There is lack of agreement in the literature about the effectiveness of photobiomodulation (PBM) for reducing pain-related symptoms in patients with knee osteoarthritis (OA).
ObjectiveTo evaluate whether PBM, when combined to exercises, provides incremental therapeutic benefits for pain, physical function, and quality of life (QoL) in patients with knee OA.
MethodsA six-month double-blind placebo-controlled randomized trial was conducted. Patients with knee OA were randomly assigned to one of three treatment groups: Exercise, Exercise plus Active PBM, or Exercise plus Placebo PBM. Treatment was provided over an eight-week period, three times per week. The primary outcomes were pain at rest and upon movement, assessed by a visual analogue scale (VAS). WOMAC global score, QoL, and a core-set of performance-based tests were measured as secondary outcomes. All outcomes were collected at baseline, immediately after treatment, and after three- and six-month post-treatment.
Results127 participants were allocated as follows: Exercise, N = 41; Exercise plus Active PBM, N = 44; and Exercise plus Placebo PBM, N = 42. There was no between-groups difference in improvement in pain, physical function, and QoL for all follow-up times. However, all groups presented significant, clinically relevant improvements in pain, physical function, and QoL immediately and three months after treatment compared with baseline measures.
ConclusionPatients with knee OA who received a strengthening exercises program did not experience incremental benefits regarding pain, physical function, or QoL when adding PBM to their therapeutic exercises.
Considered the most common musculoskeletal disease in the aging population, osteoarthritis (OA) is highly prevalent among adults and it results in substantial personal and societal costs.1 OA is a chronic disease affecting joints and periarticular tissues and is characterized by progressive degeneration of articular cartilage and by changes in the subchondral bone.2 Knee OA is clinically characterized by joint pain, morning stiffness, and decreased mobility, which may reduce the quality of life (QoL) of adults.3 Thus investigating evidence-based non-pharmacological management is of pivotal importance.
International clinical guidelines strongly recommend physical exercise as a non-pharmacological treatment for OA.4–6 The benefits of exercises for improving pain and function in people with knee OA were well established in a systematic review.7 In adequate doses, a strengthening program for lower limbs improves muscle weakness by increasing muscle recruitment and/or mass, which may decrease internal knee forces, reducing pain intensity and dysfunction.7 Therefore, muscle strengthening is recognized as core treatment for knee OA8,9 and should be individually prescribed through progressive exercise programs.10
Laser therapy or photobiomodulation (PBM), another non-pharmacological approach, may induce analgesic and anti-inflammatory effects.11 However, the body of evidence and recommendations on the use of PBM for knee OA are inconclusive.4 The American Academy of Orthopaedic Surgeons is unable to recommend for or against the use of PBM in patients with symptomatic knee OA12 and the Osteoarthritis Research Society International (OARSI) guidelines conditionally recommend the use of laser therapy for this condition.4
While some randomized controlled trials (RCTs) have investigated the isolated effect of PBM on knee OA,13–16 there is incipient research on the combination of PBM with therapeutic exercise programs.17–22 Moreover, these studies present some limitations, such as having a small sample size,18,19,21,22 lacking a control group,18,21,22 failing to simultaneously perform the PBM and the exercise program interventions,19,22 being unable to conduct a long-term follow-up,18–21 limiting participation to only female21 or male18 participants, and performing only a few treatment sessions.19
Considering that exercises should be prescribed for OA symptoms management,7 this study aimed to evaluate whether PBM provides incremental therapeutic benefits for pain, physical function (e.g. standing and sitting in a chair, walking, ascending and descending stair), and QoL in patients with knee OA.
MethodsThis study adhered to the Standard Protocol Items: Recommendations for Interventional Trials,23 the OARSI Clinical Trials Recommendations,24 and the Template for Intervention Description and Replication checklist guidelines.25 The results are reported according to the Consolidated Standards of Reporting Trials statement for RCTs of non-pharmacologic treatments.26 Due to delay in processing, the trial was registered on the Brazilian Clinical Trials Registration Platform (RBR-8f4s9d) within 3 months of the start of the study, which is before completion of data collection for any participants. The protocol was approved by the Institutional Human Ethics Committee of the Universidade Federal de São Carlos, São Paulo, Brazil (No. 65685517.9.0000.5504). Enrollment of participants began in March 2018 and data collection ceased in June 2020.
