Low back pain is the leading cause of disability worldwide. The therapeutic management of patients with chronic LBP is challenging.
ObjectivesThe aim of this study is to evaluate the effects of heat and transcutaneous electrical nerve stimulation combined on pain relief in participants with chronic low back pain.
MethodsFifty participants with chronic (≥3 months) low back pain were randomly assigned to two groups: HeatTens (n=25) and control group (n=25). Primary outcome was pain. Secondary outcomes were pressure pain thresholds, temporal summation, conditioned pain modulation, fear-avoidance and beliefs questionnaire, central sensitization inventory, quality of life, and medication use. The control group received no treatment and continued usual care. After four weeks of treatment, all measurements were repeated.
ResultsFifty individuals participated in this study. Significant higher pressure pain threshold measures after both 30min and 4 weeks for the lower back region and the second plantar toe were found only in the experimental group.
ConclusionThe combination of heat and transcutaneous electrical nerve stimulation does not reduce pain scores in patients with chronic low back pain. Pressure pain threshold values significantly improved, showing beneficial effects of the experimental treatment.
ClinicalTrials.gov: NCT03643731 (https://clinicaltrials.gov/ct2/show/NCT03643731).
Low back pain (LBP) is a highly prevalent and complex condition. It is associated with significant socio-economic costs.1,2 Currently, it is the leading cause of disability worldwide.3–6 Most episodes of LBP resolve within 6 weeks7 but 10–15% become chronic. The therapeutic management of patients with chronic low back pain (CLBP) is challenging.8 To minimize the impact of LBP on individuals’ daily lives, pharmacological treatment is regularly recommended. However, inappropriate and non-optimal drug prescribing is common.9,10 These patients may benefit from nonpharmacological treatment, such as transcutaneous electrical nerve stimulation (TENS) and heat.11,12
TENS is an inexpensive treatment modality that delivers electrical impulses through the skin. There is conflicting evidence for the beneficial effects of TENS and therefore its use in the management of CLBP is not typically recommended.13 However, recent clinical research has advanced our understanding of TENS.14 TENS triggers a complex neuronal network that activates descending inhibitory systems, resulting in reduction of hyperalgesia.15,16 There is growing evidence that the transition from acute to persistent LBP can be explained by sensitized central pain mechanisms.17,18 Symptoms of central sensitization (CS) were recently identified in a subgroup of patients experiencing CLBP.19 Numerous studies have previously demonstrated significant dysfunction in descending inhibitory pathways and widespread hyperalgesia in chronic pain conditions, including CLBP.18,20–22 CS consists of altered sensory processing in the brain,23–25 malfunctioning of descending anti-nociceptive mechanisms,23,24,26 increased activity of pain facilitatory pathways, and temporal summation of second pain or wind-up.25,26 It is important to note that CS is a neurophysiological concept27 and that the underlying processes cannot directly be measured in clinical practice.28 Persistent CS negatively affects treatment outcome and quality of life in patients with LBP.29 Not only from a clinical point of view, but also to design appropriate and tailored treatments, assessing the presence of CS is important.30,31 To study altered sensory processing, including signs of CS, quantitative sensory testing is used.32,33 Studies in people with fibromyalgia indicate restored central pain modulation (CPM) and lower pressure pain thresholds (PPT).34 However, while the effects of TENS on PPTs have been investigated in patients with CLBP,35,36 results on its effectiveness on CPM seem to be lacking. Furthermore, TENS studies show promising results for pain control in movement-evoked pain (MEP).34,35,37 MEP refers to pain that is experienced as a result of physical activity.38 Because study findings suggest that pain at rest (or spontaneous pain) and MEP are likely driven by different underlying mechanisms,39 it seems appropriate to assess both as possible treatment outcomes.
In addition to TENS, superficially applied heat is another often-used treatment modality.12,40 Moderate-quality research showed that superficial heat improved pain and function in a CLBP population (weighted mean difference: 1.06, 95% CI: 0.68, 1.45).12 In contrast, several clinical practice guidelines found insufficient evidence to support the effectiveness of superficial heat for relieving pain in patients with LBP.41,42 It is concluded that, as a sole treatment, heat may not provide enough pain relief to warrant inclusion in clinical guidelines. However, given the observed positive effects in individual studies, further studying the effects of heat in CLBP seems warranted. As both heat and TENS appear to result in small clinical improvements in patients with LBP,11,12,43 combining both interventions may result in a synergistic effect. These combined modalities have already showed promising and beneficial effects in patients with knee osteoarthritis.44 Therefore, the aim of this study is to evaluate the short-term effects of TENS combined with heat (HeatTens device (HV-F311-E, OMRON Healthcare Co., Ltd., Japan.) on pain relief (primary outcome), PPT, CPM response, quality of life, and medication use in patients with CLBP.
