Various systematic reviews and/or meta-analyses examining the effects of pre- or postoperative exercise on body function or activity in patients undergoing total knee arthroplasty (TKA) have been published. However, the interventional period needed to at least improve outcomes is unknown.
ObjectiveThe aim of this systematic review and meta-analysis was to investigate the exercise intervention period needed to effectively improve body function or activity before and after TKA in patients with knee osteoarthritis (OA).
MethodsStudies published until July 2017 were included in the review. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was applied to each meta-analysis to determine the quality of the evidence.
ResultsTwenty-seven randomized controlled trials were identified. A meta-analysis indicated that exercises performed for 8 weeks after discharge in addition to standard postoperative intervention effectively improved body function as assessed using pain level; physical function, and stiffness on the Western Ontario and McMaster Universities Arthritis Index; extension strength; active knee flexion range of motion; timed up and go test; and gait speed.
ConclusionOverall, we found low- to moderate-quality evidence that an 8-week exercise period was needed after discharge to improve body function and activity in patients with knee OA undergoing TKA.
Total knee arthroplasty (TKA) is usually performed in patients with severe knee osteoarthritis (OA).1 Interventions using exercise are often performed before and/or after TKA. Various systematic reviews2 and/or meta-analyses3–5 examining the effects of pre- or postoperative exercise on body function or activity in patients undergoing TKA were published. For example, Wallis and Taylor3 and Gill and McBurney4 reported that preoperative exercise is ineffective for pain control3 and assessed physical function3 using the Western Ontario and McMaster Universities Arthritis Index (WOMAC), knee extension strength,4 and gait speed4 after TKA. Minns Lowe et al.5 examined the effects of postoperative exercise in patients undergoing TKA and found no significant effect on walking or quality of life (QOL), although knee joint range of motion (ROM) significantly improved compared to that observed in the control group. Finally, Artz et al.6 reported that interventions including physical therapy and exercise result in short-term improvements in physical function.
However, knowledge is lacking on the effective exercise intervention period for improving outcomes. Since rehabilitation time before and after surgery affects medical cost, it is meaningful to clarify the required intervention period to improve outcomes. Furthermore, in previous systematic reviews and/or meta-analyses, the following research limitations were observed. Wallis and Taylor3 did not examine whether additional early exercise effectively improves body function and activity.3 The systematic review by Gill and McBurney4 included non-randomized controlled trials (RCTs); thus, the findings provided in this review are not indicative of high-quality evidence. Gill and McBurney4 and Minns Lowe et al.5 used data other than the mean value and standard deviation of the primary study for the meta-analysis. Artz et al.6 conducted the meta-analysis based on the difference in the intervention contents after discharge only. Therefore, the aim of this systematic review was to investigate the exercise intervention period needed to effectively improve body function or activity before and after TKA in patients with knee OA.
MethodsThe study design was a systematic review with a meta-analysis statistical approach.
Eligibility criteriaThe studies were eligible if: (1) the research design was an RCT; (2) the participants were undergoing TKA for knee OA; (3) preoperative exercise intervention or postoperative exercise intervention was performed; (4) the researchers assessed the participants’ body function and/or activity using parameters such as pain, strength, ROM, QOL, balance, and gait speed; and (5) the paper was published in English. Eligibility criteria for the control group were not set. Regarding the selection of each article, the choice of the two researchers were independent. According to the international classification of function, disability, and health proposed by the World Health Organization,8 we defined body function as physiological functions of body systems (including psychological functions) and activity as the execution of a task or action by an individual.
Information sourcesWe used the following search terms to search all trial registers and databases: “arthroplasty, replacement, knee,” “osteoarthritis, knee,” “exercise,” and “exercise therapy.” The search strategy consisted of a combination of free text words and medical subject heading terms. The search strategy is shown in Table 1. All studies published until July 2017 were included in the search. The terms “population” and “intervention” were combined with the word “AND” as an operator. Population was defined as participants with OA of the knee on a waiting list for TKA or who had undergone TKA. This RCT intended to achieve the most valid information regarding intervention effectiveness. For each concept, synonyms and Medical Subject Headings terms were combined with the “OR” operator.
Search strategy.
| PubMed | #1 arthroplasty, replacement, knee [MeSH Terms] #2 osteoarthritis, knee [MeSH Terms] #3 exercise [MeSH Terms] #4 exercise therapy [MeSH Terms] #5 #1 AND #2 AND (#3 OR #4) Limits: Randomized Controlled Trial |
| PEDro (Advanced search) | Abstract & Title: knee osteoarthritis exercise (arthroplasty or replacement) Method: clinical trials |
| CENTRAL | #1 MeSH descriptor Osteoarthritis, knee #2 MeSH descriptor Exercise #3 MeSH descriptor Exercise Therapy #4 MeSH descriptor Arthroplasty, Replacement, knee #5 (#1 AND (#2 OR #3) AND #4) |
| CINAHL | (MH “Osteoarthritis, knee”) AND ((MH “Exercise+”) OR (MH “Therapeutic Exercise+”)) AND (arthroplasty OR replacement) Limits: Randomized Controlled Trial |
The PubMed, Cochrane Central Register of Controlled Trials, Physiotherapy Evidence Database (PEDro), and Cumulative Index to Nursing & Allied Health databases were searched.
Study selectionTwo reviewers independently screened the titles and abstracts using the predetermined eligibility criteria. Disagreements were resolved by discussion. Full-text copies of articles that were not definitively excluded based on the title and/or abstract were retrieved, and the criteria were reapplied. Uncertain cases were discussed by the reviewers to achieve a consensus.
Data collection processPredesigned spreadsheets were used to extract data regarding the participants, interventions, outcome measurements, and results.
Data itemsThe database search was supplemented by a manual search of the reference lists of past systematic reviews.
