Persistent shoulder pain is common, and it is associated with substantial morbidity and healthcare costs. Approximately 21 to 50 % of people with shoulder pain treated in primary healthcare recover within six months. It is not known why at least half do not recover. One possibility is the manner underlying mechanisms related to persistent shoulder pain are managed. Being able to determine the predominant pain phenotype in people with persistent shoulder pain (i.e., nociceptive, neuropathic, or nociplastic pain) together with their underlying mechanism and tailoring management accordingly may improve outcomes for people seeking care for persistent shoulder pain. The International Association for the Study of Pain (IASP) recently developed clinical criteria and a grading system for the identification of nociplastic pain.
ObjectiveIn this paper, we aim to provide suggestions to clinicians to assist in the evaluation of pain phenotypes, underlying mechanisms, and their causal relationships.
DiscussionBased on the IASP clinical criteria and grading system for nociplastic pain, we outline pain phenotype evaluation and provide a clinical reasoning framework. To facilitate this, three case studies involving people living with persistent shoulder pain are presented.
With reported prevalence rates of up to 30 %, shoulder pain is one of the most common musculoskeletal health disorders.1–4 It encompasses conditions such as frozen shoulder, osteoarthritis, avascular necrosis, and rotator cuff related shoulder pain (RCRSP).5–7 In addition to the negative impact shoulder pain has on an individual’s ability to participate in valued daily activities,8,9 it also places a significant burden on health services and society as a whole.10–12
Persistent shoulder pain has high non-recovery rates, with up to 50 % of individuals not fully recovering after either surgical or non-surgical care.8,13–17 Furthermore, the effectiveness of interventions for persistent shoulder pain symptoms remains inconsistent, with mixed findings reported in the literature.18–22 One possible reason for the suboptimal effectiveness of current management strategies is that they may not sufficiently account for the dominant pain type(s) experienced by the patient. This suggests that treatments might fail to target the underlying mechanisms of the pain, leading to poor outcomes.23,24 For instance, current clinical practice often focuses on structural changes (e.g., surgery for rotator cuff tear, acromial spur) or mechanical diagnoses (e.g., exercises for forward head posture, scapular dyskinesia) as the cause of pain.25–27 Such thinking might overlook the possibility that chronic pain by sensitization of pain processing in the central nervous system, as a condition in itself, may be the root cause of symptoms and could require treatment tailored to specific pain phenotypes.28,29
The International Association for the Study of Pain (IASP) defines three different pain phenotypes, i.e., nociceptive, neuropathic, and nociplastic pain (Table 1), with different neurobiological mechanisms explaining the pain experience per phenotype.30,31 In other musculoskeletal pain conditions, it has already been explored if the response to exercise varies by pain phenotype. For example, Falla and Hodges highlight the differences in pain intensity reduction following a specific neck exercise program for individuals with chronic whiplash-associated disorders (i.e., a condition with typically dominant nociplastic pain) and idiopathic neck pain.32 The data indicate a 47 % reduction in neck pain intensity in patients with mild to moderate idiopathic neck pain (data from Falla et al.,33). In contrast, individuals with whiplash-associated disorders who exhibited signs of mechanical hyperalgesia experienced only a 37 % reduction in neck pain intensity, while those with whiplash-associated disorders who showed signs of widespread mechanical and cold hyperalgesia had only a 16 % reduction (data from Jull et al.34). This illustrates that the response to exercise is influenced by various factors, such a more sensitive pain processing in the central nervous system. Consequently, individuals with heightened central pain processing may benefit more from alternative exercise therapy programs.