MethodsStudy designThis two-armed, randomized controlled clinical trial was conducted at the University Hospital of Brussels and at the Vrije Universiteit Brussel. The study was approved by the local ethics committees of the University Hospital Brussels, Brussels, Belgium (B.U.N. 143201836092) and was conducted between August 2018 and June 2019. The study protocol is registered online on ClinicalTrials.gov (NCT03643731) and reported according to CONSORT guidelines45 and the TIDieR Checklist.46
Study population and sample sizeA sample of 50 participants with CLBP was recruited through posters and flyers, distributed in the University Hospital Brussels and the Vrije Universiteit Brussels and via social media. All participants provided written consent.
Participants were considered eligible when experiencing nonspecific CLBP (≥3 months)47 and aged between 25 and 80 years.
They were excluded in the following cases: (1) spinal surgery in the past 6 months, (2) diagnosed with chronic fatigue syndrome, fibromyalgia, or severe underlying comorbidities (neurological conditions, cardiovascular problems, or rheumatologic diseases), (3) pregnant or given birth in the preceding year, and (4) initiated a new LBP-treatment in the 6 weeks prior to study participation.48 They were not allowed to be actively using heat or TENS on their own but could continue usual medication use. Participants were instructed to provide a diary in which they needed to write down if they have taken any medication or received other kinds of therapy. Participants with contraindications to TENS were excluded as well.
To evaluate feasibility and to estimate the required sample size, we conducted a pilot study in which 20 participants with CLBP were included. Sample size for MEP was then calculated using G*Power software (Düsseldorf, Germany) based on the effects we found in the pilot study for MEP between experimental and control group in participants with CLBP (effect size: d=1, α=0.05, power=0.90, test family: t-tests, statistical test: means, difference between two independent groups (two groups), type of power analysis: a priori). Considering a 20% loss to follow-up, a sample size of 44 participants had to be recruited.
Blinding and randomizationThe outcome-assessor and statistician were blinded to treatment allocation.49 The allocation was conducted by an independent researcher who was not involved with other experimental procedures. Simple randomization results were concealed in sealed envelopes with consecutive numbers.45
Outcome measuresPrimary outcome measuresAn 11 points numeric rating scale (NRS) was used for pain intensity measurements.50 Participants were instructed to provide a pain rating score for average pain during the last 24h, maximum pain during the last 24h, and MEP. Pain scores ranged from “no pain” (0) to “excruciating pain” (10). A 30% decrease on the pain NRS in individuals with CLBP represents a clinically important difference.51–53 Prior to measuring MEP, pain at rest was assessed. Participants were to lay or sit in a pain-free position and needed to rate their pain after keeping this position for five minutes. MEP intensity was measured in response to two physical tasks: the Back Performance Scale (BPS),35,54 and a five-minute walk test (5MWT).55,56 The BPS consists of five functional tasks. Participants rated their level of “peak pain” during each task. “Current pain” was rated six times in relation to the five tasks (once immediately before and once immediately after). For the analysis, MEP was calculated in two ways. First, an index of MEP-Sensitivity (MEPS) was calculated. MEPS refers to an increase in pain in response to repeated physical activity. Therefore, the difference between pre- and post-walking pain ratings was used to calculate MEPS.57 Second, the mean of peak scores during each movement task (both 5MWT and BPS) was used for mean MEP scores.58