Risk of bias in individual studiesTwo researchers independently applied a validated scale (PEDro) to rate the methodological quality and statistical reporting of each trial.9 The 11 items are based upon the Delphi list.10 Each item is scored “yes” or “no” with a maximum score of 10 as one criterion is not scored. The PEDro score has demonstrated moderate inter-rater reliability (intraclass correlation coefficient=0.68 [95% confidence interval (CI), 0.57–0.76]) for clinical trials.11 A trial with a score ≥6 was considered to demonstrate high quality consistent with previous reviews.12,13
Summary measuresStandardized mean differences (SMDs) (effect sizes) and 95% CI were calculated based on the postintervention means and standard deviations (SDs).14 When the standard error or 95% CI was provided, the data were converted to the SD. The p values were used to estimate the SD.
Synthesis of resultsPositive SMD values were used to indicate that the outcome favored the intervention group. A value of 0.2–0.5 indicated a small effect size; 0.5–0.8, a moderate effect size; and >0.8, a large effect size.15 A meta-analysis was performed using the inverse variance method and a random effects analysis.16 The Review Manager Version 5.1 (The Cochrane Collaboration, Freiburg, Germany) software program was used for the meta-analysis. Before combining data, trials were categorized into subgroups based on intervention, outcome, and intervention period (4, 8, or 12 weeks). Combining the data in meta-analyses was planned in which a minimum of two trials in a subgroup were clinically homogeneous. A trial was considered clinically homogeneous if a common population and outcome measurement were used. Regarding interventions, there were no restrictions in type or intensity.
Risk of bias across studiesThe Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach17 was applied to each meta-analysis to determine evidence quality. This approach entails the downgrading of evidence from high to moderate to low and very low quality based on certain criteria. Downgrading the evidence one step (e.g., high to moderate quality) occurred if: (1) the PEDro score was ≤5 for the majority of trials (>50%) in the meta-analysis; (2) statistical heterogeneity between the trials was greater than the accepted low level (I2>25%)18; or (3) there were large confidence intervals, indicating a small number of participants. If there were serious issues with the methodological quality, for example, all trials in the meta-analysis had a PEDro score <6 without allocation concealment or the use of blinded assessors, a double step downgrade occurred (e.g., high to moderate quality). A footnote was used to explain the reason for the grade applied to each meta-analysis.
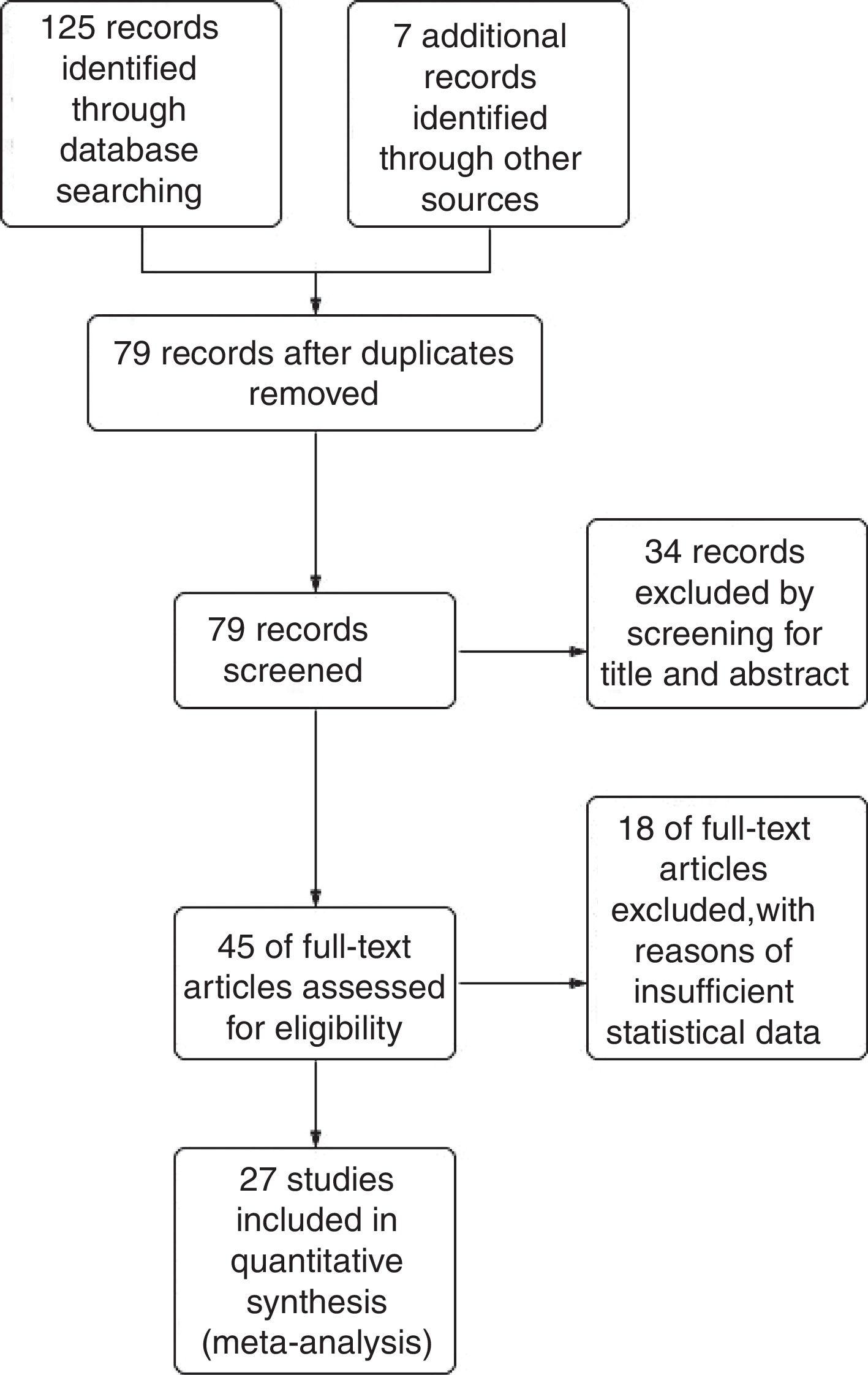
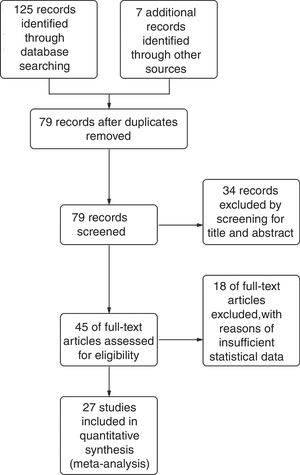
ResultsStudy selectionThe combined database search yielded 132 trials. After the adjustment for duplicates, 79 trials were considered. Of them, 34 were eliminated after abstract review for not meeting the selection criteria. The complete text of the remaining 45 studies was examined in detail. Eighteen studies did not meet the inclusion criteria as described. Finally, 27 studies19–45 fulfilled the inclusion criteria (Fig. 1).
