Spinal manipulative therapy (SMT) demonstrates small effects on pain intensity in low back pain. Combining SMT with a psychosocial intervention like pain neuroscience education (PNE) could promote additional effect.
ObjectivesTo evaluate the additional effect of PNE when combined to SMT on pain intensity and low back pain-related disability in patients with chronic low back pain (CLBP).
MethodOne hundred and four patients with CLBP of both sexes aged between 18 and 55 years were treated with PNE + SMT compared to SMT alone. The primary outcome measures were pain intensity and disability post-treatment (4 weeks). Secondary outcomes were fear-avoidance beliefs, global perceived effect of improvement, and pain self-efficacy. Results were obtained immediately post-treatment and at three follow-ups (30-days, 90-days, and 180-days).
ResultsNo significant between-group difference was observed for pain intensity and disability post-treatment. In contrast, our results showed a significantly longer additional effect for the group treated with SMT + PNE for the following outcomes: pain intensity (change baseline to 90 day follow-up = −0.90 [95% CI= −1.76, −0.4] and change baseline to 180 day follow-up = −1.19 [95% CI= −2.06, −0.32]) and low back pain-related disability, global perceived effect of improvement and pain self-efficacy (180th day follow-up).
ConclusionThe results of this trial suggest the addition of PNE to SMT did not bring any additional effect on pain intensity and disability in the short term, but SMT + PNE can result in longer-lasting effects in patients with CLBP and that such an effect could be related to a possible mediator effect of pain self-efficacy.
In the Global Burden of Diseases study,1 low back pain was the fourth cause of disability-adjusted life-years (DALY) in the 25- to 49-year age group. Most low back pain is termed non-specific, in which no specific pathoanatomic cause can be identified by imaging findings.2,3 Clinical practice guidelines recommend using the biopsychosocial model for assessing and managing low back pain,4,5 in addition to recommending non-pharmacological therapies such as spinal manipulation as an adjunct to other treatments for managing chronic low back pain (CLBP).4,5 A meta-analysis showed that spinal manipulative therapy (SMT) has similar effects to other recommended therapies for short-term pain relief, with limited clinical improvement in function in the short term for CLBP.6
There is a need to understand better the effects of multimodal treatments that address biological and psychosocial factors in treating CLBP.7 A systematic review endorsed the superior effects of biopsychosocial interventions associated with movement-based therapies compared to unimodal treatment modalities, such as movement therapies alone.8 A clinical guideline reported that randomized controlled trials with low risk of bias support administering multimodal programs for treating CLBP.9
Multimodal treatment is typically defined as using more than one type of therapy and can include more than one discipline when available (multidisciplinary).10 A previous guideline suggested three different modalities of treatment for chronic musculoskeletal (MSK) pain: education, exercise, and manual therapy.4 An education program is a psychosocial intervention based on the premise that the better an individual understands their condition, the better they will be able to manage it.11 Pain Neuroscience Education (PNE) can be defined as an educational intervention that explains the neurobiological processes to patients in easy-to-understand metaphors, examples, and pictures, as a means to alter a patient's beliefs regarding pain and to reduce its perceived threat.12 A systematic review with meta-analysis reported that a combination of PNE and physical therapy interventions resulted in greater effects on pain intensity and disability in chronic MSK pain when compared to PNE alone.11 Thus, psychosocial factors covered by the PNE can improve pain and disability and make this approach a good candidate for inclusion in multimodal treatment approaches.11
Only one previous study to be best of our knowledge has compared the additional effect of PNE when combined with SMT.13 No previous study compared the effects of PNE + SMT compared to SMT alone at six-month follow-up on pain intensity and disability in CLBP. Therefore, the objective of our study was to determine the effect of providing PNE in combination with SMT when compared to SMT alone for the primary outcomes of pain intensity and low back-related disability immediately post-treatment. The hypothesis of this study is that patients randomly assigned to the multimodal intervention (i.e., both PNE and SMT) will have better outcomes than patients receiving SMT alone.
MethodsStudy designThis study was a randomized controlled trial reported following the recommendations of the consolidated standards of reporting trials – CONSORT.14
The project was approved by the Ethics Committee of the Centro Saúde Escola Cuiabá – Ribeirão Preto Medical School, University of São Paulo (CAAE:69387916.7.0000.5414). This study was also registered prospectively at ClinicalTrials.gov (NCT03356886) and conducted in the Integrated Rehabilitation Center – NIR, a primary health care unit in São José do Rio Preto, São Paulo, Brazil. All patients who consented to participate signed an informed consent form following the Helsinki protocol.
ParticipantsParticipants of both sexes were recruited according to the following inclusion criteria: i) age between 18 and 55 years (with the aim to avoid sampling bias with different age groups15,16); ii) experience CLBP lasting at least three months; and iii) match at least three of the following criteria of the clinical prediction rule for SMT17,18: 1) hip internal rotation >35°; 2) hypomobility of one or more lumbar segments; 3) absence of symptoms distal to the knee; and 4) FABQ work subscale score <19. The clinical prediction rule for SMT was adopted with the aim to increase the likelihood of patients being responsive to SMT as suggested previously.18 In the current study, we followed the Flynn's et al.18 modified clinical prediction18 rule proposed by Dougherty et al.17 in which the criterion of symptom duration <16 days was excluded.
The exclusion criteria were: (1) pregnancy; (2) presence of red flags (e.g.: fracture or infection); (3) disc herniation with radiculopathy; (4) patients with cognitive deficits evaluated according to the Mini-mental state examination (MMSE) with a score ≤22 points19; and (5) previous physical therapy for low back in the past year or previosuly treated with any health/pain education strategy. Patients were instructed not to use pain relief medications during the intervention period of this trial, and if any medication was used, participants were encouraged to report it.