Secondary outcome measuresPPTs (i.e., the point of minimum pressure that creates an unpleasant sensation)59 were measured using a digital pressure algometer (Wagner Force Ten). Four bilateral areas were tested: (1) 2cm lateral to the L3 spinous process, (2) 2cm lateral to the L5 spinous process,34 (3) near the posterior superior iliac spines (PSIS),13 and the plantar side of the second toe.60 Temporal summation (TS) was used to evaluate endogenous pain facilitation and started two minutes after PPT measurements. TS was induced through 10 pressure pulses with the hand-held algometer at the PPT intensity.61 Participants were instructed to rate their pain level according to a NRS at the first, fifth, and tenth pulse.61,62 TS was calculated by subtracting the pain rating provided in response to the 10th stimulation by the pain rating provided in response to the first stimulation.61 Low scores indicate normal TS, whereas high scores indicate minor efficacy of TS.63 CPM was used to evaluate the efficacy of the descending inhibitory modulation of pain. The cold-pressor task (0.7±0.1°C, VersaCool™) was used as conditioning stimulus and pressure pain tolerance threshold at the second toe was used as the test stimulus.60 The water was recirculated to maintain temperature and avoid thermal barrier to be created. Pressure pain tolerance threshold was defined as the point at which the subject felt the pain as intolerable. They were instructed to say “stop” when this point was reached60 and the number on the digital pressure algometer was noted. Participants needed to put their hand, up to the wrist, in the water for a maximum of two minutes. They were instructed to withdraw the hand when the pain was experienced as intolerable. If the participant did not withdraw the hand at two min, this time was recorded. Pressure pain tolerance threshold was assessed once before and immediately after the cold pressor task.64 CPM was calculated by subtracting pressure pain tolerance-score after the cold-pressor task by the scores before the cold-pressor task.64 CPM-values were coded as numerical data. Negative values indicate impaired endogenous pain inhibition.59
The Fear Avoidance and Beliefs Questionnaire (FABQ) is a tool based on theories of fear and avoidance behavior and focuses specifically on participants’ beliefs about how physical activity and work affects their LBP.65 Self-reported mental and physical health was examined by means of the Short Form 36 (SF-36).66 The Central Sensitization Inventory (CSI) is a self-reported tool to identify key symptoms that are associated with central sensitization.67 It contains a “part A” of 25 statements related to current health symptoms, indicative of central sensitization (scored on a five-point Likert scale ranging from 0 to 4). A mean score of 40 is the cut off value for central sensitization.68,69 Next to excellent measurement properties, the CSI is found to be a responsive treatment outcome measure as well.59,70 Medication use was recorded retrospectively before starting the experiment and by means of a diary during the follow-up period. Participants needed to write down their use of medication during the four-week treatment period, using a five-point Likert scale (no medication, less than once per week, one to three times per week, three to five times per week, more than five times per week).
ProcedureParticipants completed three testing sessions at the Vrije Universiteit Brussels over a four-week period. After baseline measurements [time=0], participants were randomly assigned in two groups: HeatTens group (n=25) and control group (n=25). The HeatTens group was then treated with the HeatTens-device (HeatTens HV-F311-E, OMRON Healthcare Co., Ltd., Japan) for 30min. The treatment comprised a combination of heat and TENS and was applied using two self-adhesive electrodes. A clinician placed the electrodes on opposite sites (left/right) at the spinal level corresponding to the pain complaint.35,71 The following TENS parameters were used: pulse frequency from 0.7 to 108Hz and a pulse width of 100μs. Intensity level was instructed to be as high as tolerable.14 To control for positional intolerance, participants could choose if they wanted to sit or lie down during the intervention. Controls received no treatment and went through a 30-min waiting period. Immediately after these 30min, measurements of MEP, PPTs, TS, CPM, and CSI were repeated (T1) [time=1]. Subsequently, participants completed testing and went home. Each participant in the experimental group got a HeatTens device to take home. They were individually and face-to-face instructed to use the device daily, for 30min. Written instructions were provided as well. The clinician giving the HeatTens treatment as well as the practical demonstration on the use of the device was a trained physical therapist. The control group received no additional treatment throughout the study protocol. After four weeks of treatment, all measurements were repeated (T2; primary endpoint) [time=2].