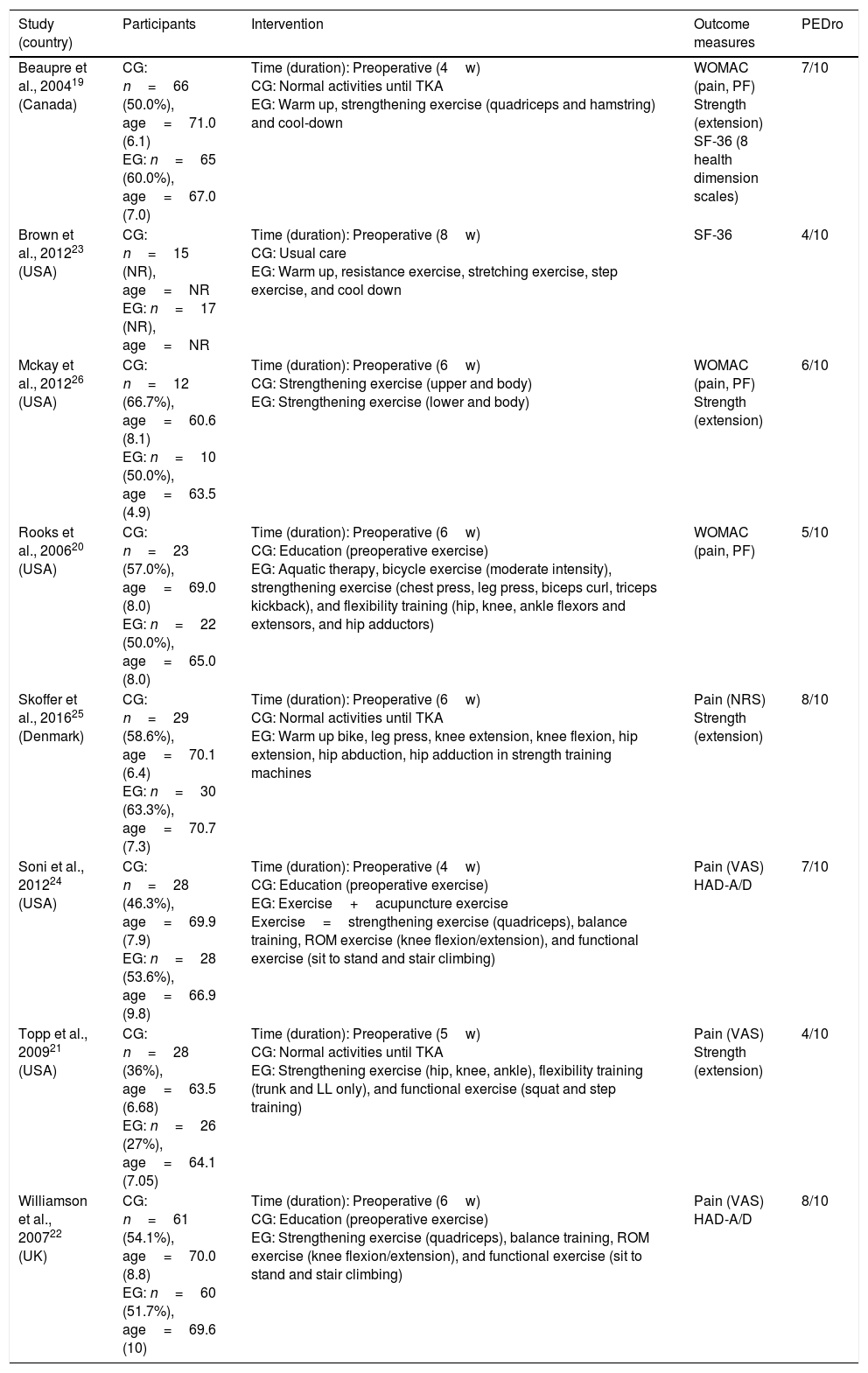
Study characteristicsThe included studies involved 2432 participants (1208 interventions and 1224 comparisons). Based on the available data of the interventions, the participants were 60.6–74.6 years of age and 27.0–95.2% were female. A summary of the included trials is shown in Tables 2 and 3.
Summary of included trials (preoperative).