InterventionsSpinal manipulative therapy (SMT)The SMT techniques administered in the current study have been widely used in the literature.20,21 In this study, we consider SMT to represent any hands-on spine treatment, including both manipulation and mobilization techniques applied to the spine.6 Mobilizations use low-grade velocity, small or large amplitude passive movement techniques within the patient's range of motion and control. In contrast manipulation uses a high-velocity impulse (thrust) over a short amplitude at or near the end of the passive or physiological range of motion.6 A theoretical framework model postulates that the mechanical stimulus from a manual therapy intervention results in neurophysiological responses within the peripheral and central nervous systems resulting in pain relief.21
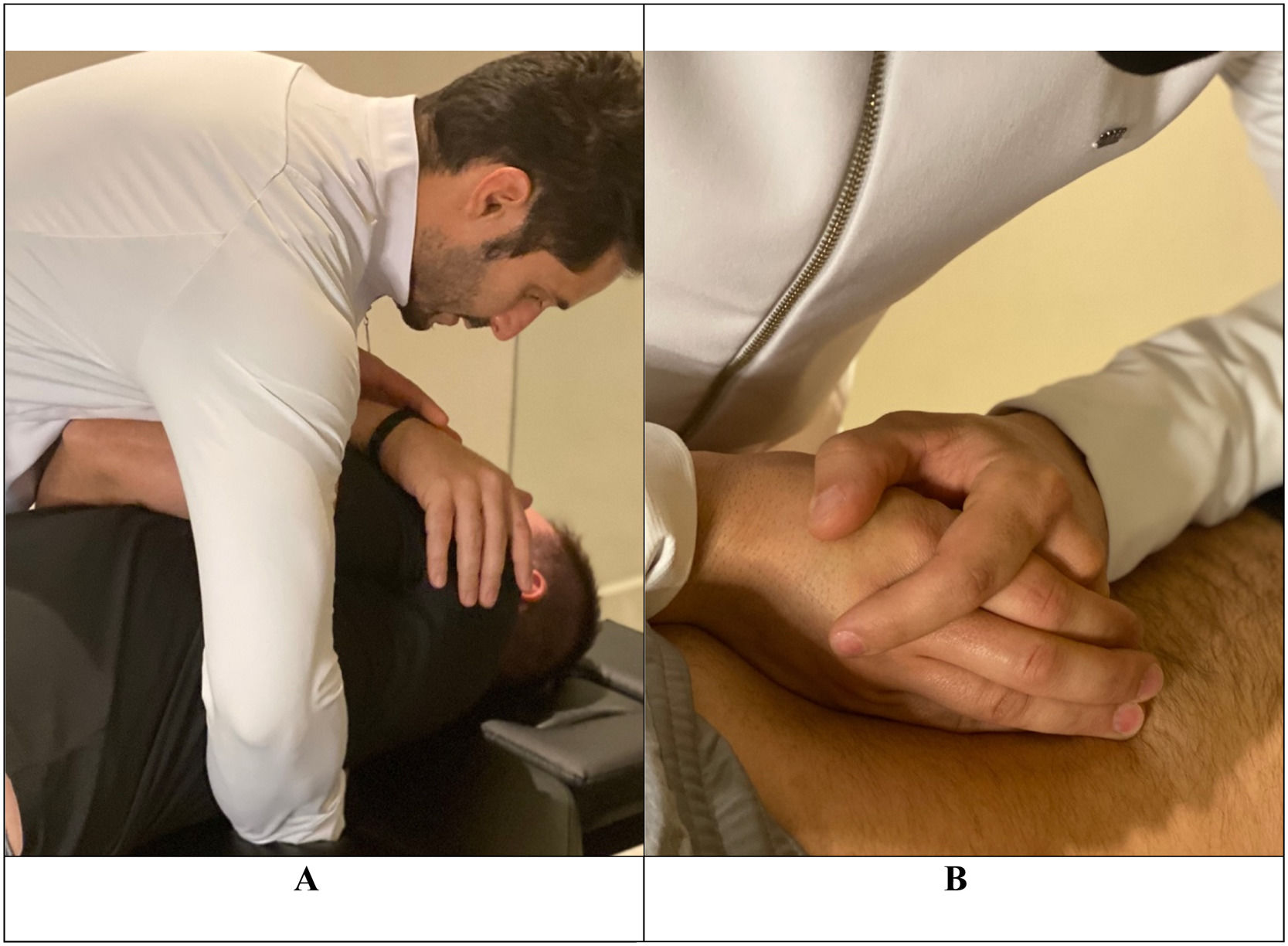
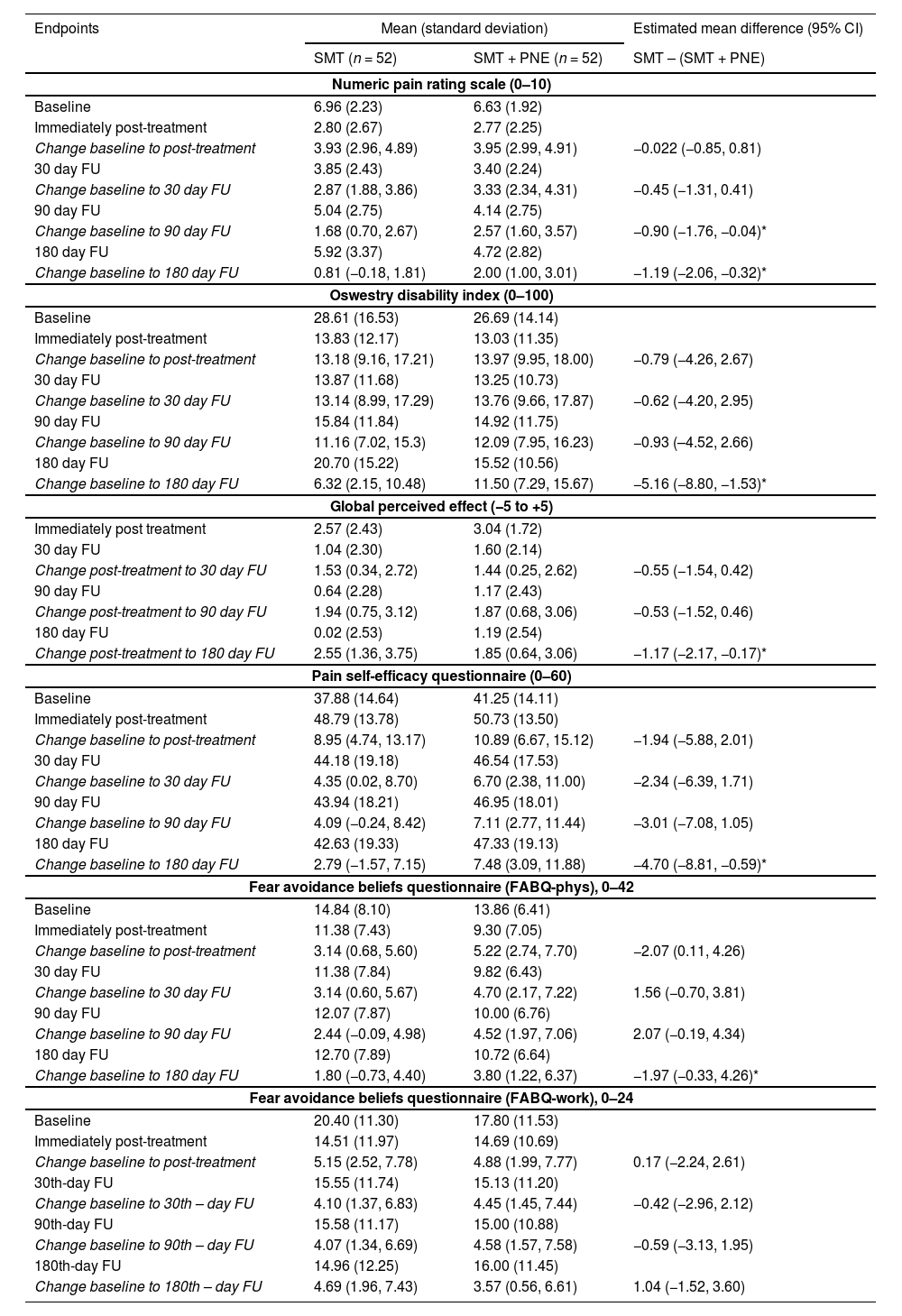
The protocol included: i) the administration of a global low-amplitude and high-speed manipulative maneuver in the upper thoracic region between T1 and T5 levels in the dorsal decubitus position and ii) techniques of posterior-anterior central mobilization applied for 30 s with an average of 30 repetitions for each lumbar vertebra (from L5 to L1) while patients were positioned in the ventral decubitus position (Fig. 1).22 The thrust manipulation was administered at the thoracic spine, considering that a previous study23 found no differences in pain intensity after lumbar spine high-velocity manipulation versus non-region-specific manipulation in patients with CLBP. This protocol of combined manipulation and mobilization techniques was adopted in view of the previous findings of a systematic review,24 in which the combination of thrust mobilization and non-thrust techniques showed greater evidence of effectiveness for CLBP when compared to each technique alone.
A trained and experienced researcher (physical therapist trained in SMT with 13 years of clinical experience) administered the SMT interventions. The treatment consisted of eight sessions, twice a week, for four weeks. The physical therapist who administered the SMT protocol was blinded to the participants who received PNE and those who did not. The fidelity of the intervention was not controlled.
Pain neuroscience education (PNE) + SMTAll PNE + SMT group participants received the PNE followed by SMT just in the first two sessions. In the remaining sessions (six), PNE + SMT group participants received just SMT. We adopted the Explain Pain approach in which educational interventions are focused on changing someone's understanding of what pain actually is, what function it serves, and what biological processes are thought to underpin it.25