Statistical analysisAll data were analyzed using RStudio version 1.1.463 running on R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). Analyses were conducted using intention-to-treat principles. Means and standard deviations (SD) were reported for continuous variables. To analyze differences between the experimental and the control group, a linear mixed effects model was built for the following variables: average pain in the last 24h, maximum pain in the last 24h, MEP, MEPS, PPT, TS, CPM, FABQ, SF-36, and CSI. Linear mixed effects modeling was performed to estimate within-subjects effects (time), between-subjects effects (interventional) and interaction effects. To evaluate the synergistic effect of the combination of heat and TENS, and medication use, we adjusted pain scores for medication use for average pain during the last 24h and maximum pain during the last 24h. However, since pain scales are affected by many more factors than just pain,72 a new parameter was included. Inspired by previous research,73 we multiplied pain scores by a factor that relates to the amount of medication the participant had used in the last week. This factor was based on the five-point Likert scale (i.e. no medication (multiplied by one, so no change in pain score), once per week (multiplied by two), two to three times per week (multiplied by three), three to five times per week (multiplied by four), and more than five times per week (multiplied by five)). Higher scores indicate higher pain intensity. A random intercept per participant was included due to the variation present between different participants. Results are expressed as mean estimates and standard deviations together with their 95% confidence intervals (CI) for the different groups. The effects were expressed as mean difference between groups and their corresponding 95% CI.
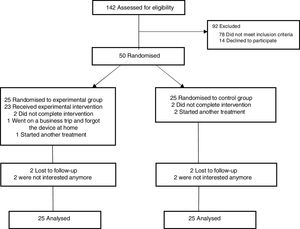
ResultsA total of 50 people with CLBP were included in the trial and received the HeatTens treatment (n=25) or no treatment (n=25) (Fig. 1). The baseline characteristics of the control and experimental group are presented in Table 1. There were no appreciable differences between the two groups with respect to age, gender, weight, height, body mass index (BMI), and duration of complaints. There was also no significant difference in the number of participants that terminated the cold pressor task before the end of two minutes (11 and 7 in the control and experimental group respectively, p=0.239).
Demographic and Baseline Characteristics.
| Experimental group (n=25) | Control group (n=25) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Demographic | ||
| Male/female, no. (%) | 12 (48)/13 (52) | 11 (44)/14 (56) |
| Age, years | 43.9±12.2 | 44.7±12.2 |
| Body weight, kg | 80.6±14.7 | 82.2±17.0 |
| Body height, cm | 174.3±8.2 | 172.6±9.0 |
| BMI, kg/m2 | 26.5±3.8 | 27.6±5.1 |
| Duration of complaints, months | 76.6±65.9 | 82.1±99.3 |
| Baseline | ||
| Average pain (24h) | 3.7±1.8 | 4.4±1.8 |
| Max. pain (24h) | 5.6±1.9 | 6.5±1.8 |
| MEP BPS | 2.3±1.8 | 2.8±1.8 |
| MEP 5MWT | 2.2±1.9 | 2.4±1.9 |
| MEPS 5MWT | 0.6±1.1 | 0.7±1.0 |
| PPT, kgf | ||
| Lower back | 7.0±3.2 | 6.7±3.0 |
| 2nd toe | 6.2±2.1 | 6.6±2.1 |
| TS, kgf | ||
| Lower back | 2.3±1.3 | 2.5±1.3 |
| 2nd toe | 2.2±1.3 | 2.0±1.5 |
| CPM, kgf | 0.6±2.3 | 0.4±2.3 |
| CPT (time), s | 84.8±41.9 | 97.4±37.6 |
| SF-36 | 98.2±6.4 | 97.8±6.7 |
| FABQ | 30.4±11.8 | 26.8±12.0 |
| CSI | 35.9±10.5 | 31.6±10.8 |
| Medication, no. (%) | ||
| No medication | 12 (48) | 8 (32) |
| <1/week | 5 (20) | 3 (12) |
| 1–2/week | 1 (5) | 4 (16) |
| 3–5/week | 1 (5) | 1 (4) |
| >5/week | 1 (5) | – |
| Missing data | 5 (20) | 9 (36) |
Abbreviations: no., number; kg, kilogram; cm, centimeter; BMI, body mass index; Max. pain (24h), maximum pain in the last 24h; MEP, movement-evoked pain; MEPS, movement-evoked pain – sensitivity; BPS, Back Performance Scale; 5MWT, five minute walk test; PPT, pressure pain threshold; kgf, kilogram force; TS, temporal summation; CPM, conditioned pain modulation; CPT, cold pressor task; SF36, 36-Item Short Form Health Survey; FABQ, fear avoidance beliefs questionnaire; CSI, Central Sensitization Inventory; SD, standard deviation.
For primary outcomes, (i.e. average pain in the last 24h, maximum pain in the last 24h, MEP, and MEPS) no significant interaction effects of condition×time were observed (Table 2; primary outcome measures).