| Study (country) | Participants | Intervention | Outcome measures | PEDro |
|---|---|---|---|---|
| Beaupre et al., 200419 (Canada) | CG: n=66 (50.0%), age=71.0 (6.1) EG: n=65 (60.0%), age=67.0 (7.0) | Time (duration): Preoperative (4w) CG: Normal activities until TKA EG: Warm up, strengthening exercise (quadriceps and hamstring) and cool-down | WOMAC (pain, PF) Strength (extension) SF-36 (8 health dimension scales) | 7/10 |
| Brown et al., 201223 (USA) | CG: n=15 (NR), age=NR EG: n=17 (NR), age=NR | Time (duration): Preoperative (8w) CG: Usual care EG: Warm up, resistance exercise, stretching exercise, step exercise, and cool down | SF-36 | 4/10 |
| Mckay et al., 201226 (USA) | CG: n=12 (66.7%), age=60.6 (8.1) EG: n=10 (50.0%), age=63.5 (4.9) | Time (duration): Preoperative (6w) CG: Strengthening exercise (upper and body) EG: Strengthening exercise (lower and body) | WOMAC (pain, PF) Strength (extension) | 6/10 |
| Rooks et al., 200620 (USA) | CG: n=23 (57.0%), age=69.0 (8.0) EG: n=22 (50.0%), age=65.0 (8.0) | Time (duration): Preoperative (6w) CG: Education (preoperative exercise) EG: Aquatic therapy, bicycle exercise (moderate intensity), strengthening exercise (chest press, leg press, biceps curl, triceps kickback), and flexibility training (hip, knee, ankle flexors and extensors, and hip adductors) | WOMAC (pain, PF) | 5/10 |
| Skoffer et al., 201625 (Denmark) | CG: n=29 (58.6%), age=70.1 (6.4) EG: n=30 (63.3%), age=70.7 (7.3) | Time (duration): Preoperative (6w) CG: Normal activities until TKA EG: Warm up bike, leg press, knee extension, knee flexion, hip extension, hip abduction, hip adduction in strength training machines | Pain (NRS) Strength (extension) | 8/10 |
| Soni et al., 201224 (USA) | CG: n=28 (46.3%), age=69.9 (7.9) EG: n=28 (53.6%), age=66.9 (9.8) | Time (duration): Preoperative (4w) CG: Education (preoperative exercise) EG: Exercise+acupuncture exercise Exercise=strengthening exercise (quadriceps), balance training, ROM exercise (knee flexion/extension), and functional exercise (sit to stand and stair climbing) | Pain (VAS) HAD-A/D | 7/10 |
| Topp et al., 200921 (USA) | CG: n=28 (36%), age=63.5 (6.68) EG: n=26 (27%), age=64.1 (7.05) | Time (duration): Preoperative (5w) CG: Normal activities until TKA EG: Strengthening exercise (hip, knee, ankle), flexibility training (trunk and LL only), and functional exercise (squat and step training) | Pain (VAS) Strength (extension) | 4/10 |
| Williamson et al., 200722 (UK) | CG: n=61 (54.1%), age=70.0 (8.8) EG: n=60 (51.7%), age=69.6 (10) | Time (duration): Preoperative (6w) CG: Education (preoperative exercise) EG: Strengthening exercise (quadriceps), balance training, ROM exercise (knee flexion/extension), and functional exercise (sit to stand and stair climbing) | Pain (VAS) HAD-A/D | 8/10 |
Abbreviations: CG, control group; EG, experimental group; Preoperative, effects of preoperative exercise interventions versus standard care; TKA, total knee arthroplasty; WOMAC, Western Ontario and McMaster Universities Arthritis Index; PF, physical function; SF-36, Short Form 36 Health Survey; HAD-A/D, Hospital Anxiety and Depression score for anxiety/depression; VAS, visual analogue scale; NR, no record; NRS, numerical rating scale; ROM, range of motion; LL, lower limb.
Summary of included trials (postoperative).
| Study (country) | Participants | Intervention | Outcome measures | PEDro |
|---|---|---|---|---|
| Beaupre et al., 200131 (Canada) | CG: n=40 (30.0%), age=69.0 (8.0) EG: n=40 (50.0%), age=67.1 (7.6) | Time (duration): Hospital postoperative (5–7 days) CG: Walking, ROM exercise (knee), strengthening exercise EG: Control group and slider board | WOMAC (pain, stiffness, PF), active knee ROM (flexion, extension), SF-36 (PCS, MCS) | 8/10 |
| Bruun-Olsen et al., 201337 (Norway) | CG: n=28 (50%), age=69.0 (10.0) EG: n=29 (62.1%), age=68.0 (8.0) | Time (duration): Discharge postoperative (6–8w) CG: Usual physical therapy care (strengthening exercise, ROM exercises, and walking) EG: The walking-skill group (Warm-up, sit to stand exercises, lunges single-leg stance, step-up/step-down exercises, stair climbing, obstacle course, throwing a ball, walking, and stretching) | Active knee ROM (flexion) KOOS (symptoms, pain, function, recreation, QOL) | 5/10 |
| Codine et al., 200433 (France) | CG: n=30 (70.0%), age=71.1 (15.0) EG: n=30 (53.3%), age=74.6 (13.0) | Time (duration): Discharge postoperative (until 30D) CG: Knee mobilization, strengthening exercise, and walking exercises EG: Control group exercise and strengthening exercise (hamstring) | Strength (flexion, extension) | 3/10 |
| Christiansen et al., 201542 (USA) | CG: n=13 (53.8%), age=66.6 (8.1) EG: n=13 (46.2%), age=68.2 (8.6) | Time (duration): Discharge postoperative (6w) CG: Home-based exercise EG: Control group exercise and weight bearing biofeedback | Gait speed | 7/10 |
| Fransen et al., 201730 (Australia) | CG: n=210 (52.0%), age=65.2 (6.0) EG: n=212 (54.0%), age=64.0 (6.5) | Time (duration): Early postoperative (8w) CG: Usual acute care EG: Post acute care (warm up, progressive functional and strengthening exercises, bicycle) | WOMAC (pain) Strength (flexion, extension) Active knee ROM (flexion, extension) | 8/10 |
| Frost et al., 201234 (UK) | CG: n=23 (47.8%), age=71.5 (5.4) EG: n=24 (50.0%), age=71.1 (5.6) | Category in this study: Discharge postoperative (NR) CG: Quadriceps strengthening exercise and knee ROM exercises EG: CG+home-based exercise (warm up, chair rise, walking, and leg lifts) | Knee score (1–5 scale), strength (extension), active knee ROM (flexion, extension), gait speed | 6/10 |