Participants watched power-point presentations presented by a researcher in a classroom format in which different concepts of pain neuroscience were discussed. The sessions were individual and face-to-face in the primary care facility. A multimedia device and a computer was used to run the presentations. These sessions were run in workshop style also involving facilitated discussion between the researcher and the patient. Live multimedia presentations included animated videos, and learning objectives were addressed with both metaphors and scientifically accurate descriptions. Learning checks that required participants to provide feedback on the material and offer behavioral responses to the material were included. Only one researcher, who was not involved in the administration of the SMT, administered the PNE program and was not blinded to group allocation. The researcher received 40 h of training to administer PNE – Explain Pain.
The PNE program was administered in the two first sessions, each session lasting 40 min. The content covered 19 thematic topics according to Explain Pain26 as previously published27 (Supplemental Material). The reader can find some illustrative slides from the PNE program administered in Supplemental Material. The fidelity of the intervention was not controlled.
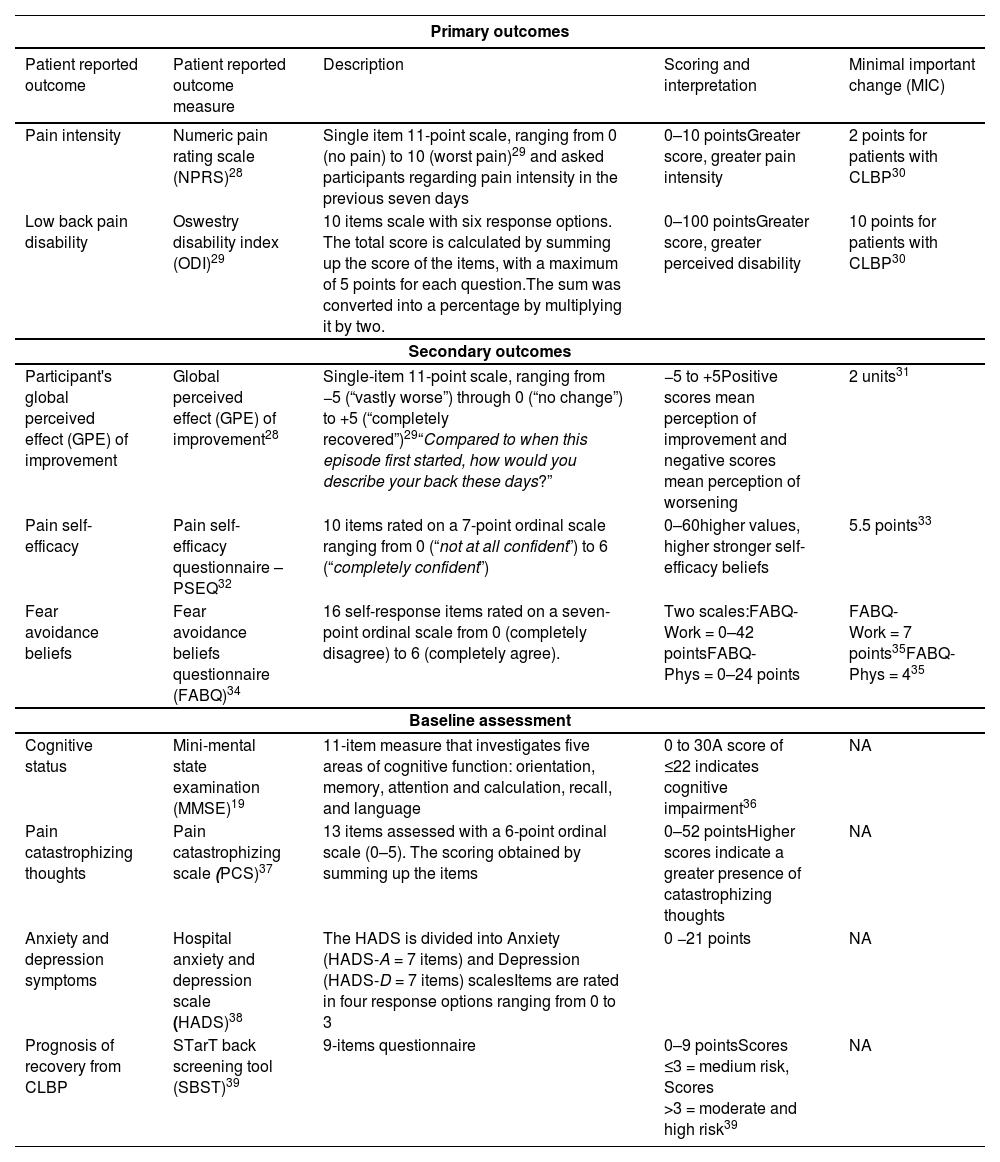
Outcome measuresPatients were assessed at baseline, immediately post-treatment and at 30, 90, and 180 day follow-ups (Fig. 2) always by the same researcher who could not be considered blinded because this study adopted just patient self-reported outcomes. However, to minimize a possible bias associated with patient's expectations regarding the treatments received, we did not inform participants about the clinical trial hypothesis, and the assessor was not aware of group allocation. The variables assessed in the baseline are described on Table 1.
Description of the primary and secondary patient reported outcomes as well as baseline variables considered in the study.
| Primary outcomes | ||||
|---|---|---|---|---|
| Patient reported outcome | Patient reported outcome measure | Description | Scoring and interpretation | Minimal important change (MIC) |
| Pain intensity | Numeric pain rating scale (NPRS)28 | Single item 11-point scale, ranging from 0 (no pain) to 10 (worst pain)29 and asked participants regarding pain intensity in the previous seven days | 0–10 pointsGreater score, greater pain intensity | 2 points for patients with CLBP30 |
| Low back pain disability | Oswestry disability index (ODI)29 | 10 items scale with six response options. The total score is calculated by summing up the score of the items, with a maximum of 5 points for each question.The sum was converted into a percentage by multiplying it by two. | 0–100 pointsGreater score, greater perceived disability | 10 points for patients with CLBP30 |
| Secondary outcomes | ||||
| Participant's global perceived effect (GPE) of improvement | Global perceived effect (GPE) of improvement28 | Single-item 11-point scale, ranging from −5 (“vastly worse”) through 0 (“no change”) to +5 (“completely recovered”)29“Compared to when this episode first started, how would you describe your back these days?” | −5 to +5Positive scores mean perception of improvement and negative scores mean perception of worsening | 2 units31 |
