The minimal important difference (MID) of the Postural Assessment Scale for Stroke Patients (PASS) remains unknown, limiting the interpretation of change scores.
ObjectivesTo estimate the MID of the PASS in patients with subacute stroke.
MethodsData at admission and discharge for 240 participants were retrieved from a longitudinal study. The “mobility” item of the Barthel Index was used as the anchor for indicating the improvement of posture control. Receiver operating characteristic (ROC) method was used to estimate the anchor-based MID of the PASS.
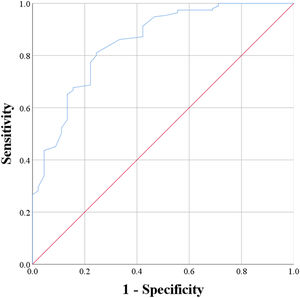
ResultsThe ROC method identified a MID of 3.0 points, with a sensitivity of 81.0 % and a specificity of 75.6 %.
ConclusionThe MID of the PASS was 3.0 points, indicating that if a patient achieves an improvement of 3.0 or more points on the PASS, they have a clinically important improvement in posture control. Our results can help in interpreting change scores and aid in understanding the clinical values of treatment outcomes.
Interpretation of the change in outcome measures is crucial for evidence-based practice.1 To date, most clinical trials have used statistical significance and effect sizes to depict the treatment effects.2,3 However, both statistical significance and effect sizes are group-level indices, which present difficulties for clinicians in predicting or interpreting the treatment effect of an individual patient when implementing evidence-based treatments in clinical settings. Additionally, statistical significance does not necessarily equate to clinical meaningfulness. A small difference may be statistically significant in a large sample. In contrast, a small sample size may be underpowered to reveal that a large and clinically meaningful difference is statistically significant.4
The minimal important difference (MID) represents the smallest change in a treatment outcome that patients or other outcome stakeholders (e.g., clinicians or researchers) should identify as meaningful or worthwhile.5,6 The MID can be interpreted at the individual level for both clinical and research purposes. For clinicians making decisions to continue or alter treatment, the MID can be used to identify whether an individual patient has a meaningful change. For researchers, the MID can be applied to quantify the proportion of patients who benefit from a treatment, showing the probability that an individual patient will respond to the treatment.
The anchor-based approach, which relates a difference in the outcome measure to a subjective or objective anchor that is a clinically relevant criterion, is recommended as the optimal approach to estimate the MID.7-9 The anchor-based MID is determined either by a mean change or a receiver operating characteristic (ROC) analysis, in reference to an anchor.6,10 Although a subjective anchor (e.g., patient global impression of change) is the most commonly used, an objective anchor may be preferable to a subjective anchor to avoid recall bias, response shift, and unreliability attributed from the patient's self-awareness.9,11,12
The Postural Assessment Scale for Stroke Patients (PASS) was specifically designed for individuals who have experienced a stroke,13 which is one of the recommended outcome measures of posture control for stroke rehabilitation.14 The psychometric properties (including reliability, validity, and responsiveness) of the PASS are satisfactory in persons with stroke.13-17 Compared to other well-known outcome measures of posture control for stroke, such as the Berg Balance Scale,18 the PASS shows slightly better psychometric characteristics, especially the ability to detect balance improvements in patients with subacute stroke who have severe balance deficits.14,19 Furthermore, the PASS has good clinical utility,20 including portability, requiring no additional cost or equipment, and ease of administration within 1–10 min (depending on the severity of balance deficits).21 However, it is unknown to what degree changes in PASS scores should be considered clinically meaningful in clinical practice or clinical trials. The lack of a MID has led to difficulty in the interpretation of changes in PASS scores. Thus, this study aimed to estimate the MID of the PASS in patients with subacute stroke using the anchor-based approach.
MethodsParticipantsA prospectively collected dataset with a sample size of 560 from a previous study was used,22 wherein persons with subacute stroke were consecutively recruited from a medical center from January 2009 to January 2012. Patients were included if they met the criteria as follows: (1) a diagnosis of stroke (intracerebral hemorrhage or ischemia); (2) stroke onset within 10 days before hospital admission; and (3) ability to follow instructions. The exclusion criteria were as follows: (1) diagnosis of other major diseases influencing their motor control before or during recruitment; (2) dysfunction of communication; and (3) unwillingness to participate. The study was reviewed and approved by the Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan. Written informed consent was obtained from all participants after screening for eligibility.
