Although the brain injury caused by cerebral palsy (CP) is not progressive, there is often a worsening of physical conditions during the transition to adolescence and adulthood. The aim of this systematic review was to summarize factors related to decline in motor function in adolescents and adults with CP.
MethodsThis review was registered in PROSPERO (CRD42022311783). Studies met the following criteria: participants diagnosed with CP, ages 10 to 59 years; scientific studies with any methodological design and emphasis on motor function, reporting quantitative analysis of the effect of age on motor function or factors relevant that declined or improved motor capacity; published in English; any publication year, in electronic databases: Pubmed/Medline, Scopus, Cinahl. Data selection and extraction used the Covidence software. Factors related to motor outcomes that were statistically significant in at least one study and present in more than one study were reported. The outcomes were grouped within major categories (gross motor function, neuromusculoskeletal functions, and gait) for summary.
Results23 studies met the inclusion criteria. Relevant factors for changes in gross motor function (n = 16 studies) were: pain, neuromusculoskeletal aspects, altered gait parameters, Gross Motor Function Classification System (GMFCS) level, age, and history of surgery; for gait (n = 11): mobility performance, altered gait parameters, age, GMFCS level, pain and neuromusculoskeletal aspects; and for neuromusculoskeletal functions (n = 4): altered gait parameters, neuromusculoskeletal aspects, age, pain, and GMFCS level.
ConclusionAge, GMFCS level, and presence of pain stood out as significant for motor decline across categories. Monitoring these factors is relevant for planning interventions and transition programs.
The life expectancy of individuals with cerebral palsy (CP) has been catching up with that of the general population as a result of the increasing survival rate of low and very low birth weight infants and also due to advances in neonatal health care.1,2 Improvements in health care have also led to a higher occurrence of individuals with CP going through the transition from childhood to adolescence and adult life, thus creating new challenges for healthcare planning.3,4
Although the brain injury caused by CP is not progressive, it is known that worsening of physical conditions often occurs during the transition to adolescence and adult life,5 including decreased mobility and increased impairments in body structures and functions.5,6 A previous review showed that gait deterioration generally occurs in early adulthood, and that 25 % or more of young adults with CP might experience a decline or gait deterioration.7 The topography of motor impairment, the presence of comorbidities, secondary musculoskeletal issues, and increased frequency and intensity of pain have been related to gait decline.7 Pain is considered the most common comorbidity among individuals with CP, and is often associated with reduced quality of life and well-being, with an increase in intensity during the transition to adulthood.8
Even though the decline in gait and associated factors have been previously explored,7 no reviews were found investigating motor decline more broadly, i.e., not only focusing on gait. This is relevant considering that a significant portion of individuals with CP do not walk and may be at risk for decline in their other motor functions. There is also a lack of studies summarizing the factors that should be monitored or addressed during the development of individuals with CP to prevent or manage motor decline. This information is crucial to support health-care practices and to guide future research about health development of young people in transition to adult life.3,9
Thus, this systematic review aims to identify factors related to overall motor outcomes in adolescents and adults with CP.
MethodsStudy designReview procedures were based on recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines.10 The study question was based on the PECO strategy: Participants: Adolescents and adults with CP; Exposure: exposure to any factors shown to influence motor outcomes; Comparator: non-applicable; Outcome: any motor-related outcomes. The research question was defined as: Which factors are relevant for the motor outcomes of adolescents and adults with cerebral palsy?
This systematic literature review was registered in the International Prospective Registries of Systematic Reviews (PROSPERO): registration number CRD42022311783.