| Pain self-efficacy | Pain self-efficacy questionnaire – PSEQ32 | 10 items rated on a 7-point ordinal scale ranging from 0 (“not at all confident”) to 6 (“completely confident”) | 0–60higher values, higher stronger self-efficacy beliefs | 5.5 points33 |
| Fear avoidance beliefs | Fear avoidance beliefs questionnaire (FABQ)34 | 16 self-response items rated on a seven-point ordinal scale from 0 (completely disagree) to 6 (completely agree). | Two scales:FABQ-Work = 0–42 pointsFABQ-Phys = 0–24 points | FABQ-Work = 7 points35FABQ-Phys = 435 |
| Baseline assessment | ||||
| Cognitive status | Mini-mental state examination (MMSE)19 | 11-item measure that investigates five areas of cognitive function: orientation, memory, attention and calculation, recall, and language | 0 to 30A score of ≤22 indicates cognitive impairment36 | NA |
| Pain catastrophizing thoughts | Pain catastrophizing scale (PCS)37 | 13 items assessed with a 6-point ordinal scale (0–5). The scoring obtained by summing up the items | 0–52 pointsHigher scores indicate a greater presence of catastrophizing thoughts | NA |
| Anxiety and depression symptoms | Hospital anxiety and depression scale (HADS)38 | The HADS is divided into Anxiety (HADS-A = 7 items) and Depression (HADS-D = 7 items) scalesItems are rated in four response options ranging from 0 to 3 | 0 −21 points | NA |
| Prognosis of recovery from CLBP | STarT back screening tool (SBST)39 | 9-items questionnaire | 0–9 pointsScores ≤3 = medium risk, Scores >3 = moderate and high risk39 | NA |
NA, not applicable.
The primary outcomes considered in this study were pain intensity and low back pain-related disability immediately post-treatment. Secondary outcomes were: pain intensity and disability assessed at the 30, 90, and 180 day follow-ups and fear-avoidance beliefs, pain self-efficacy, and global perceived effect of improvement assessed post-treatment and at the 30, 90, and 180 day follow-ups.
Primary outcomesThe primary and secondary outcomes and baseline variables are described on Table 1.
Randomization, allocation, and blindingOnce the patient accepted the invitation to participate and provided informed consent, a researcher collected their clinical assessment data and determined their eligibility, including if the participants were eligible according to the manual therapy clinical prediction rule. After this initial assessment, participants were randomly assigned to one of two treatment groups following a simple randomization procedure.
The allocation schedule was generated by a research assistant not involved in any other aspect of the study. The same researcher generated the allocation sequence and placed allocation codes into sequentially numbered, sealed, opaque envelopes. Randomizer software was used to randomize patients into the two groups of the study: PNE + SMT group or SMT group.