Primary and secondary outcomes.
| Within group differences: mean (95% CI) | Between group differences: mean (95% CI) | ||
|---|---|---|---|
| Control group | Experimental group | ||
| Mean±SD (95% CI) | Mean±SD (95% CI) | ||
| Primary outcome | |||
| Average pain (24h) | |||
| Baseline | 4.4±1.8 (3.7, 5.2) | 3.7±1.8 (3.0, 4.5) | |
| At 4 wks | 4.3±1.9 (3.5, 5.1) | 2.5±1.7 (1.8, 3.3) | −1.1 (−2.2, 0.1) |
| Change baseline → 4 wks | −0.1 (−1.0, 0.7) | −1.2 (−2.0, −0.4) | |
| Max. pain (24h) | |||
| Baseline | 6.5±1.8 (5.6, 7.3) | 5.6±1.9 (4.8, 6.4) | |
| At 4 wks | 5.7±2.0 (4.9, 6.6) | 4.0±2.0 (3.2, 4.9) | −0.8 (−2.2, 0.6) |
| Change baseline → 4 wks | −0.7 (−1.7, 0.3) | −1.5 (−2.5, −0.6) | |
| MEP BPS | |||
| Baseline | 2.8±1.8 (2.0, 3.5) | 2.3±1.8 (1.6, 3.1) | |
| At 30min | 2.8±1.8 (2.0, 3.5) | 2.2±1.8 (1.4, 2.9) | −0.2 (−0.7, 0.4) |
| Change baseline → 30min | −0.0 (−0.4, 0.4) | −0.2 (−0.5, 0.2) | |
| At 4 wks | 2.3±1.7 (1.6, 3.1) | 1.8±1.6 (1.1, 2.5) | −0.1 (−0.7, 0.5) |
| Change baseline → 4 wks | −0.5 (−0.8, −0.1) | −0.5 (−0.9, −0.1) | |
| MEP 5MWT | |||
| Baseline | 2.4±1.9 (1.6, 3.2) | 2.2±1.9 (1.4, 3.0) | |
| At 30min | 2.4±1.9 (1.6, 3.2) | 1.8±1.8 (1.1, 2.6) | −0.4 (−1.0, 0.3) |
| Change baseline → 30min | −0.0 (−0.5, 0.5) | −0.4 (−0.8, 0.1) | |
| At 4 wks | 1.9±2.0 (1.1, 2.8) | 1.2±1.9 (0.4, 2.0) | −0.6 (−1.2, 0.1) |
| Change baseline → 4 wks | −0.5 (−0.9, −0.0) | −1.0 (−1.5, −0.6) | |
| MEPS 5MWT | |||
| Baseline | 0.7±1.0 (0.3, 1.1) | 0.6±1.1 (0.2, 1.1) | |
| At 30min | 0.3±1.1 (−0.1, 0.8) | 0.3±1.0 (−0.1, 0.7) | 0.1 (−0.6, 0.7) |
| Change baseline → 30min | −0.4 (−0.8, 0.1) | −0.3 (−0.8, 0.1) | |
| At 4 wks | 0.7±0.9 (0.3, 1.1) | 0.2±1.1 (−0.3, 0.6) | −0.5 (−1.1, 0.2) |
| Change baseline → 4 wks | −0.0 (−0.5, 0.4) | −0.5 (−1.0, 0.0) | |
| Average pain×medication | |||
| Baseline | 2.5±5.8 (0.1, 4.9) | 1.7±5.3 (−0.5, 3.9) | |
| At 4 wks | 6.1±4.9 (4.0, 8.2) | 2.0±4.8 (−0.1, 4.0) | −3.3 (−7.7, 1.03) |
| Change baseline → 4 wks | 3.6 (0.4, 6.8) | 0.3 (−2.7, 3.2) | |
| Max. pain × medication | |||
| Baseline | 4.6±7.6 (1.4, 7.7) | 2.8±6.9 (−0.1, 5.6) | |
| At 4 wks | 8.2±6.4 (5.5, 11.0) | 3.0±6.2 (0.3, 5.6) | −3.5 (−9.2, 2.2) |
| Change baseline → 4 wks | 3.7 (−0.5, 7.9) | 0.2 (−3.7, 4.1) | |
| Secondary outcome | |||
| PPT lower back, kgf | |||
| Baseline | 6.7±3.0 (5.4, 8.0) | 7.0±3.2 (5.7, 8.3) | |
| At 30min | 6.8±3.2 (5.5, 8.1) | 8.3±3.6 (7.0, 10.0) | 1.2 (0.7, 1.7) |
| Change baseline → 30min | 0.1 (−0.3, 0.5) | 1.3 (0.9, 1.6) | |
| At 4 wks | 7.3±3.1 (5.9, 8.6) | 8.7±3.0 (7.4, 10.0) | 1.1 (0.6, 1.6) |
| Change baseline → 4 wks | 0.6 (0.2, 0.9) | 1.7 (1.3, 2.0) | |
| PPT 2nd Toe, kgf | |||
| Baseline | 6.6±2.1 (5.8, 7.5) | 6.2±2.1 (5.4, 7.1) | |
| At 30min | 6.5±2.1 (5.7, 7.4) | 6.8±2.1 (5.9, 7.6) | 0.6 (0.0, 1.3) |
| Change baseline → 30min | −0.1 (−0.5, 0.3) | 0.5 (0.1, 1.0) | |
| At 4 wks | 7.2±2.0 (6.3, 8.0) | 7.6±2.0 (6.8, 8.5) | 0.8 (0.2, 1.5) |
| Change baseline → 4 wks | 0.6 (0.1, 1.0) | 1.4 (0.9, 1.8) | |
| TS lower back, kgf | |||
| Baseline | 2.5±1.3 (2.0, 3.1) | 2.3±1.3 (1.7, 2.8) | |