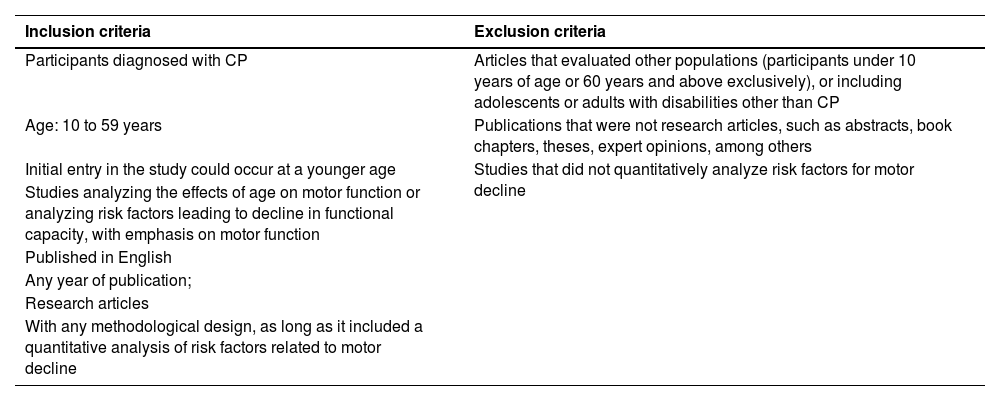
Eligibility criteriaInclusion and exclusion criteria for the studies are presented in Table 1.
Inclusion and exclusion criteria.
The search string comprised five concepts: Cerebral Palsy; Musculoskeletal development; Mobility limitation; Musculoskeletal system; and Transition to adult care. A systematic literature search was performed in three electronic databases: Pubmed/Medline, Scopus, Cinahl, including only studies in English, covering all years of publication until June 2023, with adjustments on the search string being made for each database. A manual search was also performed on the reference lists of the included articles to extract additional studies. The last search update was made in June 2023. Supplementary material 1 provides an example of a search strategy from one of the databases.
Data collection and analysisThe Covidence software was used to manage the data. Studies were screened based on titles and abstracts, followed by reading of full texts. Study selection and data extraction were performed independently by two reviewers, and a third reviewer solved conflicts.
A data collection form developed by the research team and previously tested in the first studies was used to establish the consistency of the data with the study objective. The following data were extracted: (1) Characteristics of the study; (2) Characteristics of the participants; (3) Measurement tools and motor-related outcomes. The factor was considered relevant and included in the review when it was identified as statistically significant in at least one study that had a low to moderate risk of bias based on the checklist of choice for this study (description presented next) and if the same outcome was identified as statistically significant in at least two studies with a moderate to high risk of bias.11
Even though the study initially focused on risk factors, protective factors for motor decline were also summarized if they met the criteria to be considered relevant factors. For easier presentation, the factors affecting motor outcomes were analyzed within the following major categories identified during data summary: 1) Gross motor function (e.g., measured by the Gross Motor Function Measure [GMFM-66] or Gross Motor Function Classification System [GMFCS]); 2) Gait; and 3) Neuromusculoskeletal functions (here defined as those related to the functions of joints, bones, reflexes, and muscles,12 e.g., decrease in muscle strength or joint range of motion [(ROM]).
Risk of bias assessmentThe checklist to assess study bias was scored based on the clear description of several relevant items, assigning 1 point for studies reporting the item and 0 for those that did not clearly report this item. The maximum score for each study is 16 points. Studies with scores between 12 and 16 were considered to have few methodological limitations, being classified as having good quality; scores between 7 and 11 points indicate moderate methodological limitations, with the study being classified as fair; a score below 7 points represents significant methodological limitations, and thus the study quality was classified as poor.13,14
The heterogeneity of the outcomes did not allow meta analyses, therefore the results were analyzed descriptively. The results were summarized based on the factors related to each category of motor-related outcomes.