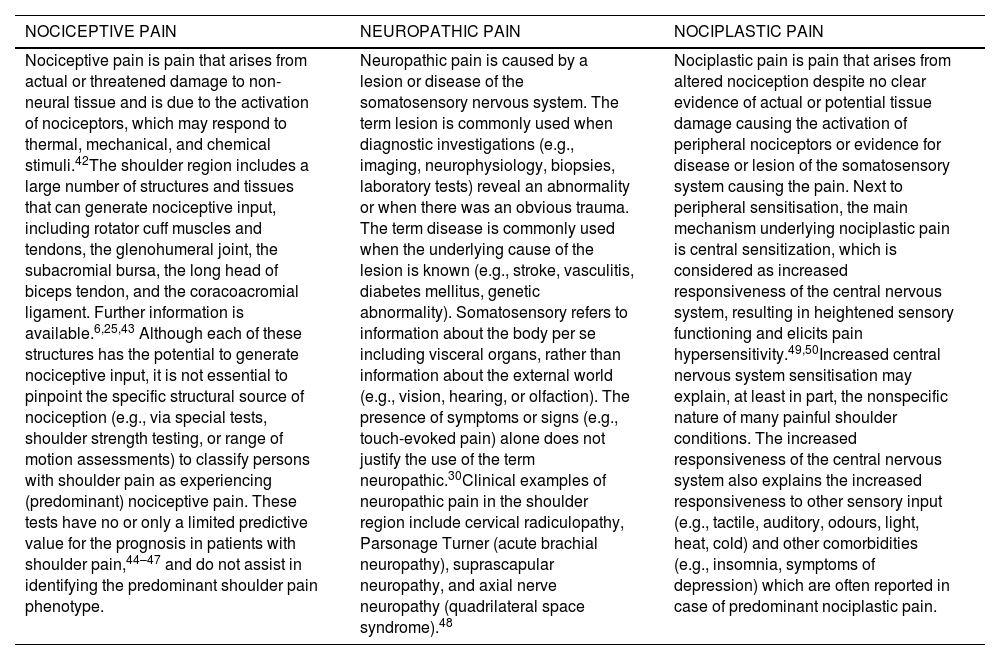
Nociceptive, neuropathic, and nociplastic shoulder pain: definitions according to the International Association for the Study of Pain (IASP)30and possible shoulder pain examples.
| NOCICEPTIVE PAIN | NEUROPATHIC PAIN | NOCIPLASTIC PAIN |
|---|---|---|
| Nociceptive pain is pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors, which may respond to thermal, mechanical, and chemical stimuli.42The shoulder region includes a large number of structures and tissues that can generate nociceptive input, including rotator cuff muscles and tendons, the glenohumeral joint, the subacromial bursa, the long head of biceps tendon, and the coracoacromial ligament. Further information is available.6,25,43 Although each of these structures has the potential to generate nociceptive input, it is not essential to pinpoint the specific structural source of nociception (e.g., via special tests, shoulder strength testing, or range of motion assessments) to classify persons with shoulder pain as experiencing (predominant) nociceptive pain. These tests have no or only a limited predictive value for the prognosis in patients with shoulder pain,44–47 and do not assist in identifying the predominant shoulder pain phenotype. | Neuropathic pain is caused by a lesion or disease of the somatosensory nervous system. The term lesion is commonly used when diagnostic investigations (e.g., imaging, neurophysiology, biopsies, laboratory tests) reveal an abnormality or when there was an obvious trauma. The term disease is commonly used when the underlying cause of the lesion is known (e.g., stroke, vasculitis, diabetes mellitus, genetic abnormality). Somatosensory refers to information about the body per se including visceral organs, rather than information about the external world (e.g., vision, hearing, or olfaction). The presence of symptoms or signs (e.g., touch-evoked pain) alone does not justify the use of the term neuropathic.30Clinical examples of neuropathic pain in the shoulder region include cervical radiculopathy, Parsonage Turner (acute brachial neuropathy), suprascapular neuropathy, and axial nerve neuropathy (quadrilateral space syndrome).48 | Nociplastic pain is pain that arises from altered nociception despite no clear evidence of actual or potential tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain. Next to peripheral sensitisation, the main mechanism underlying nociplastic pain is central sensitization, which is considered as increased responsiveness of the central nervous system, resulting in heightened sensory functioning and elicits pain hypersensitivity.49,50Increased central nervous system sensitisation may explain, at least in part, the nonspecific nature of many painful shoulder conditions. The increased responsiveness of the central nervous system also explains the increased responsiveness to other sensory input (e.g., tactile, auditory, odours, light, heat, cold) and other comorbidities (e.g., insomnia, symptoms of depression) which are often reported in case of predominant nociplastic pain. |
Apart from exercise therapy, it has also been shown that altered sensory processing (assessed via quantitative sensory testing) is prognostic for treatment outcomes of pain phenotype-specific treatments like surgery (for nociceptive pain) or pharmacological interventions.35–41
An essential first step towards implementing a pain phenotype-tailored treatment approach is to provide recommendations to clinicians to enable them to identify the predominant pain phenotype(s) underpinning the patient’s pain experience. Recently, a Delphi expert consensus study has reached agreement on features and methods that might discriminate between the different pain phenotypes in people experiencing musculoskeletal pain (Table 2).51 In parallel, the IASP clinical criteria and grading system for nociplastic pain has been developed,52 with key features identified in the Delphi study51 integrated in the different steps of this IASP clinical criteria and grading system.
