of fit index (GFI), adjusted goodness of fit index (AGFI), comparative fit index (CFI), root mean square error of approximation (RMSEA), and Akaike's Information Criterion (AIC). A low chi-square value relative to the degrees of freedom with an insignificant p-value (p>0.05), GFI>0.95, AGFI>0.95, CFI>0.95, or RMSEA<0.06 indicated a good model fit.38 AIC was used to compare the hypothetical model with the alternative model where a higher AIC indicated a better overall fitness of the model. If there were subjects with missing data, Little's test was performed to determine whether data was missing completely at random (MCAR), which tests the null hypothesis that the missing data were MCAR.39 A p-value of less than .05 usually indicates that the missing data are not MCAR (i.e., is either missing at random or non-ignorable). In the case of MCAR, Full-Information Maximum Likelihood (FIML) using the expectation-maximization (EM) algorithm was applied to impute estimated data. Statistical analyses were performed using IBM SPSS statistics 22 and IBM SPSS Missing Values (IBM SPSS, Tokyo, Japan) and AMOS 16.0.1.40
ResultsA total of 50 subjects participated in our study (Table 1). One participant had missing data for both KOOS pain and KOOS ADL score. Little's test was performed to check whether the missing values occurred at random. We did not reject the null hypothesis that the data were MCAR (p=0.840). Thus, we estimated the value for missing data using the EM algorithm.
The characteristics of participants.
| Number | 50 | |
| Age (year) | 74.0±10.4 | |
| Gender (female number) | 37 | |
| Height (average±standard deviation, cm) | 152.2±7.7 | |
| Body weight (average±standard deviation, kg) | 54.8±9.0 | |
| The Kellgren–Lawrence grade | 1 | 5 |
| 2 | 23 | |
| 3 | 12 | |
| 4 | 5 | |
| Unknowna | 5 | |
| Osteoarthritis | Spine | 20 |
| Hip | 4 | |
| Complication | Diabetes | 6 |
| (number) | Hypertension | 21 |
| Renal failure | 1 | |
| Cerebral Vascular Disorder | 3 | |
| Gastrointestinal bleeding | 1 | |
Summary measures and the results of correlation analysis are shown in Tables 2 and 3, respectively. The p-value of correlation coefficient between muscle strength and pain catastrophizing was larger than 0.10. Furthermore, the p-value of correlation coefficient between pain catastrophizing and pain was smaller than 0.10. From these results, a relationship between muscle strength and pain catastrophizing was excluded from both the hypothetical and alternative model. Moreover, a direct causal relationship between pain catastrophizing and pain was added to these models.
Summary measures.
| Average (standard deviation) | |
|---|---|
| Pain catastrophizing (0–12) | 5.1 (3.0) |
| Muscle strength: right (N/kg) | 137.9 (72.7) |
| Muscle strength: left (N/kg) | 135.2 (80.9) |
| 30CST (times/30s) | 11.3 (3.9) |
| ADL (0–100) | 69.0 (17.7) |
| Pain (0–100) | 58.9 (19.3) |
ADL, activities of daily livings; 30CST, 30-s chair stand test.
Result of correlation analysis.
| Pain catastrophizing | Muscle strength | 30CST | ADL | |
|---|---|---|---|---|
| Pain catastrophizing | ||||
| Muscle strength | −0.142 (0.326) | |||
| 30CST | −0.287 (0.044) | 0.378 (0.007) | ||
| ADL | −0.440 (0.001) | 0.314 (0.026) | 0.475 (0.000) | |
| Pain | −0.242 (0.091) | 0.398 (0.004) | 0.298 (0.035) | 0.815 (0.000) |
Value represents the Pearson's correlation coefficient. Figure between brackets indicates p values.
ADL, activities of daily livings; 30CST, 30-s chair stand test.
The results of path analyses for the hypothetical model are shown in Fig. 1. The chi-square values were not significant (chi-square=0.283, p=0.594), and the indices of goodness of fit were high, implying that the model was validated (GFI=1.000, AGFI=0.997; CFI=1.000, RMSEA=0.000, AIC=28.028). This result did not change significantly even if a subject with missing values was excluded from this path analysis (GFI=1.000, AGFI=0.995, CFI=1.000, RMSEA=0.000, AIC=28.038). Results of this analysis showed that pain was influenced significantly by muscle strength and the ADL; muscle strength was influenced significantly by the ADL via 30CST; and the ADL was influenced by pain catastrophizing. Overall, these results were concordant with the hypothesis that pain intensity was influenced by pain catastrophizing via muscle weakness and disability.