| At 30min | 2.2±1.3 (1.7, 2.8) | 2.0±1.3 (1.5, 2.6) | 0.0 (−0.4, 0.4) |
| Change baseline → 30min | −0.3 (−0.5, 0.0) | −0.3 (−0.5, 0.0) | |
| At 4 wks | 2.4±1.3 (1.8, 2.9) | 1.9±1.3 (1.3, 2.4) | −0.2 (−0.6, 0.1) |
| Change baseline → 4 wks | −0.2 (−0.4, 0.1) | −0.4 (−0.7, −0.1) | |
| TS 2nd Toe, kgf | |||
| Baseline | 2.0±1.5 (1.4, 2.6) | 2.2±1.3 (1.7, 2.8) | |
| At 30min | 1.9±1.3 (1.4, 2.5) | 2.4±1.3 (1.9, 3.0) | 0.3 (−0.4, 1.0) |
| Change baseline → 30min | −0.1 (−0.6, 0.4) | 0.2 (−0.3, 0.7) | |
| At 4 wks | 2.0±1.3 (1.4, 2.5) | 2.1±1.3 (1.6, 2.7) | −0.1 (−0.8, 0.7) |
| Change baseline → 4 wks | 0.0 (−0.5, 0.5) | −0.1 (−0.6, 0.4) | |
| CPM, kgf | |||
| Baseline | 0.4±2.3 (−0.5, 1.4) | 0.6±2.3 (−0.4, 1.5) | |
| At 30min | 0.5±2.4 (−0.5, 1.5) | 0.8±2.3 (−0.2, 1.7) | 0.1 (−1.6, 1.8) |
| Change baseline → 30min | 0.1 (−1.2, 1.3) | 0.2 (−1.1, 1.4) | |
| At 4 wks | 0.5±2.3 (−0.5, 1.5) | 0.7±2.3 (−0.3, 1.7) | 0.1 (−1.6, 1.8) |
| Change baseline → 4 wks | 0.0 (−1.2, 1.3) | 0.1 (−1.1, 1.4) | |
| CSI | |||
| Baseline | 31.6±10.8 (27.1, 36.0) | 35.9±10.5 (31.5, 40.2) | |
| At 30min | 29.3±10.8 (24.8, 33.7) | 33.2±10.5 (28.9, 37.6) | −0.4 (−4.4, 3.7) |
| Change baseline → 30min | −2.3 (−5.2, 0.6) | −2.6 (−5.5, 0.2) | |
| At 4 wks | 28.8±10.5 (24.3, 33.3) | 31.0±10.3 (26.5, 35.4) | −2.1 (−6.2, 2.0) |
| Change baseline → 4 wks | −2.8 (−5.7, 0.1) | −4.9 (−7.8, −2.0) | |
| FABQ | |||
| Baseline | 26.8±12.0 (21.9, 31.8) | 30.4±11.8 (25.6, 35.3) | |
| At 4 wks | 26.8±11.6 (21.8, 31.8) | 33.1±11.5 (28.1, 38.0) | 2.6 (−2.0, 7.3) |
| Change baseline → 4 wks | 0.0 (−3.3, 3.2) | 2.6 (−0.6, 5.9) | |
| SF-36 | |||
| Baseline | 97.8±6.7 (95.1, 100.6) | 98.2±6.4 (95.6, 100.9) | |
| At 4 wks | 101.7±6.4 (99.0, 104.5) | 101.4±6.4 (98.6, 104.1) | −0.8 (−5.0, 3.4) |
| Change baseline → 4 wks | 3.9 (0.9, 6.9) | 3.1 (0.2, 6.1) | |
Abbreviations: Max. pain (24h), maximum pain in the last 24h; MEP, movement-evoked pain; MEPS, movement-evoked pain – sensitivity; BPS, Back Performance Scale; 5MWT, five-minute walk test; SD, standard deviation; CI, confidence interval; PPT, pressure pain threshold; kgf, kilogram force; TS, temporal summation; CPM, conditioned pain modulation; CSI, Central Sensitization Inventory; FABQ, fear avoidance beliefs questionnaire; SF36, 36-Item Short Form Health Survey. Bold values indicate statistically significant results (i.e. 95% confidence intervals do not cross zero).
Significant condition×time interaction effects were found for PPT measurements (p<0.001). Higher PPT-measures after both 30min and 4 weeks were observed for the lower back region and the second plantar toe in the experimental group compared to the control group.
No significant interaction effects were found for TS, CPM, CSI, SF-36, FABQ, Pain×Medication, and medication use (Table 2; secondary outcome measures).
DiscussionThis study aimed to evaluate the short-term effects of TENS combined with heat on pain relief (primary outcome), PPT, TS, CPM, quality of life, fear and avoidance beliefs, presence of hypersensitivity, and medication use in patients with CLBP.