| NOCICEPTIVE PAIN | NEUROPATHIC PAIN | NOCIPLASTIC PAIN |
|---|---|---|
| DISCRIMINATIVE KEY FEATURES FROM THE PATIENT INTERVIEW AND CLINICAL ASSESSMENT | ||
| In most cases, the onset of the pain is clear. The pain intensity is mostly proportional to the injury/lesion/inflammation, is movement and/or posture-related, and is often predictable. The location of pain is anatomically logical (it might include the area of referred pain). There is a positive response to NSAIDS/analgesics.In the clinical examination, consistent outcomes are reported. Tests that mechanically stress injured/inflamed structures will be positive, while other tests that do not involve these structures will be negative. | There is a history of a traumatic nerve lesion or disease. The pain is neuro-anatomically logical, which means that the pain is localized in the course of the peripheral nerve.Pain is very irritable in nature, may occur/exacerbate spontaneously, may be present at rest. There is often the presence of nocturnal pain, which can interfere with sleep. The pain is usually accompanied by altered sensitivity, which can be described as pins and needles, or numbness. There is no response to NSAIDS/analgesics.Compared with nociceptive pain, neuropathic pain is often associated with higher levels of pain, greater disability, and a reduced health-related quality of life. Comorbid anxiety and depressive symptoms are also common.In the clinical examination, pain is provoked by manoeuvres that tension or compress the involved neurogenic structures in people with neuropathic pain related to heightened neural mechanosensitivity. There is hyperalgesia/allodynia in the course of the peripheral nerve. | The onset of pain is unclear and there is a variable pain provocation. The pain is disproportionally high in relation to posture/movement/activity and is not activity-related (non-mechanical). The pain extent is not anatomically logical (widespread pain area) and there is inconsistency in pain intensity, pain location, and pain provocation. There is often a strong association with psychosocial factors such as depression and pain catastrophizing,54 and with comorbidities such as sleep disturbance and obesity. In the clinical examination, outcomes on mechanical pain provocation test are inconsistent. There is mostly widespread hyperalgesia/allodynia, with positive static or dynamic mechanical allodynia. Static allodynia can be assessed using digital palpation with a weight of approximately 4 kg (i.e., nailbed blanching), with reporting pain in response to such digital palpation considered as mechanical allodynia. Dynamic mechanical allodynia can be assessed by gently stroking the skin in the painful area with a brush or a cotton pad. If this triggers pain, it is considered dynamic allodynia. Painful aftersensations can be present as well. See elsewhere for more details on clinical sensory testing tests batteries that can be applied to determine nociplastic pain.52 |
| KEY FEATURES FROM QUANTITATIVE SENSORY TESTING (QST)* | ||
| There may be sensory aberrations, allodynia, and hyperalgesia in the area of pain. | Decreased pressure and heat/cold pain thresholds are possible, together with hyperalgesia in the area of pain. | There is an abnormal somatosensory functioning linked to sensitisation of peripheral and/or central pain pathways.31,50,52 There are decreased pressure and heat/cold thresholds, widespread hyperalgesia (in the area of pain and at remote pain free sites), facilitated temporal summation of pain, and/or impaired conditioned pain modulation. |
| *: QST is widely used to assess (ab)normal somatosensory functioning, and includes a panel of static (e.g., pressure, heat or cold pain thresholds) and dynamic (temporal summation and conditioned pain modulation) diagnostic tests | ||
Recently, the IASP clinical criteria and grading system has been applied to different patient populations,55–57 to help clinicians in determining the probability that nociplastic pain is in part responsible for the patient’s pain experience.52 Such papers are arguably important as they create awareness in clinicians about the potential value of pain phenotyping and tailored management in musculoskeletal care. Furthermore, they provide a first direction on which components to assess in the patient’s history and clinical examination with regard to identifying the pain phenotype(s) involved in the patient’s complaint.