| Jakobsen et al., 201443 (Denmark) | CG: n=37 (56.7%), age=63.0 (NR) EG: n=35 (60.0%), age=66.0 (NR) | Time (duration): Discharge postoperative (7w) CG: Warming up, ROM exercise, stretching exercise, functional training, balance training, icing and elevation EG: Control group exercise and progressive strength training | Active knee ROM (extension) | 7/10 |
| Jorgensen et al., 201745 (Denmark) | CG: n=24 (58.3%), age=64.4 (8.7) EG: n=31 (48.3%), age=64.8 (8.3) | Time (duration): Discharge postoperative (8w) CG: Home based exercise (functional capacity, muscle strength, range of motion of knee, pain management) EG: Home based exercise+progressive resistance training (leg press, knee extension and flexion exercises) | Pain (VAS) Strength (extension) Gait speed | 8/10 |
| Labraca et al., 201127 (Spain) | CG: n=135 (81.5%), age=65.5 (4.8) EG: n=138 (73.2%), age=68.5 (8.6) | Time (duration): Early postoperative (until discharge) CG: Rehabilitation was started between 48 and 72h post-surgery EG: Rehabilitation was started within the first 24h post-surgery | Pain (VAS) | 7/10 |
| Liao et al., 201336 (Taiwan) | CG: n=55 (67.3%), age=72.9 (6.6) EG: n=58 (79.3%), age=71.4 (6.6) | Time (duration): Discharge postoperative (8w) CG: Functional training program Strengthening exercise (quadriceps, hamstrings, hip abductors, and extensors), knee mobility, advice about knee positioning, ice application, and walking EG: Control group exercise and balance training program | TUG | 7/10 |
| Lessen et al., 200632 (Netherlands) | CG: n=22 (77.3%), age=67.0 (7.0) EG: n=21 (71.4%), age=74.9 (5.0) | Time (duration): Hospital postoperative (about 4 days) CG: Strengthening exercise, ROM exercise (knee), and ADL exercise EG: Exercise group was similar to that of the control group | WOMAC (pain, stiffness, PF), active knee ROM (flexion, extension) | 8/10 |
| Liebs et al., 201228 (Germany) | CG: n=87 (70.1%), age=69.6 (7.2) EG: n=98 (73.5%), age=70.9 (7.5) | Time (duration): Early postoperative (5w) CG: Aquatic therapy beginning on POD14 EG: Aquatic therapy beginning on POD6 | WOMAC (pain) | 7/10 |
| Moffet et al., 200435 (Canada) | CG: n=39 (56.0%), age=66.7 (8.7) EG: n=38 (63.0%), age=68.7 (8.3) | Time (duration): Discharge postoperative (6–8w) CG: Home-based exercise Strengthening exercise (quadriceps, hamstrings, hip abductors, and extensors), knee mobility, advice about knee positioning, ice application, and walking EG: Control group exercise and training. Training: Warm-up and stretching exercise, specific strengthening exercise, functional task-oriented exercise, endurance exercise, and cool down | WOMAC (pain, stiffness, PF) | 7/10 |
| Monticone et al., 201338 (Italy) | CG: 55 (61.8%), age=68.0 (7.1) EG: n=55 (65.4%), age=67.0 (6.1) | Category in this study: Discharge postoperative (12w) CG: General advice and usual ADL EG: Early walking training, moving from a sitting to a standing position, ascending/descending stairs, climbing obstacles, weight-bearing exercises, walking, standing on unstable surfaces, and stationary cycling | Pain (NRS) | 8/10 |
| Munin et al., 199829 (USA) | CG: n=21 (95.2%), age=73.2 (7.7) EG: n=24 (91.7%), age=72.2 (6.5) | Time (duration): Early postoperative (until a discharge) CG: Inpatient rehabilitation on POD3 EG: Inpatient rehabilitation on POD7 | Pain (FSI) | 5/10 |
| Rajan et al., 200441 (UK) | CG: n=60 (61.7%), age=68.0 (10.0) EG: n=56 (64.3%), age=69.0 (9.3) | Time (duration): Discharge postoperative (6w) CG: Inpatient physical therapy only EG: Inpatient physical therapy and outpatient physical therapy | Active knee ROM (flexion) | 7/10 |
| Russell et al., 201140 (Australia) | CG: n=34 (NR), age=69.6 (7.2) EG: n=31 (NR), age=66.2 (8.4) | Time (duration): Discharge postoperative (6w) CG: ROM exercise, strengthening exercise, mobility, swelling management, education, and the home exercise program EG: Exercise group was similar to that of the control group | WOMAC (pain, stiffness, PF) Strength (extension) Active knee ROM (flexion) TUG | 8/10 |
| Vuorenmaa et al., 201439 (Finland) | CG: n=55 (65%), age=69.9 (9.0) EG: n=53 (57.0%), age=69.0 (8.0) | Time (duration): Discharge postoperative (4M) CG: Normal care EG: Strengthening exercises (quadriceps, hamstring), functional exercise (squat and step training) | Strength (flexion, extension) Active knee ROM (flexion, extension) TUG Gait speed | 6/10 |
| Villadsen et al., 201444 (Denmark) | CG: n=40 (60.0%), age=65.1 (9.0) EG: n=41 (60.9%), age=67.1 (8.8) | Time (duration): Discharge postoperative (8w) CG: Education EG: Education+neuromuscular exercise program | Strength (extension) Pain (KOOS) | 8/10 |
Abbreviations: CG, control group; EG, experimental group; Early postoperative, effects of early standard postoperative interventions versus late standard postoperative interventions; Hospital postoperative, effects of exercise starting in hospital in addition to standard postoperative interventions versus standard postoperative interventions only; Discharge postoperative, effects of exercise starting after discharge in addition to standard postoperative interventions versus standard postoperative interventions only; ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; PF, physical function; SF-36, Short Form 36 Health Survey (PCS, physical component summary; MCS, mental component summary); TKA, total knee arthroplasty; w, week; NR, not recorded; KOOS, Knee Injury and Osteoarthritis Outcome Score; QOL, quality of life; VAS, visual analogue scale; h, hour; TUG, timed up and go test; POD, postoperative day; ADL, activities of daily living; NRS, numerical rating scale; FSI, Functional Status Index.