Statistical analysisFor pain intensity, we aimed to detect a between-group difference of 2 points on an 11-point (0–10) numeric pain rating scale (NPRS), based on the minimal important change (MIC) described in the literature for CLBP.30 However, for low back pain-related disability (using the Oswestry disability index [ODI]), 90 participants were needed to detect a difference of 10 units30 with an effect size of 0.62. Considering a sample loss of 15% (n = 90), the required sample size was 104. This provided a statistical power of 90% and α = 0.05. The software used to calculate the sample size calculation was G*Power 3.0.10, (University of Kiel, Germany).
Statistical analysis was performed using SPSS statistics version 22.0 (IBM) and following an intention-to-treat approach. Linear mixed-models with repeated-measures analysis, random effect models, and restricted maximum likelihood were used to assess between-group differences in response to treatment. The model included treatment, time, and treatment × time interaction as fixed effects, and time as repeated measures (post-treatment, at the 30, 90, and 180 day follow-ups). Pairwise comparisons with Bonferroni adjustment were used when group x time interaction effect was significant and change scores (compared with baseline) for all the end points of the study. The statistical analysis was conducted by a blinded researcher to group allocation who was not involved in any of the phases of data collection and received data in a coded form.
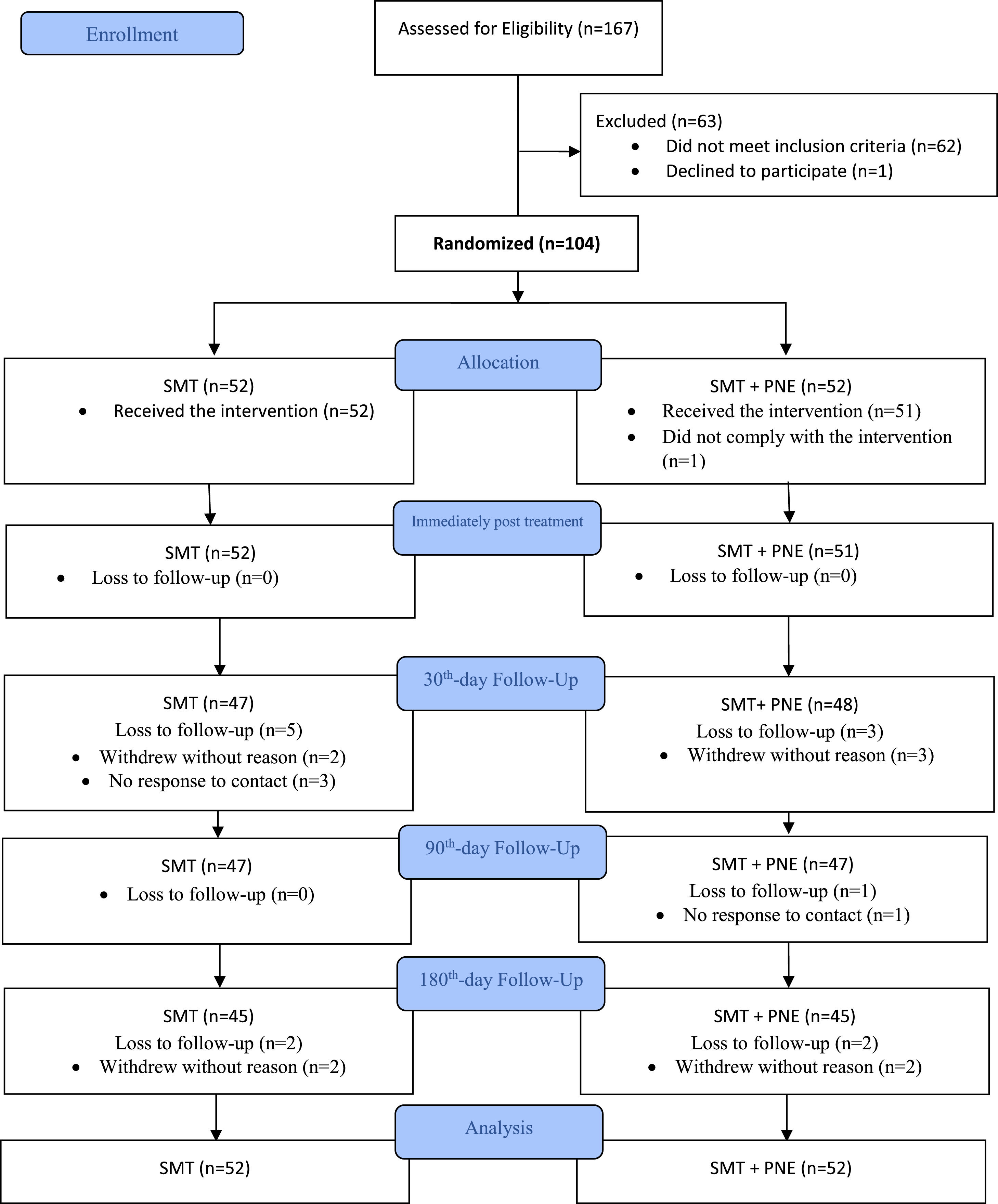
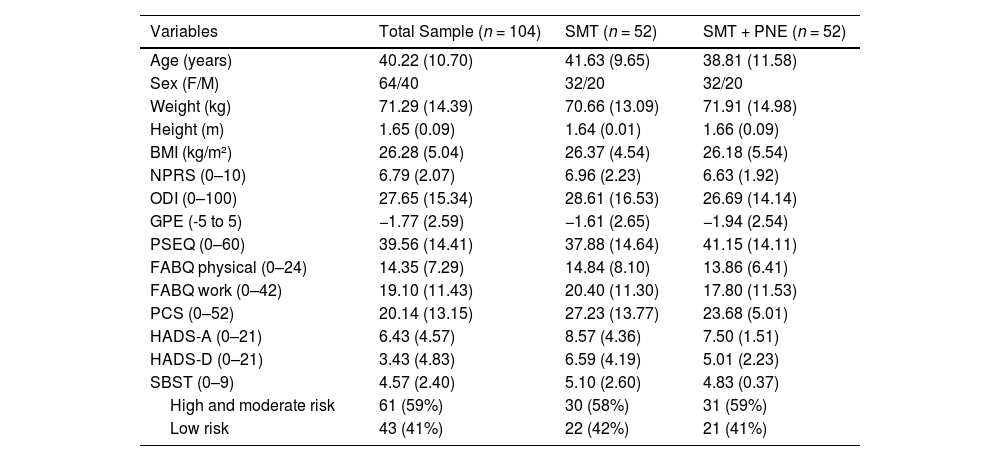
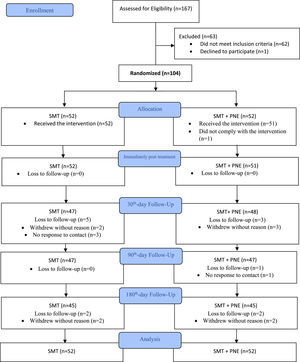
ResultsAdherence to interventions and loss of participantsA total of 167 participants were assessed for eligibility in the study between December 2017 and September 2019, and we excluded 63 patients who did not meet the inclusion criteria (Fig. 2). One-hundred four (n = 104) patients with CLBP were randomized into two groups: the SMT + PNE group (n = 52) and the SMT group (n = 52). The analysis was conducted by considering the originally assigned groups. The mean age of the participants was 40 years, with 61% females, the mean pain intensity was 6.7 and the mean disability score 27% (Table 2). The flow of participants through the trial is shown in Fig. 2. Just one participant was not measured at the immediate post-treatment time point (primary outcome) in the PNE + SMT group. However, we lost additional participants during the follow-ups: 9 participants (9.6%) at the 30-day follow-up, 1 additional participant (10%) at the 90-day follow-up, and 4 additional participants (14%) at the 180-day follow-up (Fig. 2).