Various reviews of the shoulder pain literature have been published, aiming to provide a deeper understanding of the mechanisms underlying the shoulder patient’s pain experience.6,29,58–64 These reviews generally conclude that significant knowledge gaps persist regarding the distinct characteristics of patients per specific shoulder pain phenotypes and this across various shoulder pain conditions (impingement syndrome,65–67 rotator cuff tendinopathy,60 RCRSP,29,68 frozen shoulder,59,69–71 post-stroke shoulder pain,72 non-specific persistent shoulder pain,54,73,74 and in mixed populations at a pre-surgical phase75). These results highlight that the pain phenotypes responsible for persistent shoulder pain can be complex and dynamic in nature.53,76 A person with shoulder pain may have one pain phenotype that predominantly explains the experienced pain in the acute phase after an injury/surgery, while another pain phenotype may be predominantly responsible for the pain experience at a later stage.11 Also, different pain phenotypes may coexist (i.e., mixed pain phenotypes). For example, a person with neuropathic shoulder-upper limb pain due to cervical radiculopathy and with axillary nerve-related deltoid muscle weakness may experience additional rotator cuff-related (nociceptive) shoulder pain from rotator cuff muscles, tendons, and related structures subjected to unaccustomed physical and / or lifestyle load.
Notwithstanding the complexity and lack of robust evidence on shoulder pain phenotyping, this paper aims to stimulate clinicians to implement a pain phenotype-based assessment and clinical reasoning in people with persistent shoulder pain, based on the IASP clinical criteria and grading system. To help clinicians make sense of persistent shoulder pain presentations, this paper outlines the pain phenotype evaluation and subsequent clinical reasoning of three people with persistent shoulder pain, as based on the IASP clinical criteria and grading system for nociplastic pain,52 at multiple points in their recovery process.
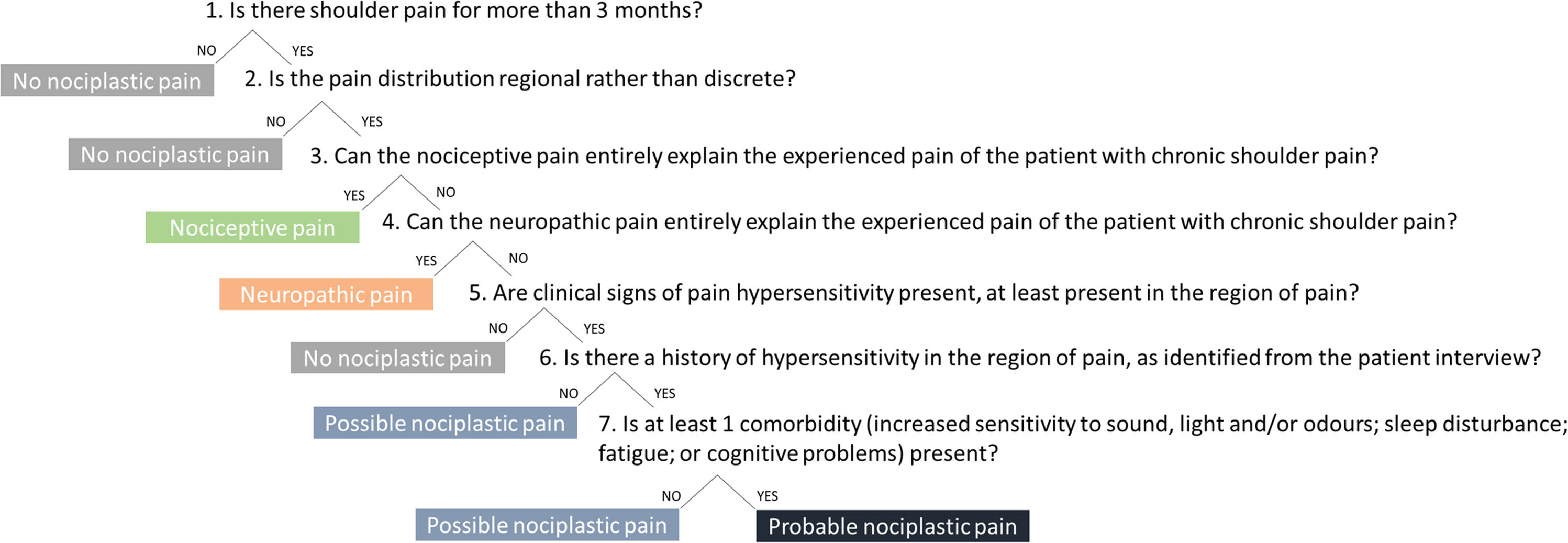