Study designA six-month-long single-center double-blind prospective parallel-design placebo-controlled RCT was conducted. This study was considered double-blind because evaluators and data analysts did not know in which group the participants were allocated. Verbal and written explanations of possible risks and benefits of the study were provided at baseline assessment to all participants, who signed the informed consent approved by the ethics committee.
ParticipantsParticipants were periodically recruited via advertisements on social media and local news. They went through a remote and face-to-face screening involving a clinical assessment to confirm their eligibility. Knee OA diagnosis was based on the clinical and radiographic criteria of the American College of Rheumatology (ACR).27 Radiography of both knees was performed to determine the radiographic grade of knee OA according to the Kellgren-Lawrence criteria,28 on the basis of the joint space narrowing, sclerosis, osteophytes formation, and joint deformity classified as 0 (no OA), 1 (doubtful), 2 (minimal), 3 (moderate), and 4 (severe).
Inclusion criteria: pain intensity at rest of ≥ 4 on a 10-centimeter visual analogue scale (VAS) in the prior week, a radiographic OA grade of 2 or 3 in at least one knee compartment,24 and being between 40 and 75 years of age. Patients were excluded if they were performing moderate/intense training for > 120 min/week; had a body mass index (BMI) ≥ 35 kg/m2; had undergone physical therapy sessions in the past three months; had received intra-articular knee injections in the past six months; had cardiorespiratory, neurological, or any other rheumatology conditions that could impose restrictions; had previous hip, knee, or ankle surgeries; or had any condition that leads to chronic general pain or dysfunction. A medical clearance to perform exercises was collected from each patient.
RandomizationEligible participants were randomly allocated into one of three groups: Exercise, Exercise plus Active PBM, or Exercise plus Placebo PBM. A block randomization was created in a computer-generated randomization list by an independent researcher who had no contact with participants, assessments and treatment procedures, and who oversaw the allocation concealment. This list was kept in an opaque, sealed envelope that was locked in a central location. The assignment of the participants to the groups was only revealed at the first session by the physical therapist providing the intervention.
InterventionsThe trial protocol has been published elsewhere.29 Participants received an eight-week supervised group strength training program with or without PBM. Sessions were performed 3 times/week on alternating days at the health unit of the Universidade Federal de São Carlos totaling 24 sessions. Participants were advised not to practice any other type of regular physical exercise that could compete with the protocol.
Briefly, individually tailored progressive resistance exercises with free weights, elastic bands, and body weight were prescribed. The conditioning phase targeted lower limbs and trunk muscles and neuromuscular training involving balance exercises. A cool-down period involved static stretching exercises. The comprehensive description of the exercises program has been previously published.29
The PBM protocol was based on previous RCTs for knee OA13,15,16 and on recommendations of the World Association of Laser Therapy. Thus, active and inactive (sham or placebo) commercial hand-held diode laser devices (Recover, MMOptics) were used, and randomly labeled "A" and "B" to blind therapists, participants, and evaluators. Irradiation parameters were: wavelength of 808 nm, maximum output power of 100 mW ± 20%, continuous waveform (CW) mode, laser beam spot size of 0.03 cm2, and power density of 3.33 W/cm2. The lasers were regularly verified using an optical power meter (LabMax-TOP, Coherent Inc.). Four points on the medial and lateral aspect of the most affected knee (8 points total) were irradiated perpendicular to the knee joint line15 with an energy of 6 joules (J) per point, totaling an energy of 48 J per session.13,16
Outcome measurementsPain intensity at rest and upon movement was assessed using a 10-cm VAS as the primary outcome. As secondary outcomes, the WOMAC global score (range 0–96), physical performance assessed by the 30-second chair stand test, the stair climb test, and the 40-meter fast-paced walk test, and general QoL (SF-36 questionnaire) were collected. For a comprehensive description of all variables and their minimal clinically important difference (MCID) values, please refer to the published protocol.29 Participants were assessed at baseline, immediately after treatment, and at three- and six-month follow-ups by the same blinded evaluator.