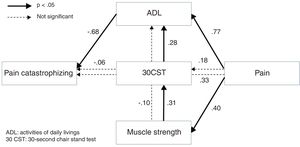
The results of path analyses for the alternative model are shown in Fig. 2. There was good fitness of this model (chi-square=0.115, p=0.735, GFI=0.999, AGFI=0.986, CFI=1.000, RMSEA=0.000, AIC=28.115), but the AIC was lower than the hypothetical model.
DiscussionThis study focuses on the reasons behind increased pain in people with symptomatic knee OA. We referred to the fear-avoidance model and hypothesized that the relationship between pain catastrophizing and increased pain would be mediated by muscle weakness and disability in patients with symptomatic knee OA. Results of our path analysis supported this hypothesis.
It is known that pain worsening may be caused by loss of the descending pain inhibitory mechanisms in knee OA.41 While lower levels of vigorous and total physical activity inactivates the descending pain inhibitory mechanisms,42 severe pain catastrophizing increased disability22 or muscle weakness via pain-related fear.43 Muscle weakness causes joint instability that promotes an inflammatory intraarticular milieu.44 Our results showing that increased pain was influenced by catastrophizing, weakness, and functional changes may reflect these mechanisms.
Our simplified model showed a significant causal relationship between muscle strength and pain. This result agrees with the high-quality evidence reported in the Cochrane database that muscle strengthening exercises reduced pain.2 Furthermore, our results showed that pain was influenced by the ADL that was emphasized by pain catastrophizing. A randomized placebo-controlled clinical trial reported that cognitive intervention decreased clinical pain in knee OA.45 Our results and the results of this trial suggest that cognitive factors and muscle strength could be an intervention target to reduce pain intensity. Our model could be a rationale for cognitive intervention for pain catastrophizing and muscle strengthening exercises to relieve pain for patients with chronic knee OA.
There are several studies46,47 that have tried to validate the fear-avoidance model. These studies addressed mainly psychological factors such as depressive symptoms, fear-avoidance beliefs, pain-related fear, and pain anxiety. However, they did not focus on factors related to disuse or disability, which is a concept included in the fear-avoidance model. Furthermore, the above studies46,47 collected mainly subjective data using questionnaires that should be carefully examined for psychological bias. In contrast, our study collected objective data of muscle strength using a hand-held dynamometer and 30CST, respectively. We also subsequently validated our results by showing the goodness-of-fit of the model to data, and the significant relationships between each variable supported the hypothesis. Thus, our results are valuable findings that support the validity of the fear-avoidance model, at least in part, in patients with knee OA.
Cognitive intervention can change self-efficacy for managing pain,48 as well as pain catastrophizing. Greater self-efficacy was associated with lower levels of pain and disability.49 These findings support the effectiveness of cognitive intervention in decreasing disability via increasing self-efficacy. In addition, our results from path analysis showed a significant relationship between pain intensity and muscle weakness. It is suggested that pain could also be improved by muscle strengthening without improving ADL level. Several completed randomized controlled trials have supported this effect.2 Thus, cognitive intervention for disability, and muscle strengthening may be valuable tools for pain relief.
There are three limitations in this study. First, this study design was cross-sectional and cannot conclude strict causal relationship. As the fear-avoidance model includes a time course, a longitudinal study design is needed to confirm model validity. Our findings suggesting the validity of this model should be reexamined by a longitudinal study in future. A second limitation is negativity bias, where the convenience sampling used in this study might influence our results. For example, people with negative emotions such as anxiety may produce negative responses. If the ratio of those having such negative emotions in the sample population was larger compared to the target population of knee OA, negative responses might strengthen the correlations among pain catastrophizing, the ADL, and pain that were subjectively scored. The possibility of an overestimate for relationships among these variables should be considered. A third limitation is measurement bias. Muscle weakness was quantified using a hand-held dynamometer, while disability was quantified using 30CST and KOOS ADL. Results using another measurement tool are unknown. In order to reexamine and generalize our finding, future studies should include other measurement tools as indices of muscle weakness and disability.
ConclusionPain intensity was influenced by pain catastrophizing via muscle weakness and disability. Cognitive intervention for disability and muscle strengthening may be valuable tools for pain relief.
FundingThis study was funded by the Ministry of Education, Culture, Sports, Science and Technology [Grant-in-Aid for Young Scientists (B)].
Conflicts of interestThe authors declare no conflicts of interest.