Defining the predominant pain phenotype(s) in people with persistent shoulder painHere and in the supplementary material we present information from the patient history, medical file, and the clinical assessment of three people with persistent shoulder pain and describe the different steps of the IASP clinical criteria and grading system for nociplastic pain for each case (Fig. 1 provides the different stages of the IASP algorithm).52 We highlight the potential change in the identified predominant pain phenotype during the recovery process of these people with shoulder pain, together with suggested treatment approaches.
The seven-steps clinical decision-making algorithm of the IASP clinical criteria for nociplastic pain applied to shoulder pain52.
Case presentation – ‘Sarah’
InterviewSarah is 29-years old and a single parent of a 16-months old son. She has been on sick leave for 5 years. Before, she used to work as a nurse in a hospital. Her lived experiences suggest she has experienced many challenges. Although she is a non-smoker and non-alcohol drinker, she has slept poorly since adolescence. She has problems falling asleep and wakes frequently most nights of the week. This leads to feeling exhausted and fatigued during daytime, which detrimentally impacts on her motivation to play and interact with her son. By definition, Sarah is overweight (body mass index - BMI of 28 kg/m2), and she does not meet physical activity target levels.77 Levels of psychological distress are high due to financial difficulties and social isolation.
Sarah was diagnosed with Ehlers–Danlos syndrome when she was 20 years old, with characteristic spinal and peripheral joint hypermobility (Beighton score for hypermobility: 7/9 points). When aged 22 years, she underwent bilateral capsular shift surgery for recurrent glenohumeral (sub)luxations. Following the surgery, she did not experience any new glenohumeral (sub)luxations. Most likely associated with Ehlers–Danlos syndrome, Sarah suffers from multilevel joint pain at rest (Numeric Pain Rating Scale (NPRS) of 3/10 on average) and during activity (NPRS of 6/10 on average). She has also been diagnosed with related soft-tissue disorders (Crohn disease). Sarah also experiences “sensitive skin” and suffers general itchiness. She does not take any pain medication.