Measurements used to assess body function (or impairment) varied and included pain, physical function, stiffness, muscle strength, and ROM. Pain was rated using the WOMAC, visual analog scale, Functional Status Index, and Knee injury and Osteoarthritis Outcome Score. Self-reported information regarding physical function and stiffness was evaluated using the WOMAC. Muscle strength was measured for the knee extensors or flexors. The ROM was measured at maximum active knee extension or flexion. Other than these measurements, measures regarding walking ability (50-m timed walk, gait speed) were included in 6 trials.22,24,26,32,37,40 Health-related QOL was assessed using the Short Form 36 Health Survey. Depressive symptoms were rated using the Hospital Anxiety and Depression score in two trials.22,24 The Oxford Knee Score or timed up and go (TUG) test was used to assess activity limitations.
Risk of bias within studiesThere were 27 high-quality trials (PEDro score >5/10), with a mean score of 6.6/10 across all trials. The total PEDro scale scores were 8 points for five trials,24,29,31,32,37,39,43 7 points for eleven trials,18,21,23,26,27,34,35,40–42,44 6 points for three trials,25,28,33,38 5 points for two trials,19,36 4 points for two trials,20,22 and 3 points for one trial.32 The most adhered to items on the PEDro scale were random allocation, the use of similar groups at baseline, measurements of variability for at least one key outcome, and between group comparisons, which were evident in almost all trials. None of the trials blinded the participants or therapists, which is expected given that these items are the most difficult to adhere to in trials of interventions involving exercise. Sixteen trials reported employing an intention-to-treat analysis, while 21 used allocation concealment, 17 used blinded outcome assessors, and 19 used measurements of at least one key outcome for >85% of the participants.
Synthesis of resultsPreoperative exercise interventions versus standard care after surgeryEight19–26 trials were considered to constitute preoperative exercise intervention in participants awaiting knee joint replacement. Seven trials19–25 investigated the effect of preoperative exercise intervention compared to standard care. In four trials,19–22 exercise programs were provided by a physical therapist or other therapist. Other types of intervention included exercise combined with education programs22 or acupuncture.24 One trial26 compared the effects of a lower-body strength training program and an upper-body strength training program prior to surgery.
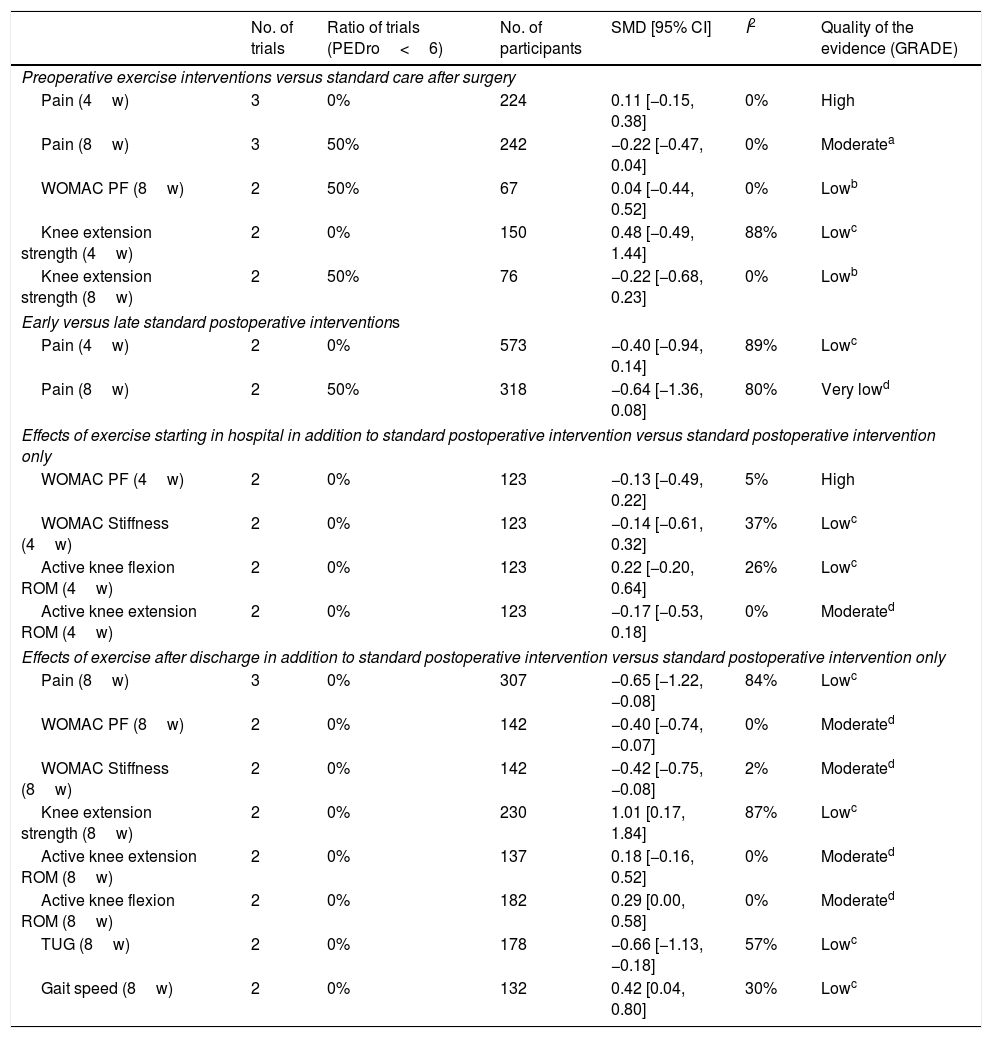
The effects of preoperative exercise interventions (experimental group) versus standard care (control group) are shown in Table 4. The intervention period of the exercise being considered in the previous study was 4 and 8 weeks. A meta-analysis showed no intergroup differences in pain,19–22,24–26 WOMAC physical function,20,26 or knee extension strength.19,21,25,26 Evidence levels were from low to high.