In this study, we retrieved the data of those who met the criteria as follows: (1) stayed in the rehabilitation ward longer than 14 days to ensure enough training; (2) were assessed at both admission and discharge with the PASS and the mobility item of the Barthel Index (BI-mobility); (3) had no deterioration in mobility between admission and discharge, as defined by the change score of BI-mobility. The decision to use the BI-mobility as the objective anchor to estimate the MID of the PASS was because the BI is one of the most prevalent outcome measures for stroke.23 Furthermore, the mobility item specifically targets the clinically important outcome of postural control training.24
ProcedureEligible patients were assessed at admission within 7 days and re-assessed within 3 days before discharge by the same research assistant. During the study period, all participants received usual inpatient stroke rehabilitation, including occupational, physical, and speech therapy where necessary. Each therapy was administered approximately 30 min per day, 5 days per week. Postural control and mobility training were integrated into both physical and occupational therapy. This study adhered to the COSMIN study design checklist to ensure the quality of the study.25
MeasuresThe PASS was specifically designed for assessing posture control in persons with stroke.13 It was developed as an adaptation of the Fugl-Meyer Assessment balance subscale.13 The PASS contains 12 items assessing postural performance in lying, sitting, and standing postures. Each item is scored on a four-point scale (0–3). The total score ranges from 0 to 36, with higher scores indicating better posture control and balance. The PASS has good internal consistency, inter- and intra-rater reliabilities, convergent validity, predictive validity, and responsiveness.13-17
The BI is a commonly used measure for assessing dependence in basic activities of daily living in persons with stroke. The BI consists of 10 items: feeding, bathing, grooming, dressing, bowel control, bladder control, toileting, chair transfer, mobility, and stair climbing. Each item is scored 0 to 1, 0 to 2, or 0 to 3. The reliability, validity, and responsiveness of the BI were sufficient in persons with stroke.26-28 This study used the change in BI-mobility as the anchor to estimate the MID of the PASS. BI-mobility is scored on a 4-point scale (0–3), with higher scores indicating better mobility.
Statistical analysisStatistical analyses were performed using SPSS software ver. 25.0 (IBM, Armonk, NY, USA). Demographic and clinical data were analyzed by descriptive statistics. The correlation of the change scores between the PASS and BI-mobility was computed by the Spearman rank correlation. A correlation of at least 0.30–0.35 was recommended to ensure that BI-mobility was useful for establishing the MID of the PASS.29
The MID of the PASS was computed using the ROC method. Participants were dichotomized into 0 (if change in BI-mobility score between admission and discharge = 0, labeled unchanged group) and 1 (if change of BI-mobility score between admission and discharge ≥ +1, labeled improved group). We set 1 point of change as the cutoff because one point represents the smallest but important change on the BI-mobility rating scale, which aligns with the patients’ priorities for meaningful mobility gains, like wheelchair use or increased walking distance.30 By plotting sensitivity versus false positive rate, the ROC curve analysis identifies the optimal cut-off value (in this case, the MID of the PASS) that best distinguishes the statuses (0 and 1). The point on the ROC curve closest to the upper left corner was considered as the optimal cut-off value.
ResultsAfter deleting 234 samples with incomplete PASS and BI-mobility scores at admission or discharge, 73 samples with unknown hospitalization time or no longer than 14 days, and 13 samples with deterioration in mobility during hospitalization, we retrieved the complete data records of 240 participants for analysis. Table 1 shows the detailed patient demographic and clinical characteristics. The mean age of these participants was 66.0 years. Most participants were men (61.7 %) with a diagnosis of ischemic stroke (67.5 %). The mean interval between admission and discharge assessments was 38.0 days. The mean number of days after stroke at admission and at discharge were 19.7 and 56.2, respectively. At admission, the mean PASS score was 16.2, with most of the participants (80.8 %) immobile. Twelve participants (5.0 %) scored the lowest possible PASS scores, while two (0.8 %) achieved the highest possible scores. At discharge, the mean PASS score was 27.5, with most participants able to walk with the help of one person (45.4 %) or independently (37.9 %). Two participants (0.8 %) scored the lowest possible PASS scores, while 17 (7.1 %) achieved the highest possible scores.