Sample size calculationThe sample size was calculated using the statistical functions available on the software Microsoft Excel 2019 (Microsoft, Redmond, WA, USA). We aimed to detect an MCID of 1.75 cm units in a VAS for knee pain30 and 30 non-normalized units on the WOMAC global score.31 Thus, the sample size per group (n) was estimated a priori by the T-test, assuming between-patient standard deviations (SDs) of 2.0 cm for pain and 45 non-normalized units for the WOMAC global score. Based on these criteria, we defined a statistical power at 0.8 and a significance level (α) at 0.05, which required 37 participants per group, considering the highest value between both variables, in this case, the WOMAC variable. To allow for possible dropouts (estimated at 10%), a target of 40 participants per group was intended, totaling a sample of 120 participants.
Statistical analysisThe data were collected using free online surveys (Google Forms), automatically compiled in an electronic database (Google Sheets) and stored in a password-protected cloud-based management system. Statistical analysis was performed by two blinded assessors using the software SPSS 23.0 (SPSS Inc, Chicago, IL, USA). For data distribution, the Kolmogorov-Smirnov test was applied, and after normality has been confirmed (p > 0.05), a two-factor ANOVA was conducted for an intention-to-treat (ITT) data analysis, with groups as the between-group factor and time (baseline, post-intervention, and follow-up periods) as the within-group factor to compare trajectories between groups. In addition, the Tukey test was performed for post hoc analysis. The expectation maximization approach was used as an imputation method for the missing data of all variables.27 Between-group differences and 95% CIs were reported and interpreted against the established thresholds for MCID.
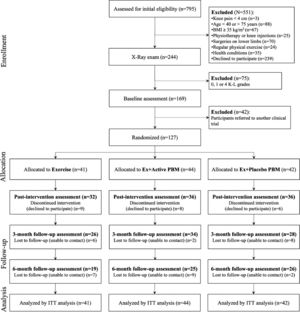
ResultsA total of 794 participants were assessed for eligibility, and 127 were randomized as follows: 41 were assigned to the Exercise group, 44 to the Exercise plus Active PBM group, and 42 to the Exercise plus Placebo PBM group. Dropout rates of 18% and 45% occurred at the post-intervention and at the 6-month follow-up assessments, respectively. A detailed flow diagram of the participants’ progress through the trial is shown in Fig. 1.
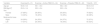
Participants attended 17 (of planned 24) treatment sessions on average. Anthropometric and clinical characteristics at baseline are presented in Table 1. Values are expressed as means (standard deviation) for continuous variables and N (%) for frequency variables.
Baseline characteristics of the participants by treatment group.
Data expressed as mean ± standard deviation and number (percentage). BMI, body mass index.
Table 2 summarizes the primary and secondary outcome scores. Self-reported measures and physical performance were similar between groups at baseline. Although all groups presented significant clinically relevant improvement (p<0.05) in all outcome measures and assessments, there were no significant differences between the groups in all outcomes immediately after treatment, and at three or at six months (Table 3).
Outcome measures over time according to group from an intention-to-treat analysis.