Meta-analysis of the effect of exercise therapy.
| No. of trials | Ratio of trials (PEDro<6) | No. of participants | SMD [95% CI] | I2 | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|---|
| Preoperative exercise interventions versus standard care after surgery | ||||||
| Pain (4w) | 3 | 0% | 224 | 0.11 [−0.15, 0.38] | 0% | High |
| Pain (8w) | 3 | 50% | 242 | −0.22 [−0.47, 0.04] | 0% | Moderatea |
| WOMAC PF (8w) | 2 | 50% | 67 | 0.04 [−0.44, 0.52] | 0% | Lowb |
| Knee extension strength (4w) | 2 | 0% | 150 | 0.48 [−0.49, 1.44] | 88% | Lowc |
| Knee extension strength (8w) | 2 | 50% | 76 | −0.22 [−0.68, 0.23] | 0% | Lowb |
| Early versus late standard postoperative interventions | ||||||
| Pain (4w) | 2 | 0% | 573 | −0.40 [−0.94, 0.14] | 89% | Lowc |
| Pain (8w) | 2 | 50% | 318 | −0.64 [−1.36, 0.08] | 80% | Very lowd |
| Effects of exercise starting in hospital in addition to standard postoperative intervention versus standard postoperative intervention only | ||||||
| WOMAC PF (4w) | 2 | 0% | 123 | −0.13 [−0.49, 0.22] | 5% | High |
| WOMAC Stiffness (4w) | 2 | 0% | 123 | −0.14 [−0.61, 0.32] | 37% | Lowc |
| Active knee flexion ROM (4w) | 2 | 0% | 123 | 0.22 [−0.20, 0.64] | 26% | Lowc |
| Active knee extension ROM (4w) | 2 | 0% | 123 | −0.17 [−0.53, 0.18] | 0% | Moderated |
| Effects of exercise after discharge in addition to standard postoperative intervention versus standard postoperative intervention only | ||||||
| Pain (8w) | 3 | 0% | 307 | −0.65 [−1.22, −0.08] | 84% | Lowc |
| WOMAC PF (8w) | 2 | 0% | 142 | −0.40 [−0.74, −0.07] | 0% | Moderated |
| WOMAC Stiffness (8w) | 2 | 0% | 142 | −0.42 [−0.75, −0.08] | 2% | Moderated |
| Knee extension strength (8w) | 2 | 0% | 230 | 1.01 [0.17, 1.84] | 87% | Lowc |
| Active knee extension ROM (8w) | 2 | 0% | 137 | 0.18 [−0.16, 0.52] | 0% | Moderated |
| Active knee flexion ROM (8w) | 2 | 0% | 182 | 0.29 [0.00, 0.58] | 0% | Moderated |
| TUG (8w) | 2 | 0% | 178 | −0.66 [−1.13, −0.18] | 57% | Lowc |
| Gait speed (8w) | 2 | 0% | 132 | 0.42 [0.04, 0.80] | 30% | Lowc |
(), intervention duration; GRADE, GRADE working group grades of evidence; WOMAC, Western Ontario and McMaster Universities Arthritis Index; PF, physical function; ROM, range of motion; TUG, timed up and go test; SMD, standardized mean difference; CI, confidence interval.
Four trials27–30 investigated early versus late standard postoperative interventions. An exercise program provided by a physical therapist or other therapist after TKA was the most common intervention in 4 trials.27–30 The effects of early (experimental group) versus late (control group) standard postoperative interventions are shown in Table 4. The intervention periods of the exercise being considered in the previous study were 4 and 8 weeks. A meta-analysis showed no differences with respect to pain.27–30
Effects of exercise starting in hospital in addition to standard postoperative intervention versus standard postoperative intervention onlyTwo trials31,32 investigated the efficacy of exercise starting after discharge in addition to standard postoperative intervention versus standard postoperative intervention only.
The effects of exercise starting in hospital in addition to standard postoperative intervention (experimental group) versus standard postoperative intervention only (control group) are shown in Table 4. The intervention periods of the exercise being considered in the previous study were 4 weeks. A meta-analysis revealed no intergroup difference in pain,31,32 WOMAC physical function31,32 and stiffness,31,32 active knee flexion, or extension ROM.31,32 Evidence levels were from low to high.
Effects of exercise after discharge in addition to standard postoperative intervention versus standard postoperative intervention onlyThirteen trials33–45 investigated the efficacy of exercise starting after discharge in addition to standard postoperative intervention versus standard postoperative intervention only. An exercise program provided by a physical therapist or other therapist after TKA was the most common intervention in 13 trials.33–45 Other interventions included exercise combined with an internet-based tele-rehabilitation program.40 One trial provided no description of the exercise intervention.41
The effects of exercise starting after discharge in addition to standard postoperative intervention (experimental group) versus standard postoperative intervention only (control group) are shown in Table 4. The intervention period of the exercise being considered in the previous study was 8 weeks. A meta-analysis showed that pain (SMD=−0.65; 95% CI, −1.22 to −0.08; I2=84%; evidence level=low),35,40,44,45 WOMAC physical function (SMD=−0.40; 95% CI, −0.74 to 0.07; I2=0%; evidence level=moderate),35,40 and stiffness (SMD=−0.42; 95% CI, −0.75 to 0.08; I2=2%; evidence level=moderate),35,40 knee extension strength (SMD=1.01; 95% CI, 0.17 to 1.84; I2=87%; evidence level=low),40,44 active knee flexion ROM (SMD=0.29; 95% CI, 0.00 to 0.58; I2=0%; evidence level=moderate),40,41 TUG (SMD=−0.66; 95% CI, −1.13 to −0.18; I2=57%; evidence level=low),36,40 and gait speed (SMD=0.42; 95% CI, 0.04 to 0.80; I2=30%; evidence level=low)39,42,45 were more improved in the experimental group at 8 weeks after the start of exercise after discharge than in the control group. A meta-analysis showed no intergroup difference with respect active knee extension ROM.40,43 Evidence levels were moderate to high.