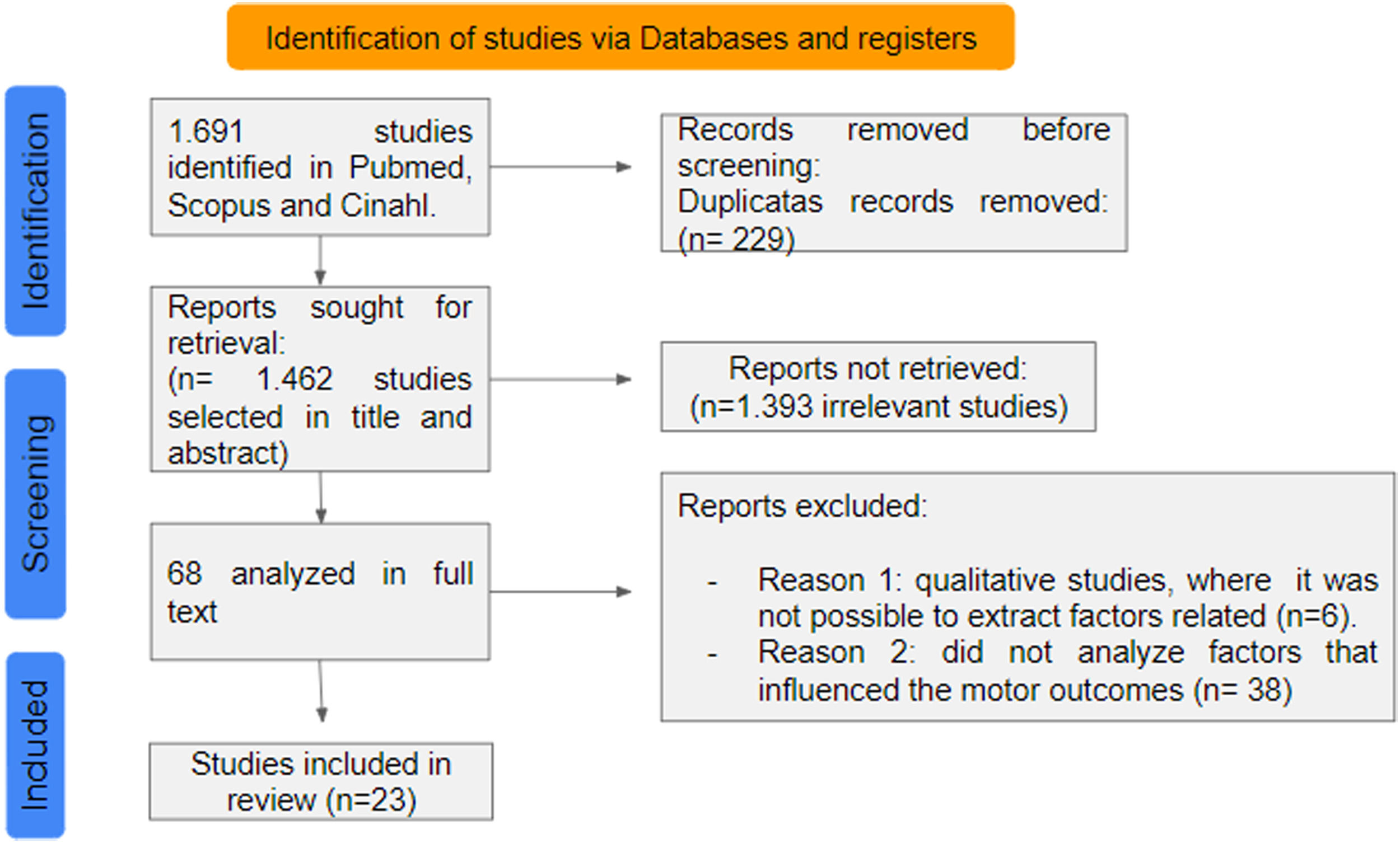
ResultsThe search resulted in 1691 articles, of which 23 met all inclusion criteria and were included in the review and proceeding with data extraction (Fig. 1).