| Outcomes | Pre-Intervention | Post-Intervention | 3 months | 6 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex(N = 41) | EPP (N = 42) | EAP (N = 44) | Ex(N = 41) | EPP (N = 42) | EAP (N = 44) | Ex(N = 41) | EPP (N = 42) | EAP (N = 44) | Ex(N = 41) | EPP (N = 42) | EAP (N = 44) | |
| Primary | ||||||||||||
| Pain a | ||||||||||||
| at rest | 5.74 ± 2.56 | 5.48 ± 2.70 | 5.62 ± 2.42 | 2.04 ± 1.99 | 1.94 ± 1.40 | 1.86 ± 1.83 | 1.51 ± 1.87 | 1.64 ± 1.88 | 2.36 ± 2.65 | 3.26 ± 2.28 | 1.99 ± 1.76 | 2.83 ± 2.17 |
| standing | 3.38 ± 2.49 | 3.54 ± 2.79 | 3.08 ± 2.96 | 1.14 ± 2.04 | 0.97 ± 1.37 | 0.61 ± 1.28 | 1.14 ± 1.80 | 1.31 ± 2.18 | 1.19 ± 2.17 | 2.03 ± 2.15 | 0.83 ± 0.96 | 1.65 ± 2.01 |
| on climbing stairs | 4.05 ± 2.77 | 4.61 ± 2.72 | 3.91 ± 3.00 | 1.20 ± 1.73 | 1.09 ± 1.44 | 1.06 ± 1.96 | 0.99 ± 1.61 | 1.21 ± 2.40 | 1.52 ± 2.87 | 2.05 ± 2.15 | 1.20 ± 1.28 | 1.50 ± 1.84 |
| walking | 2.64 ± 2.63 | 2.95 ± 2.61 | 2.88 ± 2.67 | 0.53 ± 1.15 | 0.53 ± 0.89 | 0.33 ± 1.02 | 0.74 ± 2.03 | 0.48 ± 1.90 | 0.97 ± 2.62 | 1.53 ± 1.86 | 0.53 ± 1.06 | 1.03 ± 1.88 |
| Secondary | ||||||||||||
| WOMAC global b | 38.0 ± 19.1 | 40.1 ± 17.3 | 34.4 ± 15.6 | 12.8 ± 16.5 | 11.0 ± 11.3 | 8.8 ± 9.0 | 9.7 ± 13.2 | 10.4 ± 13.7 | 12.4 ± 16.6 | 20.7 ± 16.0 | 14.8 ± 10.9 | 17.5 ± 11.9 |
| 30-second chair stand test c | 8.5 ± 2.9 | 8.2 ± 3.2 | 8.7 ± 3.3 | 10.6 ± 2.3 | 10.3 ± 2.4 | 11.3 ± 2.9 | 11.6 ± 1.9 | 11.6 ± 2.3 | 11.3 ± 3.6 | 10.4 ± 2.2 | 11.6 ± 2.1 | 10.9 ± 3.1 |
| Stair climb test d | 15.3 ± 6.5 | 15.2 ± 7.2 | 19.1 ± 11.9 | 12.3 ± 4.7 | 12.3 ± 4.4 | 12.9 ± 5.8 | 10.6 ± 3.6 | 11.0 ± 4.6 | 14.6 ± 13.8 | 14.6 ± 6.2 | 12.1 ± 4.1 | 13.7 ± 7.1 |
| 40-meter fast-paced walk test e | 1.4 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.5 ± 0.2 | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.3 | 1.6 ± 0.3 | 2.4 ± 4.9 | 1.6 ± 0.8 |
| SF-36 f | ||||||||||||
| physical domain | 55.9 ± 19.6 | 51.6 ± 16.5 | 51.9 ± 18.5 | 82.1 ± 31 | 78.3 ± 19.5 | 84.3 ± 12.6 | 77.9 ± 17.7 | 81.4 ± 15.7 | 76.5 ± 21.5 | 67.4 ± 17.6 | 75.9 ± 16.1 | 73.1 ± 18.4 |
| mental domain | 63.6 ± 21.8 | 66.6 ± 17.6 | 63.5 ± 20.2 | 82.7 ± 14.3 | 84.5 ± 11.8 | 88.5 ± 7.7 | 78.2 ± 16.7 | 84.0 ± 12.1 | 77.6 ± 20.3 | 70.9 ± 18.9 | 79.4 ± 15.4 | 77.1 ± 17.9 |
EAP, exercise and active photobiomodulation; EPP, exercise and placebo photobiomodulation; Ex, exercise; MCID, minimal clinically important difference; SF-36, 36-item short-form questionnaire; VAS, visual analogue scale.
Data expressed as mean ± standard deviation (SD).