Primary outcomesNo significant interaction effects of condition×time were observed for primary outcome measures and so the combination of heat and TENS does not seem to affect pain in a population with CLBP. However, after 4 weeks, average pain during the last 24h and MEP (5MWT) decreased by 32.0% and 46.4% respectively in the experimental group, compared to 3.2% and 19.4% in the control group. These results suggest clinically important pain relief (i.e. 30%) in the experimental group.
Although the present study found no significant interaction effects for MEP, the results published by Simon et al.35 suggest that TENS significantly improved MEP during the BPS compared to no TENS (F1,54=35.8, p<0.001) in a CLBP population. There are some differences between both studies that possibly explain these differences in results. In the study of Simon et al.35 participants completed five experimental sessions over a four-week period, in which TENS was provided by a physical therapist. Participants were instructed to verbalize when a “strong but tolerable” stimulus was experienced, corresponding to a score of 70/100 on a pain rating scale. This intensity was set and used throughout the TENS-treatment. In the present study, participants used the HeatTens device independently and were instructed to use a strong but tolerable impulse intensity. However, we had no control over the intensity when the HeatTens device was used at home.
Further, the present study assessed MEP shortly after the application of HeatTens treatment. Simon et al.35 measured MEP (BPS) during TENS treatment and found significantly lower pain scores for MEP during TENS when compared to no TENS (F1,54=35.8, p<0.05). Sluka et al.14 suggested measuring the effects of TENS during or immediately after TENS therapy. The results of the present study suggest that measuring MEP during active TENS, and so during TENS’ peak effect, might enhance the pain-relieving effects when compared to measuring MEP after TENS treatment.
Another important question we need to ask ourselves is if the BPS is sensitive enough to capture/provoke MEP. Recent research74 identified important aspects of structuring physical tasks that can evoke activity-related pain. Three strategies have been identified: self-paced, tailored, and standardized tasks. A tailored approach seemed to be most effective and most associated with temporal summation of pain. When identifying physical tasks to provoke MEP, these aspects should be considered. This study was however only recently published, and as such, these insights were not considered when designing the present study.
Secondary outcomesIn evaluating MEPS, the assessment of (mechanical) temporal summation is often included as well,58 because it describes the progressive increase of pain as a result of painful stimulation. Temporal summation has been reported as related to MEPS in response to walking in people with knee osteoarthritis.57 In the present study, neither MEPS, nor temporal summation changed subsequent to the HeatTens intervention, suggesting that the combination of heat and TENS is not effective for improving pain summation.