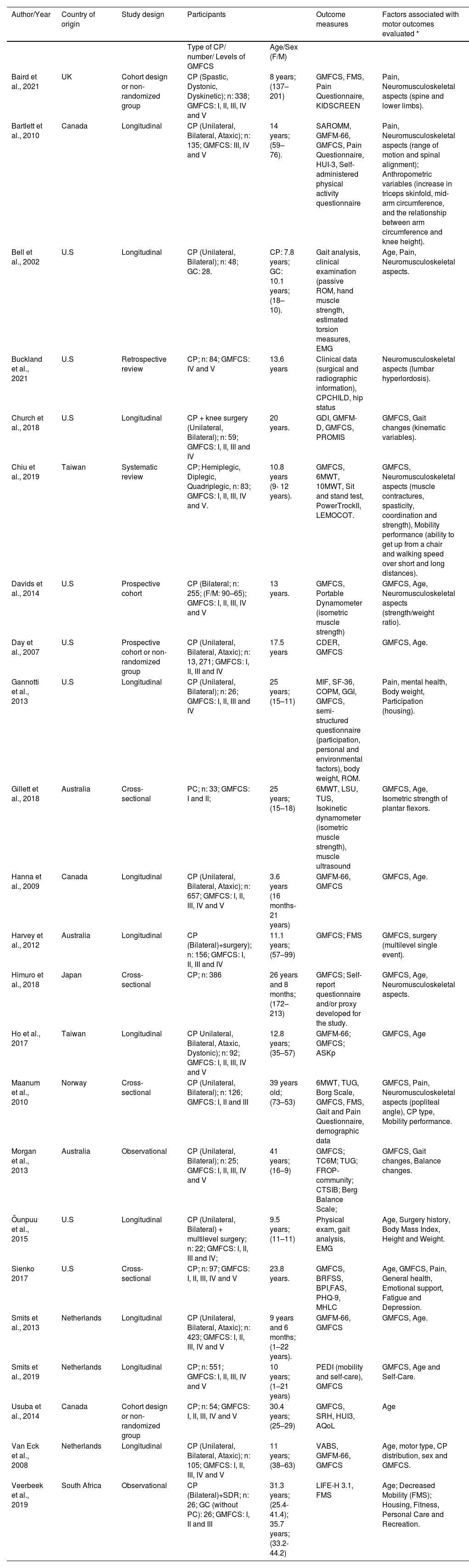
As shown in Table 2, the most frequent study design was longitudinal (n = 11); followed by cross-sectional (n = 4); cohort (n = 4); observational (n = 1); and retrospective (n = 1). The countries of the selected studies were: United States (n = 8); Netherlands (n = 3); Canada (n = 3), Australia (n = 2), and Taiwan (n = 2). Norway, United Kingdom, and Japan presented one study each. The sample size ranged from 25 to 13,271 participants. The age of the participants ranged from 3 to 41 years, GMFCS levels were I-II (n = 20 studies), III (n = 19); IV (n = 18), and V (n = 17). Six studies did not report the GMFCS level. Participants' motor impairment was unilateral in 17 studies and bilateral in 30 studies. In total, 33 factors were identified in the studies, seven of which were considered relevant.
Characteristics of the included studies.
Caption: *= all factors analyzed in the study; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; GMFM, Gross Motor Function Measure; LIFE-H, Assessment of Life Habits; FMS, Functional Mobility Scale; PEDI, Pediatric Assessment of Disability Inventory; TC6, 6-minute walk test; TUG, Timed Up and Go; BBS Berg Balance Scale; LSU, lateral step-up; TSU, Timed up-stairs; BPI, Brief Pain Inventory; GGI, Gillette Gait Index; SF-36, Quality of life questionnaire; VABS, Vineland Adaptive Behavior Scales; HUI-3, Health Utilities Index; EMG, dynamic electromyography; CPCHILD, Caregiver Priorities and Child Health Index of Life with Disabilities Questionnaire; PROMIS, Global Health of PROMIS; CDER, Client Development Evaluation Report; COPM, Canadian Occupational Performance Measure; ASKp, Children's Activity Scale; FROP-community, Falls risk for older People in the community; CTSIB, Clinical Test of Sensory Interaction and Balance; BRFSS, Behavioral Risk Factor Surveillance System; BPI, Scale of the Brief Pain Inventory; FAS, Fatigue Rating Scale; PHQ-9, Patient Health Questionnaire-9; MHLC, Health Locus of Control Scale; ROM, Range of motion; SAROMM, Spinal Alignment and Range of Motion Measure; F, female; M, male; NA, not applicable.