Description [mean and standard deviation (SD)] of baseline data for patients recruited for the study. SMT, spinal manipulative therapy group; PNE, pain neuroscience education.
| Variables | Total Sample (n = 104) | SMT (n = 52) | SMT + PNE (n = 52) |
|---|---|---|---|
| Age (years) | 40.22 (10.70) | 41.63 (9.65) | 38.81 (11.58) |
| Sex (F/M) | 64/40 | 32/20 | 32/20 |
| Weight (kg) | 71.29 (14.39) | 70.66 (13.09) | 71.91 (14.98) |
| Height (m) | 1.65 (0.09) | 1.64 (0.01) | 1.66 (0.09) |
| BMI (kg/m²) | 26.28 (5.04) | 26.37 (4.54) | 26.18 (5.54) |
| NPRS (0–10) | 6.79 (2.07) | 6.96 (2.23) | 6.63 (1.92) |
| ODI (0–100) | 27.65 (15.34) | 28.61 (16.53) | 26.69 (14.14) |
| GPE (-5 to 5) | −1.77 (2.59) | −1.61 (2.65) | −1.94 (2.54) |
| PSEQ (0–60) | 39.56 (14.41) | 37.88 (14.64) | 41.15 (14.11) |
| FABQ physical (0–24) | 14.35 (7.29) | 14.84 (8.10) | 13.86 (6.41) |
| FABQ work (0–42) | 19.10 (11.43) | 20.40 (11.30) | 17.80 (11.53) |
| PCS (0–52) | 20.14 (13.15) | 27.23 (13.77) | 23.68 (5.01) |
| HADS-A (0–21) | 6.43 (4.57) | 8.57 (4.36) | 7.50 (1.51) |
| HADS-D (0–21) | 3.43 (4.83) | 6.59 (4.19) | 5.01 (2.23) |
| SBST (0–9) | 4.57 (2.40) | 5.10 (2.60) | 4.83 (0.37) |
| High and moderate risk | 61 (59%) | 30 (58%) | 31 (59%) |
| Low risk | 43 (41%) | 22 (42%) | 21 (41%) |
F, female; M, male; NPRS, numerical pain rating scale; ODI, Oswestry low back pain disability index; PCS, pain catastrophizing scale; FABQ, fear-avoidance beliefs questionnaire; HADS-A, anxiety and depression scale-anxiety; HADS-D, anxiety and depression scale-depression; PSEQ, pain self efficacy questionnaire; SBST, STarT back screening tool; BMI, body mass index.
This study has a departure from the initial protocol registered. The 90-day and 180-day follow-ups after randomization were not previously planned, but during the study course, it was feasible, so the research team decided to collect the data. No participants reported adverse effects.
Primary outcomes and secondary outcomesSignificant time-by-group interaction was found for pain intensity at 90 and 180 day follow-ups. The mean difference (MD) between groups for pain intensity was −0.9 (95% CI= −1.76, −0.04) and −1.19 (95% CI= −2.06, −0.32) at the 90 and 180 day follow-ups, respectively, favoring the SMT + PNE group (Table 3).
Between-group comparisons for the primary (pain intensity and disability) and secondary outcomes (global perceived effect of improvement, pain self-efficacy and fear beliefs). SMT, spinal manipulative therapy group; PNE, pain neuroscience education.