For PPTs, significant interaction effects were found after 30min of HeatTens treatment and can be interpreted as clinically important (>15% increase of PPT values)75 for the lower back (+17.8%), but not for the 2nd toe (+8.7%). Also after four weeks of treatment, clinically important increased PPT values could be observed in the intervention group for both lower back (+23.5% in the experimental versus +8.3% in the control group) and 2nd toe (+22.2% in the experimental versus +8.3% in the control group). These results suggest that the combination of heat and TENS activates local analgesic effects.
Despite significant differences found for PPT measurements, TS, and CPM values did not change, suggesting that HeatTens did not influence normalization of pain inhibitory function. The results found in the present study conflict with the results found by Dailey et al.34 In addition to significant MEP improvement, they found increased PPTs in response to CPM for TENS treatment compared to placebo TENS (p<0.05) and no TENS (p<0.01) in people with fibromyalgia. However, CPM was assessed during TENS treatment.
Despite no significant effects on the FABQ scores, it is surprising that the experimental group reported higher fear-avoidance beliefs after four weeks of treatment (an increase of 8.6% in the experimental group, compared to no changes in the control group). A person's sense of control over pain often seems to be related to coping mechanisms. In the present study, participants might have felt to be more in control of their pain, self-regulating the HeatTens device. On the other hand, we instructed the participants to use the device while lying/sitting down, possibly resulting in a more passive coping strategy and external locus of control. Using the HeatTens device, not as a sole treatment, but combined with physical exercise or active therapy modalities might minimize this increase in fear-avoidance beliefs and should be considered for future research. From a more clinical point of view, results from qualitative research indicate that pain is an important barrier to activity participation in patients with CLBP.76 As the therapeutic management of this population is challenging, eliminating these barriers seems logic and necessary. It is recommended for patients with CLBP to stay physically active, as inactivity will negatively affect recovery.77 The findings of the present study regarding a clinically important decrease in average pain can therefore be of use in future research and treatment decision-making.
Strengths and limitationsMultiple strengths are present in this study. In addition to randomized allocation of groups, the outcome assessor was blinded for treatment allocation. According to Bennett et al.,49 it is highly recommended to blind the outcome assessor, because of its role in data collection. Further, participants were not aware of the randomization sequence since it was done by an independent researcher.
The study has some limitations as well. Because uniform protocols for performing CPM are still lacking,78 we consulted a systematic review79 in which different protocols for CPM were compared. Based on our target population and methodological quality of the available CPM protocols, we followed the protocol of Neziri et al.60 However efforts were made to implement recommendations regarding CPM-testing,78 we acknowledge that generalization of our results is limited. In addition, we did not assess treatment compliance and therefore potential bias and underestimation of the treatment effect should be contemplated. There was no placebo condition and patients were not blinded. Comparing the effects of HeatTens to a sham-treatment would have increased the methodological quality. Therefore, non-specific treatment effects (e.g. treatment expectation) should be considered when interpreting these results.
Future directionsFuture research should focus on investigating the effects of HeatTens on MEP while TENS is applied and later in combination with other, more active therapy modalities for patients with CLBP. There is a need for standardized assessment tools, which are sensitive enough to capture and evoke MEP. This is required to better understand and more easily integrate MEP in standard pain measures.
ConclusionThe combination of heat and TENS seems not to reduce pain scores in patients with CLBP. PPT values significantly improved, showing beneficial effects of the experimental treatment. Increased PPT but unchanged TS and CPM suggest that a combination of heat and TENS stimulates normal central pain processing but has no influence on normalization of pain inhibitory function and that the treatment activates local rather than brain-orchestrated analgesic effects.
AcknowledgementThis research was funded by Omron Healthcare Co., Ltd., Japan. A clinical study agreement between Omron and the Vrije Universiteit Brussel was set up, allowing the Vrije Universiteit Brussel to perform independent research. Omron Healthcare was not involved in data collection data analysis or writing processes. We thank the University hospital of Brussels, the Department of Physical Medicine and Rehabilitation, and the Frailty in Ageing research department of the Vrije Universiteit of Brussel for the use of their infrastructure. We thank C. Dewaele, I. Deurinckx, and T. Secretin for their hard work during the experiment.
Conflicts of interestThe authors declare no conflicts of interest.