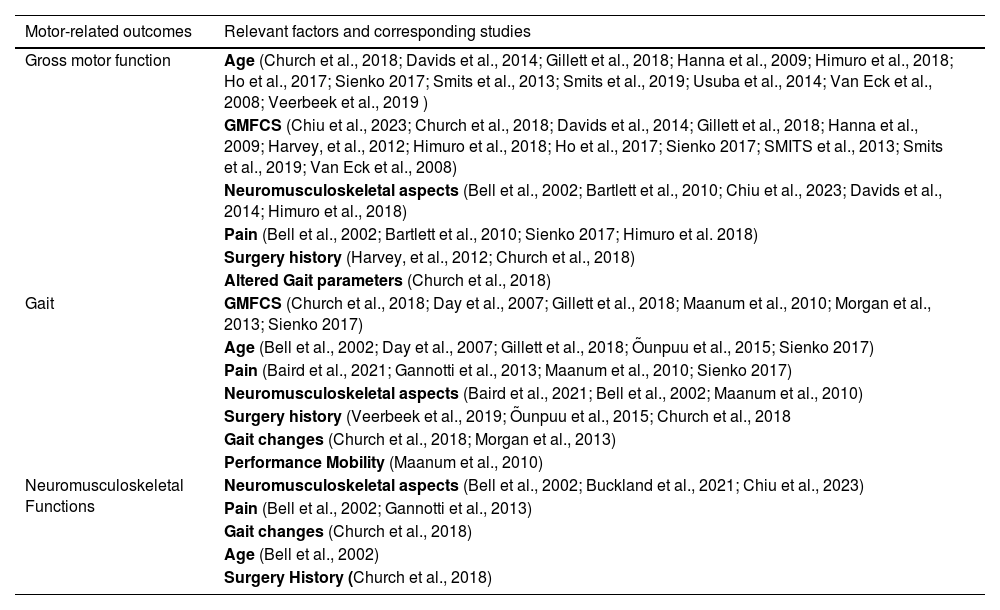
Table 3 presents the relevant factors influencing motor outcomes identified in this review.
Factors related to motor outcomes according to each category of interest.
Caption: GMFCS = Gross Motor Function Classification System.
Regarding gross motor function, factors relevant were identified in 16 studies and included: age, GMFCS level, neuromusculoskeletal aspects, pain, history of surgeries, and altered gait parameters. History of orthopedic surgeries was identified as a protective factor for gross motor function in three studies. For gait deterioration, 11 studies identified more severe GMFCS level, advancing age, pain, neuromusculoskeletal aspects (muscle strength, ROM, worsening of hip condition among others), altered gait parameters, and mobility performance as relevant factors. Regarding neuromusculoskeletal functions, four studies indicated factors relevant to decline such as pain, abnormal gait parameters, and increasing age (Table 3).
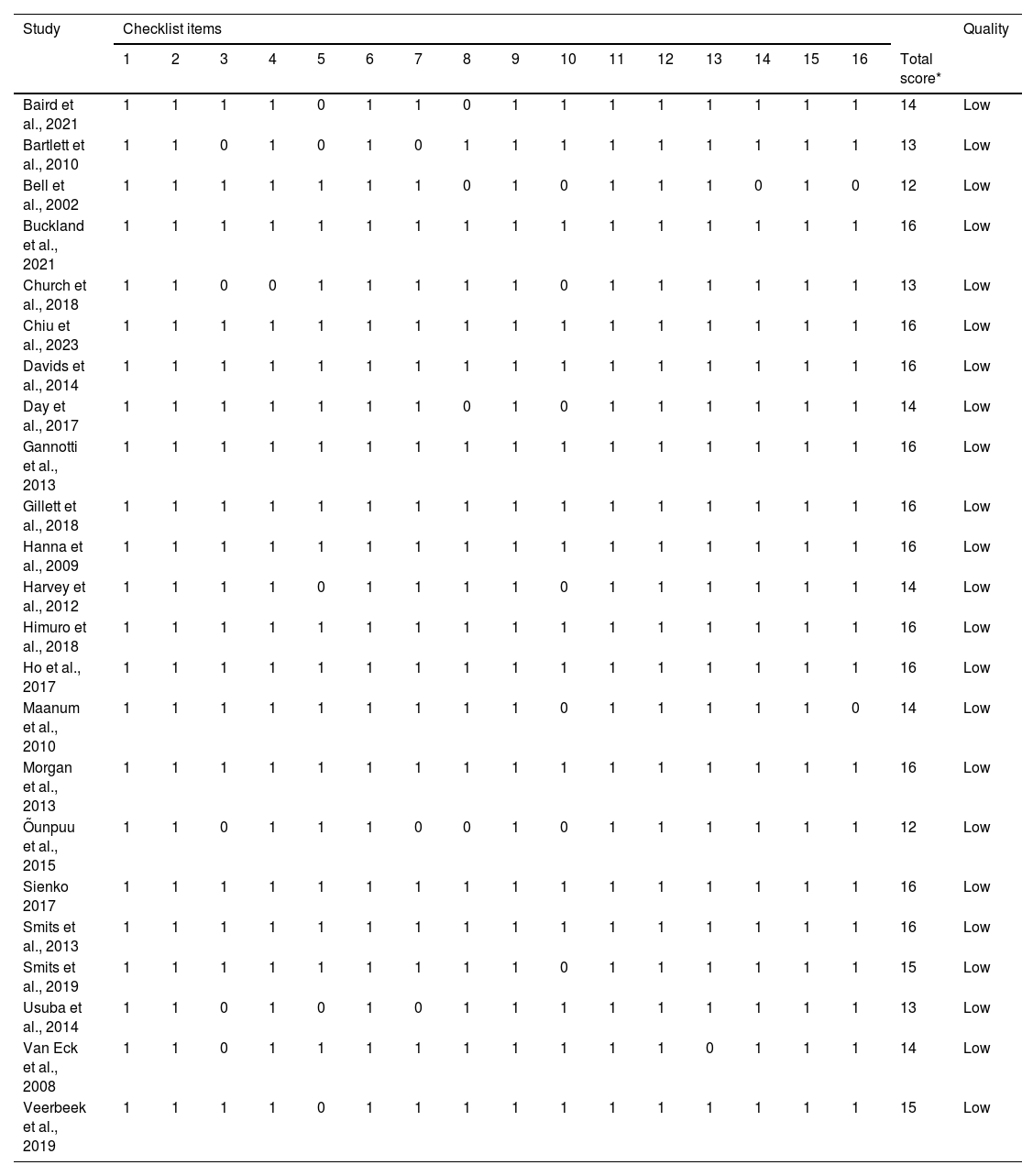
When assessing risk of bias in the included studies, all studies were classified as low risk (Table 4).
Risk of bias of the 24 studies included in the review.
Additional (non-motor) outcomes demonstrating functional decline over time included self-care skills (n = 1), health-related quality of life (n = 1), and performance and satisfaction with life habits (n = 1), however they were infrequently reported. Other factors related to motor outcomes but not classified as significant as they did not reach the criteria established in this review included depression, emotional support,15 mental health,16 housing,17 balance,18 fatigue,15 and CP type.19
DiscussionThis review aimed to identify factors relevant for motor decline in adolescents and adults with CP. Factors such as pain, mobility performance, changes in gait parameters, GMFCS level, age, history of surgery, and neuromusculoskeletal aspects were the most recurrent and relevant factors reported in the literature.
Most studies in the review were conducted in high-income countries, which has been highlighted in other reviews on individuals with CP.20,21 This result may also reflect larger awareness and resources directed towards research on the period of transition to adult life in these countries, and highlights the need for information on factors relevant for motor decline in low- and middle-income countries.