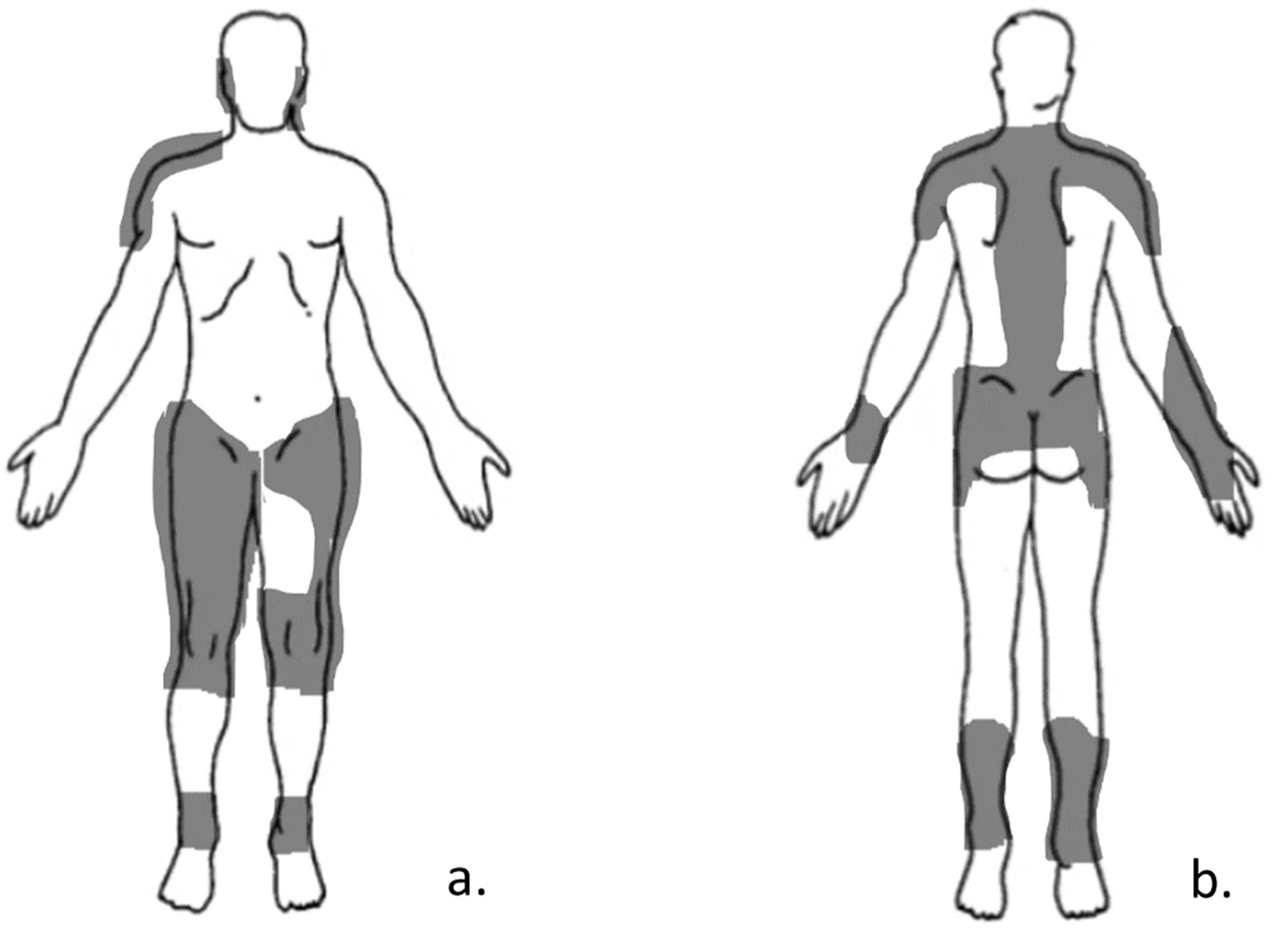
She is currently seeking care for six months of shoulder pain on her right (dominant) side. The shoulder pain was primarily provoked by carrying her son, holding her son while breastfeeding, lifting objects, and raising her arms. Currently, she experiences increased pain at rest. The rating of shoulder pain intensity (NPRS) fluctuates between 7–10/10 during abovementioned activities and is 5/10 at rest which disables her to perform her activities of daily living. A recent diagnostic ultrasound report suggests supraspinatus tendinopathy. Her current pain drawing is depicted in Fig. 2.
Clinical examinationDuring the physical examination, active abduction and external rotation of the right arm, with no external load were limited by pain (NRPS: 8/10) to 70° abduction and 30° external rotation. Pain prevented the assessment of shoulder muscle performance. Sarah demonstrated nearly full passive abduction and external rotation range of motion (right side), which is limited at the end-range by muscle tensioning.
Sarah reported an increased pain intensity while being touched on her right as well as left forearm and when the physical therapist gently stroked her skin with the finger (NRPS: 6/10), suggesting abnormal somatosensory functioning.