| Endpoints | Mean (standard deviation) | Estimated mean difference (95% CI) | |
|---|---|---|---|
| SMT (n = 52) | SMT + PNE (n = 52) | SMT – (SMT + PNE) | |
| Numeric pain rating scale (0–10) | |||
| Baseline | 6.96 (2.23) | 6.63 (1.92) | |
| Immediately post-treatment | 2.80 (2.67) | 2.77 (2.25) | |
| Change baseline to post-treatment | 3.93 (2.96, 4.89) | 3.95 (2.99, 4.91) | −0.022 (−0.85, 0.81) |
| 30 day FU | 3.85 (2.43) | 3.40 (2.24) | |
| Change baseline to 30 day FU | 2.87 (1.88, 3.86) | 3.33 (2.34, 4.31) | −0.45 (−1.31, 0.41) |
| 90 day FU | 5.04 (2.75) | 4.14 (2.75) | |
| Change baseline to 90 day FU | 1.68 (0.70, 2.67) | 2.57 (1.60, 3.57) | −0.90 (−1.76, −0.04)* |
| 180 day FU | 5.92 (3.37) | 4.72 (2.82) | |
| Change baseline to 180 day FU | 0.81 (−0.18, 1.81) | 2.00 (1.00, 3.01) | −1.19 (−2.06, −0.32)* |
| Oswestry disability index (0–100) | |||
| Baseline | 28.61 (16.53) | 26.69 (14.14) | |
| Immediately post-treatment | 13.83 (12.17) | 13.03 (11.35) | |
| Change baseline to post-treatment | 13.18 (9.16, 17.21) | 13.97 (9.95, 18.00) | −0.79 (−4.26, 2.67) |
| 30 day FU | 13.87 (11.68) | 13.25 (10.73) | |
| Change baseline to 30 day FU | 13.14 (8.99, 17.29) | 13.76 (9.66, 17.87) | −0.62 (−4.20, 2.95) |
| 90 day FU | 15.84 (11.84) | 14.92 (11.75) | |
| Change baseline to 90 day FU | 11.16 (7.02, 15.3) | 12.09 (7.95, 16.23) | −0.93 (–4.52, 2.66) |
| 180 day FU | 20.70 (15.22) | 15.52 (10.56) | |
| Change baseline to 180 day FU | 6.32 (2.15, 10.48) | 11.50 (7.29, 15.67) | −5.16 (−8.80, −1.53)* |
| Global perceived effect (−5 to +5) | |||
| Immediately post treatment | 2.57 (2.43) | 3.04 (1.72) | |
| 30 day FU | 1.04 (2.30) | 1.60 (2.14) | |
| Change post-treatment to 30 day FU | 1.53 (0.34, 2.72) | 1.44 (0.25, 2.62) | −0.55 (−1.54, 0.42) |
| 90 day FU | 0.64 (2.28) | 1.17 (2.43) | |
| Change post-treatment to 90 day FU | 1.94 (0.75, 3.12) | 1.87 (0.68, 3.06) | −0.53 (−1.52, 0.46) |
| 180 day FU | 0.02 (2.53) | 1.19 (2.54) | |
| Change post-treatment to 180 day FU | 2.55 (1.36, 3.75) | 1.85 (0.64, 3.06) | −1.17 (−2.17, −0.17)* |
| Pain self-efficacy questionnaire (0–60) | |||
| Baseline | 37.88 (14.64) | 41.25 (14.11) | |
| Immediately post-treatment | 48.79 (13.78) | 50.73 (13.50) | |
| Change baseline to post-treatment | 8.95 (4.74, 13.17) | 10.89 (6.67, 15.12) | −1.94 (−5.88, 2.01) |
| 30 day FU | 44.18 (19.18) | 46.54 (17.53) | |
| Change baseline to 30 day FU | 4.35 (0.02, 8.70) | 6.70 (2.38, 11.00) | −2.34 (−6.39, 1.71) |
| 90 day FU | 43.94 (18.21) | 46.95 (18.01) | |
| Change baseline to 90 day FU | 4.09 (−0.24, 8.42) | 7.11 (2.77, 11.44) | −3.01 (−7.08, 1.05) |
| 180 day FU | 42.63 (19.33) | 47.33 (19.13) | |
| Change baseline to 180 day FU | 2.79 (−1.57, 7.15) | 7.48 (3.09, 11.88) | −4.70 (−8.81, −0.59)* |
| Fear avoidance beliefs questionnaire (FABQ-phys), 0–42 | |||
| Baseline | 14.84 (8.10) | 13.86 (6.41) | |
| Immediately post-treatment | 11.38 (7.43) | 9.30 (7.05) | |
| Change baseline to post-treatment | 3.14 (0.68, 5.60) | 5.22 (2.74, 7.70) | −2.07 (0.11, 4.26) |
| 30 day FU | 11.38 (7.84) | 9.82 (6.43) | |
| Change baseline to 30 day FU | 3.14 (0.60, 5.67) | 4.70 (2.17, 7.22) | 1.56 (−0.70, 3.81) |
| 90 day FU | 12.07 (7.87) | 10.00 (6.76) | |
| Change baseline to 90 day FU | 2.44 (−0.09, 4.98) | 4.52 (1.97, 7.06) | 2.07 (−0.19, 4.34) |
| 180 day FU | 12.70 (7.89) | 10.72 (6.64) | |
| Change baseline to 180 day FU | 1.80 (−0.73, 4.40) | 3.80 (1.22, 6.37) | −1.97 (−0.33, 4.26)* |
| Fear avoidance beliefs questionnaire (FABQ-work), 0–24 | |||
| Baseline | 20.40 (11.30) | 17.80 (11.53) | |
| Immediately post-treatment | 14.51 (11.97) | 14.69 (10.69) | |
| Change baseline to post-treatment | 5.15 (2.52, 7.78) | 4.88 (1.99, 7.77) | 0.17 (−2.24, 2.61) |
| 30th-day FU | 15.55 (11.74) | 15.13 (11.20) | |
| Change baseline to 30th – day FU | 4.10 (1.37, 6.83) | 4.45 (1.45, 7.44) | −0.42 (−2.96, 2.12) |
| 90th-day FU | 15.58 (11.17) | 15.00 (10.88) | |
| Change baseline to 90th – day FU | 4.07 (1.34, 6.69) | 4.58 (1.57, 7.58) | −0.59 (−3.13, 1.95) |
| 180th-day FU | 14.96 (12.25) | 16.00 (11.45) | |
| Change baseline to 180th – day FU | 4.69 (1.96, 7.43) | 3.57 (0.56, 6.61) | 1.04 (−1.52, 3.60) |
For low back pain-related disability, a significant time-by-group interaction was found at the 180 day follow-up. The between groups difference for low back pain-related disability was −5.16 (95% CI = −8.8, −1.53) favoring the SMT + PNE group (Table 3).
Additionally, a significant time-by-group interaction was found for global perceived effect of improvement (MD= –1.17, 95% CI=–2.17, –0.17) and pain self-efficacy (MD = −4.7, 95% CI = −8.81, −0.59) at 180 day follow-up favoring the SMT + PNE group (Table 3).
DiscussionOur study compared the additional effect of providing PNE in combination with SMT for patients with CLBP and no significant between-group difference for the primary outcomes was observed in the short-term follow-up. As a result, we did not confirm our hypothesis that a multimodal treatment consisting of PNE and SMT would lead to significantly greater reduction of pain intensity and disability when compared to receiving SMT alone immediately post-treatment. In contrast, our results showed the following significant difference in long term follow-up favoring the group treated with SMT + PNE: pain intensity (at 90 and 180 day follow-ups) and low back pain-related disability, global perceived effect of improvement, and pain self-efficacy six months after the randomization.