The most frequent methodological design was longitudinal, suitable for monitoring changes in functioning over time and obtaining knowledge about the development of these individuals.22,23 Of the 11 longitudinal studies, the majority included adolescents, followed by young adults. However, the total amount of studies underscores the need for more research to understand the determinants of motor changes in individuals during the transition to adulthood, as well as the impact of motor decline on broader aspects of functioning, including participation and quality of life.22
Regarding the characterization of participants, bilateral CP was the most frequently reported subtype among the included studies, which may reflect epidemiological trends.24 Most studies included individuals with CP at GMFCS levels I to III, which may be related to the fact that they are independent walkers or can walk with assistive devices, and given that gait is a common concern. Similar findings were also observed by Furtado et al.,25 reporting a higher frequency of studies on children with CP with GMFCS levels I to III. Although individuals with GMFCS levels IV and V are the most vulnerable to motor decline over the years,25 literature is still scarce on motor changes after childhood. In the following sections, we will discuss the evidence on the main factors identified.
Decline in gross motor functionSixteen studies identified factors relevant that resulted in gross motor function decline. The decline in gross motor function in individuals with CP has been consistently described through GMFM, both in overall and in standing scores over development.26,27 This may be a relevant tool to follow-up youth with CP across transition, however validity for adults needs to be demonstrated. A prominent relevant factor that may contribute to the decrease in gross motor scores is the presence of pain.15,28,29 Pain is therefore a factor that should not be neglected by healthcare professionals working with individuals with CP as it may precede relevant functional losses.