Estimated mean differences between groups post intervention and at 3 and 6 months from an intention-to-treat analysis.
| Outcomes | Post-Intervention | 3 months | 6 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EPP (N = 42) xEx (N = 41) | EPP (N = 42) xEAP (N = 44) | Ex (N = 41) xEAP (N = 44) | EPP (N = 42) xEx (N = 41) | EPP (N = 42) xEAP (N = 44) | Ex (N = 41) xEAP (N = 44) | EPP (N = 42) xEx (N = 41) | EPP (N = 42) xEAP (N = 44) | Ex (N = 41) xEAP (N = 44) | |
| Primary | |||||||||
| Pain*,a | |||||||||
| at rest | −0.1 (−1.04, 0.83) | 0.08 (−0.84, 1.00) | 0.18 (−0.74, 1.11) | 0.12 (−1.04, 1.28) | −0.72 (−1.87, 0.42) | −0.85 (−2, 0.3) | −1.26 (−2.73, −0.15) | −0.83 (−1.92, −0.26) | 0.43 (−0.67, 1.54) |
| on standing | −0.17 (−1.02, 0.68) | 0.35 (−0.48, 1.19) | 0.52 (−0.32, 1.36) | 0.16 (−0.93, 1.26) | 0.12 (−0.96, 1.2) | −0.05 (−1.13, 1.04) | −1.21 (−2.16, −0.25) | −0.82 (−1.76, 0.11) | 0.38 (−0.56, 1.33) |
| on climbing stairs | −0.11 (−1.03, 0.81) | 0.03 (−0.87, 0.93) | 0.14 (−0.77, 1.05) | 0.23 (−1.03, 1.49) | −0.31 (−1.55, 0.93) | −0.54 (−1.79, 0.71) | −0.85 (−1.88, 0.18) | −0.60 (−1.61, 0.41) | 0.25 (−0.77, 1.27) |
| on walking | 0.00 (−0.54, 0.55) | 0.20 (−0.34, 0.74) | 0.20 (−0.34, 0.74) | −0.26 (−1.44, 0.92) | −0.49 (−1.65, 0.67) | −0.23 (−1.4, 0.93) | −1.01 (−1.89, −0.13) | −0.49 (−1.37, 0.37) | 0.51 (0.48, 1.38) |
| Secondary | |||||||||
| WOMAC global*,b | −1.8 (−8.5, 4.9) | 2.3 (−4.3, 8.9) | 4.1 (−2.6, 10.7) | 0.7 (−7.1, 8.5) | −2 (−9.7, 5.7) | −2.8 (−10.5, 5.0) | −5.9 (−12.9, 1.1) | −2.7 (−9.6, 4.2) | 3.2 (−3.7, 10.1) |
| 30-s chair stand test⁎⁎,c | −0.3 (−1.7, 1.1) | −1.0 (−2.4, 0.3) | −0.8 (−2.1, 0.6) | 0.1 (−1.4, 1.5) | 0.3 (−1.1, 1.7) | 0.2 (−1.2, 1.7) | 1.2 (−0.1, 2.5) | 0.7 (−0.6, 2.5) | −0.5 (−1.8, 0.8) |
| Stair climb test*,d | 0.1 (−2.6, 2.8) | −0.6 (−3.2, 2.1) | −0.6 (−3.3, 2.0) | 0.4 (−4.3, 5.1) | −3.5 (−8.1, 1.1) | −3.9 (−8.6, 0.7) | −2.5 (−5.7, 0.7) | −1.5 (−4.6, 1.6) | 0.9 (−2.1, 4.1) |
| 40-m fast-paced walk test⁎⁎,e | 0.1 (−0.1, 0.2) | 0.0 (−0.1, 0.2) | −0.1 (−0.2, 0.1) | 0.0 (−0.1, 0.2) | 0.1 (−0.1, 0.2) | 0.1 (−0.1, 0.2) | 0.8 (−0.7, 2.3) | 0.8 (−0.7, 2.3) | 0.0 (−1.5, 1.5) |
| SF-36⁎⁎,f | |||||||||
| physical domain | −3.7 (−15.5, 8.1) | −5.9 (−17.5, 5.7) | −2.2 (−13.9, 9.5) | 3.5 (−6.4, 13.4) | 4.9 (−4.8, 14.6) | 1.4 (−8.4, 11.1) | 8.4 (−0.9, 17.7) | 2.7 (−6.4, 11.9) | −5.7 (−14.9, 3.5) |
| mental domain | 1.7 (−4.4, 7.9) | −4.0 (−10.1, 2.0) | −5.8 (−11.8, 0.3) | 5.9 (−3.1, 14.8) | 6.4 (−2.4, 15.2) | 0.6 (−8.3, 9.4) | 8.6 (−0.4, 17.6) | 1.7 (−7.1, 10.6) | −6.8 (−15.7, 2.1) |
EAP, Exercise and Active Photobiomodulation; EPP, Exercise and Placebo Photobiomodulation; Ex, Exercise; SF-36, 36-item short-form questionnaire; VAS, visual analogue scale.