Educational approaches combined with SMT in patients with CLBP were administered in previous studies.40,41 One study compared two groups that received SMT combined with a neuroplasticity educational approach to explain manual therapy or a biomechanical educational approach.40 Another study investigated the additional effect of adding a cognitive behavioral therapy program (administered virtually) to standard care intervention (manual therapy + exercise).41 In contrast to our findings, no between-group difference was observed for disability or pain intensity in these previous studies. We propose that the nature of the educational approaches administered in the previous studies40,41 can explain this difference.
Conversely, our findings are in agreement with the only previous study13 in which patients with CLBP were treated with SMT + PNE + home exercise program (HEP) compared to a group not treated with PNE. In that study,13 the group that received the combination of PNE + SMT + HEP showed a significant reduction in pain intensity compared to the group treated with SMT + HEP, particularly at the 12-week follow-up. The group treated with PNE had a mean pain intensity of 3.05 and 2.09 while the SMT + HEP group had a mean pain intensity of 4.42 and 4.52 at post-treatment and 12-week follow-ups, respectively.13 In our study, the pain intensity increased more for the SMT alone group particularly at the 90 and 180 day follow-ups. Nevertheless, the difference observed between-group in the current study for pain intensity is below the MIC estimate reported previously30 and endorsed by others42,43 particularly when comparing different treatments.43 The decrease in disability post-treatment in the SMT + PNE group also lasted consistently at the six-month follow-up, but it was not true for the SMT group which showed an increase in ODI score. However, the mean change difference between groups from baseline to six-month follow-up observed in our study was 5.16 which is below the MIC estimate reported in the literature for ODI score.30
In our study, we found between-groups differences in the global perceived effect of improvement and pain self-efficacy at the 180 day follow-up. Our results showed a less pronounced decrease for the global perceived effect (2.55 vs. 1.85, respectively for SMT vs. SMT + PNE) and higher self-efficacy scores for the SMT + PNE group at the six-month follow-up (42 vs. 47). There is no previous study that investigated the global perceived effect and pain self-efficacy outcomes in CLBP after SMT combined with PNE. However, in patients with chronic temporomandibular disorders, a previous study27 reported larger global perceived effect and pain self-efficacy scores for patients treated with a combination of manual therapy + exercise + PNE at all time points considered (6-week, 10-week, and 18-week follow-ups). The inclusion of exercise in association with PNE can account for the differences observed. The between-group difference observed for pain self-efficacy in the current study (4.7) is considered slightly below the MIC estimate described previously which is 5.5 for patients with CLBP.33
These findings together suggest that SMT combined with PNE can result in longer-lasting effects than SMT alone in patients with CLBP and it could be related to a mediator effect of pain self-efficacy. Mediation is an indirect effect of an intervention whereby changes in one outcome are associated with changes in another.44 PNE programs are designed to reconceptualize pain, change cognitions and maladaptive beliefs, as well as motivate patients to cope with pain through changes in lifestyle like being more active, which could be mediated by an increase in self-efficacy levels. Pain self-efficacy could be defined as the confidence in the ability to perform specific tasks or behaviors, like the adoption of strategies to cope with pain.45 Both groups, in the current study, showed a consistent increase in the pain self-efficacy levels post-treatment. However, the group receiving SMT showed a more pronounced decrease from post-treatment values on pain self-efficacy mean score than the SMT + PNE group (6-point decrease for SMT group vs. 3 points for the SMT + PNE group). One can suppose that the adoption of more active behaviors, which caused the maintenance of the pain self-efficacy levels in the PNE group boosted by the short-term pain relief promoted by SMT, can explain the lower pain intensity and disability reported by the patients.
Manual therapy is a recognized intervention for the management of chronic musculoskeletal pain4 and a previous systematic review with meta-analysis showed moderate quality evidence suggesting that SMT has similar effects to other recommended therapies for pain and disability in CLBP but with just short-term effects.6 Conversely, it is noteworthy that manual therapy approaches are recognized as passive treatments46 whereas the PNE message is to motivate patients to increase self-efficacy and autonomy for pain control. One can argue that the implicit message communicated by SMT (“a health professional is the only one who can reduce my pain”) could be in conflict with the PNE message which may have contributed to the lack of additional effect of PNE in the short-term.
One can argue that the PNE delivered in the current study was not effective to reconceptualize beliefs which could be the first step to promoting changes in pain intensity and disability indirectly. However, we did not assess the pain knowledge of the patients after the PNE program; in this way, we cannot firmly state that patients reconceptualize maladaptive cognitions. We suggest that future studies include the assessment of pain concepts, beliefs, and knowledge in RCTs.
This study has several limitations: 1) The outcome assessor in the current study was unaware of group allocation, but this procedure could not be called blinding because we only used patient self-reported outcomes.We can not exclude a possible bias related to patients' expectations regarding the effects of the treatment. To minimize such a problem the participants were not informed about the study hypothesis. Conversely, the researcher who administered SMT was blinded to group allocation, and a different researcher delivered the PNE; 2) We did not evaluate the knowledge acquired by the patients after the PNE program (which could be considered a treatment fidelity assessment) to investigate the efficacy of the educational approach (PNE); thus, future studies should adopt a strategy to control for learning and beliefs changes; and 3) Ultimately, we did not investigate the consistency of the interventions administered in the present study (treatment fidelity) among participants. Particularly for SMT, as we administered a prescriptive protocol, we consider that SMT was not influenced by inconsistencies during the treatment delivery. We recommend that future studies could include fidelity analysis, particularly considering PNE.
ConclusionIn this study, we found no additional effect of combining PNE with SMT intervention for pain intensity and disability in patients with CLBP in comparison to SMT alone in the short-term. Conversely, our results showed a significant long-term effect for the group treated with SMT + PNE for the following outcomes: pain intensity (90 and 180 day follow-ups) and low back pain-related disability, global perceived effect of improvement and pain self-efficacy at the six-month follow-up. These findings suggest that SMT + PNE can result in long-term effects in patients with CLBP and that such an effect could be related to a possible mediator effect of pain self-efficacy.
None.