Self-reported outcomesThe Central Sensitisation Inventory (CSI) score was 54/100, indicating a severe level of symptoms of central sensitisation.78
Based on the final seventh step (Table 3), it is concluded that Sarah, who was referred to physical therapy for a local shoulder complaint, suffers ‘probable nociplastic pain’. However, it is likely that an additional source of nociception from the supraspinatus tendinopathy possibly due to acute local overload79 is also responsible for her currently increased level of shoulder pain. In step 3, we have argued that nociceptive pain is not entirely responsible for her pain complaints, but this does not exclude a mixed nociplastic-nociceptive pain phenotype with a nociceptive peripheral input from the supraspinatus tendinopathy. The increased responsiveness of the central nervous system, underlying the nociplastic pain, might also explain the difficulties Sarah experiences when trying to sleep together with frequent waking at night time. It might also clarify her reported general feeling of exhaustion and fatigue during daytime,80 as well as increased skin sensitivity.
aligns the clinical reasoning process with the IASP clinical criteria and grading system for nociplastic pain52.
| Table 3. Clinical reasoning in accordance with the IASP clinical criteria and grading system for nociplastic pain,52 as based on Sarah’s interview, clinical examination and self-reported outcomes | |
|---|---|
| Step 1 | Sarah has experienced shoulder pain for a considerable period of time. The current episode of right shoulder pain started 6 months previously. Therefore, the criterion for persistent pain (i.e., pain of at least 3 months duration) is fulfilled. |
| Step 2 | According to the pain diagram (Fig. 2), Sarah’s pain distribution is widespread, with the pain being present in regions other than the ones that are neuro-anatomically plausible. Indeed, the pain is spreading to a larger area than would be expected if only peripheral nociceptive mechanisms were present. Therefore, the criterion of regional (instead of discrete) pain distribution is met. |
| Step 3 | Although the diagnosis of Ehlers–Danlos syndrome has been established, features of the patient interview and clinical examination suggested that her pain response cannot solely be explained by peripheral nociceptive input from the Ehlers–Danlos syndrome-related spinal and peripheral joint hypermobility or from supraspinatus tendinopathy (due to acute local overload). More specifically, pain at rest fluctuates in intensity. Generally, high pain intensity, the extent of the pain, and the pain experienced while touching her forearms in the clinical examination indicate that nociceptive pain cannot be considered entirely responsible for her experienced pain. |
| Step 4 | Sarah has no history of a lesion or disease of the somatosensory nervous system. Furthermore, according to the Margolis pain diagram (Fig. 2), her pain distribution is neuro-anatomically illogical. That is, the pain is not in the course of a peripheral nerve. Furthermore, typical sensory signs like numbness and burning are not present. Hence, it can be concluded that her pain could not be entirely explained by neuropathic pain mechanisms. |
| Step 5 | During the clinical examination, increased pain sensitivity was observed. There is clear static and dynamic mechanical allodynia (pain reported on palpation and when the physical therapist is gently stroking Sarah’s skin with the finger, respectively). As a hypersensitive pain response was identified, we may conclude that she has, according to the algorithm, possible nociplastic pain. For increasing the likelihood to ‘probable’ nociplastic pain, additional requirements need to be fulfilled. |
| Step 6 | Sarah has a clear history of (pain) hypersensitivity within and beyond the region of pain. Indeed, she has been suffering from pain at rest for 10 years. Furthermore, there are also other hypersensitivity signs like her sensitive skin, with continuous itchiness. Given that she has a history of pain sensitivity, this sixth step is fulfilled, and the next step will be needed to conclude whether she has possible or probable nociplastic pain. |
| Step 7 | Sarah reports multiple comorbid symptoms that are included in the IASP clinical criteria for nociplastic pain. This is clear from the interview where she discloses having sleep problems and suffers disabling fatigue. This is furthermore evident from the item-based analysis of the Central Sensitisation Inventory. |
Two other examples of clinical cases applying the different steps of the IASP clinical criteria and grading system for nociplastic pain52 are provided in Supplementary Material.