Data expressed as adjusted mean differences between groups (95% confidence interval).
This study aimed to evaluate whether PBM provides incremental therapeutic benefits for pain, physical function, and QoL in patients with knee OA. The findings indicate that the addition of on average 17 PBM sessions to an exercise program did not result in incremental benefits for pain, physical function, or QoL in this population.
The 2019 OARSI guidelines strongly recommended land-based exercise as a core treatment for individuals with knee OA.4 Also, there is a large body of high-quality evidence for the effectiveness of therapeutic exercises for people with knee OA.7,32,33 According to this literature, a progressive exercise program can improve muscle weakness, limited range of motion, deficient proprioception, impaired balance, and poor cardiovascular fitness in people with knee OA. An appropriate strengthening program for lower limbs enhances muscle strength, which may decrease internal knee forces, reducing pain and dysfunction.7 Other advantages of exercise prescription for this population include weight loss, emotional well-being, and improvement in overall health.8 It is noteworthy that exercise has similar effect sizes for pain intensity as pharmacologic interventions, such as nonsteroidal anti-inflammatory drugs.34 This is consistent with our findings, which show significant improvements—greater than the MCID—in pain within groups over time, which further supports the benefits of a regular exercise program.
PBM is suggested to provide an anti-inflammatory effect, decreasing the inflammatory infiltrate and the release of pro-inflammatory cytokines.35 Specifically, for chronic joint diseases, PBM is performed to reduce pain intensity and modulate inflammatory processes.36,37 But, based on inconsistent conclusions from systematic reviews38–40 and conditional recommendations from practice guidelines the clinical effectiveness of PBM for knee OA is still unclear.4
The authors of a systematic reviews with meta-analysis (9 RCTs, n = 518) found no significant differences in improvements in pain and WOMAC scores between active and placebo PBM for knee OA.38 The findings of this review (e.g. the lack of effectiveness of PBM treatment) were attributed to the heterogeneity of the methodological designs of the RCTs analyzed. In contrast, the meta-analysis by Rayegani et al.39 (14 RCTs, n = 678) indicated a significant reduction in pain at rest and upon activity and in WOMAC scores for patients who received active PBM compared to placebo PBM, however there was no significant difference in the WOMAC pain subscale between groups. Finally, Stausholm and colleagues40 meta-analyzed 22 trials (n = 1063 patients) and also reported significant improvements in pain and disability with PBM. They concluded that PBM therapy is effective in knee OA both with and without exercise therapy as cointervention. Nevertheless, the authors stated that “strength training was seemingly only used as an adjunct to PBM in two of the included trials, and thus more trials with this combination of treatments are needed.” The lack of consistency in results may be due to differences in study design and treatment parameters.
Alfredo and colleagues17 investigated PBM combined with exercises for knee OA. The participants were randomly assigned to active or placebo laser groups. PBM was performed with an energy of 3 J/point to nine points for 9 sessions, prior to the strengthening program phase, which was performed three times a week for eight weeks. The authors observed significant improvement in pain and function for both groups with no differences between groups. Also, there were no significant differences between the active and placebo PBM groups in all outcomes at the three- and six-month follow-ups.22 The limitations of the study were the small sample size (n = 20), the lack of a control group, and not having performed the PBM simultaneously with the exercise phase.17 While these limitations were addressed in the current RCT, our results also indicated no additional benefits of PBM.