As expected, results highlighted age as a frequent factor relevant for the decline in gross motor function in adolescents and adults with CP, along with GMFCS levels.29–36 The combination of age and GMFCS predicted Pediatric Evaluation of Disability Inventory (PEDI) mobility scores, with higher development and longer period in development for individuals with mild to moderate GMFCS levels (I-III), compared to those at more severe GMFCS levels.35 Similarly, these factors predicted earlier peak GMFM scores and significant declines in adolescence and adulthood in those between levels III to V.31,36 Several other studies supported the influence of the GMFCS level as a factor relevant for the decline in gross motor function in individuals with CP in the first decade of life and in adults, with results similar to those previously discussed.15,30,31,33,34,36–39 Collectively, these findings underscore the vulnerability of non-ambulant individuals to early motor decline.
Musculoskeletal conditions were also pointed out as relevant factors for gross motor change. These included muscle strength,30 ROM in the upper limbs and in hip, knee, and ankle joints, spinal alignment,28 and presence of stiffness and deformities.29 Such findings point to the relevance of monitoring and intervening early in life on these factors because interventions addressing these issues may be strategies to prevent motor decline. Among the interventions targeting musculoskeletal aspects, single-event multilevel surgery (SEMLS) has been suggested to favor stability of GMFCS levels.38
In summary, the findings are quite consistent in demonstrating that during transition to adulthood there is a decline in gross motor function and mobility, with age, GMFCS level, and body structure and function aspects such as pain and neuromusculoskeletal aspects being the primary relevant factors. Thus, monitoring these factors throughout the development of children, adolescents, and young adults with CP is important so timely interventions to prevent decline and/or maintain the functional level are offered.
Decline in gaitGait deterioration was reported as an indicator of motor decline in 11 studies. GMFCS level was pointed out as a relevant factor in several studies,15,16,19,26,31,39,40 often interacting with age to determine changes in gait.15,31,39,41,42 Other aspects of gross motor function such as the performance of the Timed Up and Go (TUG) test, the ability to walk and climb stairs, and the need for assistive devices were also relevant predictors of gait stability and decline.19 It is therefore relevant to consider the implementation of validated tests in transition programs to help predict the risk of gait deterioration.
The presence of pain was another relevant factor, predicting gait endurance,19 and gait in general,15 with reports of loss of the ability to walk in individuals who had pain.16 However, the impact of the association of pain and gait decline on functioning was not explored in the included studies, and should be addressed in the future. Another limitation was that the assessment of pain did not include detailed information such as type and location of the pain, which warrants further investigation.
The presence of musculoskeletal deformities was also reported as an important contributor to gait loss,40 and to the performance in gait tests.19,41 However, there is conflicting evidence on the impact of interventions targeting such deformities on gait. Bell et al.41 showed that gait function declined over 4 years in both functional and less functional walkers without surgery, illustrated by decline walking velocity and other kinematic parameters. In contrast, a group that underwent orthopedic surgery had a more positive prognosis, suggesting that this intervention may minimize the decline over time. Possible positive effects of surgical interventions were also reported by Gannotti et al.16 and Veerbeek et al.17 These findings are however in contrast with the decline after multilevel surgery over an 11-year period reported in another study.42 Also, Church et al.26 have suggested a mild decline (increased popliteal angle and decreased gait speed) in a 12-year follow-up after multilevel surgery. Given these findings, particularities such as type of surgery, participants' age, and other characteristics should be explored in future studies to elucidate the impact of surgery on motor prognosis.
The findings of this review highlight that gait decline is prevalent and relevant in the motor decline context. When untreated, it can result in loss of mobility and quality of life.43 Thus, the identification of relevant factors for changes in gait can prevent a cascade of other potentially aggravating events for health in general. Monitoring these factors should be a priority in transition planning.