Discussion and future researchBased on the clinical cases, we aimed to assist clinicians in identifying predominant pain phenotypes in people with persistent shoulder pain. We used the IASP clinical criteria and grading system for nociplastic pain as an assisting tool.52 It is important to keep in mind that while the IASP clinical criteria and grading system for nociplastic pain are helpful and provide confidence to perform the pain phenotyping, the proposed graded approach with clinical criteria per step has not yet been validated in people with musculoskeletal pain conditions (including shoulder pain) and cannot replace expert clinical reasoning. For example, when the IASP step-by-step algorithm52 is strictly followed, not considering 'nociplastic pain' in step 2 because pain is localized within a discrete distribution may incorrectly lead to the rejection of the presence of the ‘possible nociplastic pain’ or ‘probable nociplastic pain’, in case other symptoms of central nervous system sensitization are present in the patient’s story or clinical examination (which are only assessed in later steps of the algorithm).
Validating diagnostic criteria for pain phenotyping for persistent shoulder pain, which are clinically applicable and therefore easy to use by trained clinicians, represents a key area for further research. Quantitative sensory testing is recommended as a tool to investigate altered sensory processing. However, specifically for shoulder pain, the psychometric properties of quantitative sensory testing (i.e. pressure pain thresholds, conditioned pain modulation, and temporal summation of pain) are unknown.81 However, for clinical implementation, the value and psychometric properties of simple bed-side sensitivity testing need to be explored in people with shoulder pain. It is also well-known that many psychosocial (e.g. pain catastrophizing, attention to pain stimulus) and lifestyle factors (e.g., physical activity, sleep) influence the result of quantitative sensory testing, and should thus be taken into account when interpreting the results of the quantitative sensory testing.82–88 This suggests that different underlying mechanisms are responsible for altered pain modulation, and that these influential factors might need consideration in diagnostic criteria for pain phenotyping. In this context, it has already been shown in people with knee pain that the combination of different quantitative sensory measures with relevant clinical pain-related outcomes improves the predictive value of pain phenotyping on treatment response.89 Furthermore, it should be investigated whether care tailored to the pain phenotype(s) involved leads to greater effectiveness on pain and related disability in people with persistent shoulder pain as compared to unmatched treatments.
Potential benefits of identifying pain phenotypesPain assessment-based pharmacological, surgical, exercise, or psychology-informed behavioural therapy should take into account the causal determinants and underlying mechanisms of the pain phenotypes (e.g., low grade system inflammation due to disturbed sleep or obesity; inappropriate load – load capacity balance due to poor activity management, etc.). If care tailored to the pain mechanism involved leads to larger long-term effect sizes than the currently reported small to moderate effects of first-line exercise therapy in people with persistent shoulder pain,18 it seems valuable to apply diagnostic labels for pain phenotype rather along diagnostic labels for anatomical pain locations (e.g., shoulder pain, knee pain, neck pain).23 The use of such pain phenotype diagnoses rather than pain location diagnoses might optimize and accelerate the application of pain phenotype-based clinical reasoning in painful conditions localized within the musculoskeletal system and might be the required step towards precision pain medicine.90
ConclusionDefining the predominant pain phenotype(s) responsible for the pain experience in people with persistent shoulder pain, and tailoring treatments towards these pain phenotypes is an emerging issue. Therefore, the IASP developed a clinical criteria and grading system for nociplastic pain, to assist clinicians and researchers in determining the probability that nociplastic pain is (partly) responsible for the patient’s pain experience. In this paper, the use of this algorithm in people with shoulder pain is presented and discussed by means of different case studies. Further research should focus on investigating the validity of the IASP algorithm in people with persistent shoulder pain. Furthermore, clinical trials assessing the effectiveness of treatments tailored to the predominant pain phenotype versus untailored treatments are a next step towards the development of more precise care for people with shoulder pain.
CRediT authorship contribution statementLiesbet De Baets: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Kevin Kuppens: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Céline Labie: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Melina Nevoeiro Haik: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Eleni Kapreli: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Paraskevi Bilika: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Filip Struyf: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Dorien Borms: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. César Fernández-de-Las-Peñas: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Eva Kosek: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Enrique Lluch: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Marco Testa: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Jeremy Lewis: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Zosia Goossens: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Marc Schilz: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Inge Bonneux: Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. Jo Nijs: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The authors declare no competing interest.
None.