DiscussionSummary of evidenceFrom among the outcomes observed as having a significant effect in our study, those that were not confirmed in preceding studies included pain, physical function and stiffness evaluated by the WOMAC, knee extension strength, active knee flexion ROM, TUG, and gait speed by exercise for 8 weeks after discharge in addition to standard postoperative intervention. The effect size of each was small, and the quality of evidence was low to moderate. Our meta-analysis showed that an exercise-intervention period ≥8 weeks is needed after discharge to improve body function and activity. These results have not been reported in preceding studies. Regarding active knee flexion ROM, Minns Lowe et al.5 reported a significant effect due to exercise intervention by integrating knee joint flexion and extension. In contrast, our study integrated the data of knee flexion ROM only. The results obtained by Minns Lowe et al.5 and in our study indicate that the improvement in knee ROM due to exercise starting after discharge in addition to standard postoperative interventions was led by improved flexion ROM and required at least 8 weeks.
In our study, the following types of intervention were not observed as having any significant effects in several outcomes: 4- or 8-week preoperative exercise intervention for pain, physical function evaluated using the WOMAC, knee extension strength; early exercise intervention for pain; 4- or 8-week early versus late standard postoperative interventions; 4-week exercise starting in the hospital added to the standard postoperative intervention for pain, physical functions, and stiffness evaluated using the WOMAC and active knee extension/flexion ROM; 8 weeks exercise starting after discharge in addition to standard postoperative intervention for active knee extension ROM. The results of our meta-analysis did not suggest that the early start of exercise demonstrated an effect on body function and activity before and/or after TKA. These evidences were discovered for the first time in the current systematic review and meta-analysis. However, the quality of this evidence ranged from very low to high, so the degree of effect may vary in subsequent studies.
ImplicationsThe results of this study suggest that exercise intervention for 8 weeks after discharge would improve pain, physical function, and stiffness measured by the WOMAC, knee extension strength, active knee flexion ROM, TUG results, and gait speed. These results are an important fact for clinicians since insurers may worry about an increase in medical costs due to the continuous exercise intervention. However, the physical function in patients who performed exercise for 8 weeks was more improved than that of controls. Femoral fractures occur at a rate of 2.5% after TKA.46 The risk of this fracture can be decreased by using exercise to improve one's physical function.47,48 Therefore, our findings might be used by clinicians to support the claim that insurers should accept the need for an exercise intervention period ≥8 weeks after discharge.
Major study strengthsThe strengths of our study are as follows. Prior to our study, Wallis and Taylor,3 Gill and McBurney,4 Minns Lowe et al.,5 and Artz et al.6 conducted systematic reviews and meta-analyses regarding the effect of exercise intervention in patients who underwent TKA. However, Wallis and Taylor3 and Gill and McBurney4 did not investigate the effect of postoperative exercise intervention. Additionally, the research design by Gill and McBurney4 includes primary studies other than RCT. Moreover, Gill and McBurney4 and Minns Lowe et al.5 used data other than the mean value and standard deviation of the primary study for their meta-analysis and did not investigate evidence level. Artz et al.6 conducted a meta-analysis based on the differences in the follow-up period. Accordingly, these reports have room for examinations regarding the effective exercise intervention period. Meanwhile, regarding our findings, a meta-analysis was performed based on the subgroup of similar intervention periods. Consequently, the results obtained from our study are the effective intervention period of exercise started after discharge. Our findings will be helpful to clinicians who are planning the exercise program after discharge in patients with knee OA undergoing TKA.
LimitationsThe current study has three main limitations. The first limitation involves the evidence level. The GRADE system used in our study determines the evidence level based on the results of investigations regarding all five items. One such item is the presence of publication bias, which is investigated by a visual analysis using funnel plotting or statistical testing. However, in the case of a statistical test, if the primary study has a score of ≤10, then a β error is suspected in the investigation results.49 The primary study included in the meta-analysis of our study had a maximum score of 3, and performing a detailed investigation regarding publication bias was difficult. Therefore, the evidence level determined in our study is provisional, with the possibility that the evidence may have been downgraded by one level due to the results of future studies depending on the outcome thereof. The second limitation regards the individual effect of the exercise intervention. The results of the exercise intervention are expected to differ depending on the time of commencement and program content. Accordingly, in our study, an investigation was performed by dividing the primary study into the following four items in terms of content: preoperative exercise intervention; postoperative exercise intervention; early postoperative exercise intervention; and exercise intervention after discharge. Thereby, the data from primary studies with similar exercise content is more fully integrated than in past reviews,3–6 and it may be said that the range of generalization is clearer than in past reviews. However, because most primary studies combine a plurality of exercise interventions, an investigation into the effect of each individual exercise could not be performed. Therefore, the effects of individual exercise intervention remain unknown. The third limitation regards the frequency of the exercise intervention. The primary studies extracted in our study did not have a completely matching frequency. Regardless, because there were only a few articles, the data could not be stratified and investigated. Therefore, the influence of the differences in frequency on the effect cannot be eliminated from the analysis results. Finally, fourth limitation is that some studies not published in a non-English language were not included in our review. A systematic review of intervention researchers working in non-English speaking countries might have published some of their research in local journals. However, English reports are more likely to have better methodological quality than reports written in other languages.50 Thus, in this review the results are considered to be largely unchanged if some studies not published in a non-English language were included. To overcome these four limitations, it is necessary to carry perform a statistical analysis to investigate the presence of publication bias, stratify the primary studies included in the meta-analysis by content and frequency, and add investigations after the number of articles on RCT increases in the future.
ConclusionWe investigated the effective exercise intervention period in patients undergoing TKA. A meta-analysis indicated that the exercises performed for 8 weeks after discharge in addition to standard postoperative intervention effectively improved body function. However, the effective pre- and postoperative periods of exercise before discharge were not identified.
FundingNone.
Conflicts of interestThe authors declare no conflicts of interest.