Another RCT studied the effects of PBM concurrently with exercises in patients with knee OA.18 Fifty-three men were randomly allocated into one of three groups: high-intensity laser therapy and exercise; low-intensity laser therapy and exercise; and placebo low-intensity laser therapy and exercise. The PBM was applied twice a week for six weeks while the participants performed the exercise program at home three times a week for eight weeks. Pain (VAS) and knee function (WOMAC) were assessed before and immediately after treatment. In contrast to our study, active PBM in combination with exercise resulted in additional improvements in pain and function when compared with exercise alone. Limitations of this study were the absence of follow-up beyond the end of the intervention and the inclusion of males only. de Paula Gomes and colleagues19 combined PBM therapy, delivered by a cluster device of laser and light-emitting diodes, with an exercise program for knee OA. Comparing the three groups (exercise, exercise plus active PBM, and exercise plus placebo PBM) immediately after treatment, active PBM (23.55 J per session) combined with the exercise program was significantly more effective at reducing pain compared with exercise alone or exercise plus placebo PBM. However, the combination was not more efficacious in terms of WOMAC physical function, which is similar to our findings. Limitations included a small sample size (n = 20), a short-period intervention (10 sessions), and recruiting people from only one physical therapy center, which were addressed in our study.
An RCT20 investigated the efficacy of an exercise program and PBM, simultaneously and separately, for knee OA. Thus, participants were allocated into control group, laser group, exercise group, or laser plus exercise group (n = 28 per group). WOMAC questionnaire and gait measures were assessed at baseline and immediately after treatment. Only those in the exercise group had a significant improvement in WOMAC scores immediately after treatment. As an important limitation, the study presented a total dropout rate of 46.4%.
Finally, our findings are consistent with the outcomes of an RCT21 that investigated the use of PBM combined with strengthening exercises. Other than the light source, the PBM parameters in that study were very similar to our protocol, with an output power of 100 mW on continuous near-infrared wavelength and a total energy of 56 J applied per knee. Both the active PBM plus exercise and placebo PBM plus exercise groups presented an improvement in WOMAC scores at the end of treatment; however, there were no significant differences between groups – as we have observed in our groups. As mentioned by the authors,21 this observation reaffirms “the positive effects of the exercise and the lack of an extra effect of PBM” on this condition.
Major strengths of this study were the inclusion of a sham treatment to minimize bias in the PBM groups; and to the best of our knowledge, we have performed the longest therapeutic sessions period and the largest sample size of any studies conducted on this topic to date. Because the per protocol analysis states the effect of treatment assignment under adherence to the treatment protocol, and the ITT analysis provides a consistent effect estimate according to the treatment assignment, both analyses were performed.41 We only reported the most conservative approach, e.g. the ITT analysis, because results were similar.
The main limitation of this study was the high dropout rate. Most participants abandoned the trial during the interventional period because they were not treated with PBM in our study. Although in this period the dropout rate was below 20%, the 3- and 6-month follow-up assessments surpassed this rate, showing dropout rates of 30% and 45% respectively. It is worth noting that the COVID-19 pandemic contributed in 2020 to an important sample loss, as social distancing and restrictions occurred. Also, it was not possible to blind participants from the exercise group to the exercise plus active or placebo PBM groups.
ConclusionIn conclusion, PBM did not provide incremental therapeutic benefits over a strengthening exercise program for pain, physical function, or quality of life for our patients with knee OA.
The authors thank Dr. Vanderlei Salvador Bagnato for the PBM support; Vitor Luiz Innocentini for the exercise technical support; and Oswaldo Jorge Neto for obtaining the radiographic images at the University Hospital.
This work was supported by the São Paulo Research Foundation (FAPESP, #2017/00062-7); and the National Council for Scientific and Technological Development (CNPq, #401333/2016-7). MMOptics provided the PBM devices.
Trial registration number: RBR-8f4s9d (https://ensaiosclinicos.gov.br/rg/RBR-8f4s9d)