Decline in neuromusculoskeletal functionsThe decline in neuromusculoskeletal functions (muscle strength, ROM, worsening of hip condition, among others) was related to factors such as GMFCS level, altered gait parameters, and age26,37,38,41,44 Interestingly, worsened conditions in the hips, knees, and ankles may already appear during childhood, as noted by Bell et al.41 in children followed over 4 years starting at ages as young as 5 years. Less functional walkers presented particular characteristics, such as more changes in the foot and ankle, which may relate to the increased risk of mobility decline in this subgroup and points to the importance of prevention by means of appropriate tone management and physical activity early on.41 Over a 12-year follow-up, however, changes in knee angles did not result in significant decline in the gait of young adults,16 which suggests that outcomes related to ROM must be accompanied by measures that inform about the capacity and performance of individuals, so that the proposed interventions can be functionally relevant.
One study focused more directly on hip status of non-ambulant children with CP,44 pointing that a postoperative hyperlordosis greater than 60° was related to the increased risk of worsening the hip condition at 5 years after surgical correction of scoliosis. The implementation of adequate postural management through positioning and assistive devices is therefore a critical strategy to prevent such complications. Although not reported in the included studies, other studies indicate that in addition to spinal alignment, other factors such as age, GMFCS level, and type of gait as determinants for hip dislocation.26,41 Considering the incidence of hip dislocation of up to 35 % (increasing with GMFCS) in CP, which can result in pain, reduced function, and reduced quality of life,44 the factors relevant for this condition should be closely monitored and addressed as early as possible. The implementation of hip surveillance, which is the strategy with the highest level of evidence to minimize complications related to hip dislocation, is necessary from the first year of life of children with CP and in some cases needs to continue until skeletal maturity.45,46
Given these findings, it is clear that musculoskeletal comorbidities and multimorbidities arise early and deteriorate with advancing age in adults with CP and may be more critical in the non-ambulant individuals. Thus, access to preventive services and therapeutic interventions at the right time can have a positive impact on the functioning and well-being of individuals with CP, especially those at higher risk for musculoskeletal complications, thus providing more favorable health conditions as they transition to adulthood.
Quality appraisal and future research needsIn general, the reviewed studies showed a low risk of bias, which gives more credibility and support to the conclusions made in this review. However, heterogeneity in the use of assessment instruments was found, for example, the GMFCS in some studies was used as a criterion to indicate gait ability, while in others as an indicator of general gross motor function. There was also a lack of validated assessments for older age groups of people with CP. Although the GMFM is the gold standard in CP assessment, it is not validated for adults yet. This aspect also deserves attention in future studies.
Limitations of the study include the fact that the factors were selected based on their statistical significance and the lack of measures of the strength of the recommendations. We suggest that future reviews also take into account the clinical significance of the findings and use tools to provide accurate recommendations. Interestingly, indicators of functional decline that were not directly related to motor outcomes were assessed in some studies, including decline in self-care skills, health-related quality of life, and performance and satisfaction with lifestyle habits. These indicators should not be disregarded as they illustrate areas of functioning that are essential for wellbeing. Other factors identified as relevant for motor outcomes but not classified as significant as they did not reach the criteria established in this review included depression, emotional support,15 mental health,16 housing,41 balance,18 fatigue,15 and CP type.19 Their contribution should be investigated in future, high quality studies. Still, although the distribution of motor impairment and the presence of comorbidities have been previously pointed out as relevant factors in the literature,18 they were not identified in the results of this review. Such variability of outcomes and results is probably due to the multifactorial nature of motor decline, and indicates that individualized biopsychosocial care must be assured when planning transition services to adult life.
Regarding implications for physical therapy, the results of this review support the continuity of follow-up of individuals with CP during the transition to adulthood, especially the ones at higher risk for motor decline, while discharge due to lack of motor progress is not recommended as it may contribute to further deterioration in health status. Future studies should summarize evidence on recommended physical therapy interventions for this population. Studies that longitudinally follow adolescents and young adults with CP are also scarce, especially in low and middle-income countries, and the determinants for the motor outcomes in these countries need to be investigated in future studies.
ConclusionThis review summarized the factors that may influence the motor outcomes of adolescents and adults with CP, highlighting the role of age, GMFCS level, and presence of pain, which stood out as significant for decline in gross motor function, gait, and neuromusculoskeletal functions. Knowledge of these factors is relevant for professionals and caregivers of people with CP, as monitoring them during the transition to adolescence and adulthood may help reduce the impact of limitations caused by motor decline.
The authors declare no competing interest.