Demographic and clinical characteristics of the participants.
BI-mobility: Mobility item of the Barthel Index; PASS: Postural Assessment Scale for Stroke Patients.
The correlation between the change scores of the PASS and those of BI-mobility was adequate, with coefficient of 0.56 (95 % confidence interval: 0.46, 0.67), indicating that BI-mobility was an appropriate anchor for indicating the improvement of posture control.
ROC methodAmong the 240 participants, 45 participants were categorized as the unchanged group, and the remaining 195 participants were categorized as the improved group. The ROC analysis specified an optimal cutoff of 3.0 PASS change points, with a sensitivity of 81.0 % and a specificity of 75.6 %. The area under the ROC curve was 0.851. Fig. 1 illustrates the ROC curve.
DiscussionThe extent to which changes in PASS scores should be deemed clinically meaningful in clinical practice or clinical trials remains unknown. Thus, in this study, we used ROC method to estimate the anchor-based MID of the PASS in patients with subacute stroke. The results demonstrated that the MID of the PASS was 3.0 points. Such findings imply that if a patient with subacute stroke achieves 3.0 or more points of improvement on the PASS, they have a meaningful change in function. Our results can help users interpret change scores of the PASS and further understand the values of treatment outcomes on postural control.
An increasing emphasis on personalized medicine has shifted the focus of clinical trials from documenting the statistical significance and effect sizes toward not only demonstrating treatment efficacy but also characterizing those who benefit from the treatment.31 Traditionally, treatment efficacy has been demonstrated by the statistical significance and magnitudes of between-group differences in improvement. However, a statistically significant difference is simply a difference unlikely to be caused by chance. A statistically significant and non-trivial difference may be of little or no importance to the patients. For example, a previous study examined the effect of home rehabilitation on posture control in patients within 7 days after stroke onset.32 The mean change score on the PASS was 1.21 (standard deviation of 0.92 points), and the effect size d was 1.31, which was considered as a statistically significant and large effect. However, the mean change score of 1.21 is much lower than the MID of the PASS, as found in this study, which indicates that the patients did not achieve important change after receiving the home rehabilitation. The MID detects the magnitude of improvement that is meaningful to patients and the value that patients place on the change.9 The MID can aid in interpreting an individual patient's meaningful change and demonstrate the proportion of patients who benefit from treatment (i.e., responders). Furthermore, the MID may be helpful to advance personalized medicine by comparing characteristics between responders and non-responders to identify the characteristics of patients who are the most likely to respond to treatment.
Anchor-based MIDs have been recommended over distribution-based MIDs,7,8 although there is no consensus on the appropriate approaches to use. Anchor-based MIDs use a clinically interpretable criterion indicating a meaningful change in status. However, distribution-based MIDs rely on purely statistical indicators. Distribution-based MIDs, such as minimal detectable change,8,11 can only identify a change that is unlikely to be attributable to random measurement error, rather than a change that is clinically meaningful. Minimal detectable change can be useful to provide supplementary information for anchor-based MIDs about whether an important change exceeds random measurement error. For example, combining the evidence on the 95 % confidence minimal detectable change of 2.2 points of the PASS,15 an improvement of 2.2–2.9 points indicates a real (beyond random measurement error) but unimportant change, and an improvement of equal to or larger than 3.0 points indicates a real and important change. Thus, the anchor-based approach should be the optimal means to estimate MIDs,7,8 and distribution-based MIDs can be regarded as temporary substitutes for or supplements of anchor-based MIDs.
This study used an objective anchor (i.e., the BI-mobility) rather than a subjective anchor, which is more commonly used in typical MID studies.12 Due to the subjective nature of a subjective anchor, the calibration of scales is relative to internal standards in patient's minds. For example, when assessing the magnitude of change on a 15-point Likert-type scale, it is the patient who defines what is “a little bit better” and how much change in the “true” level of posture control constitutes a shift of one unit in the scale. Therefore, subjective anchors have been criticized for their inaccuracy, which results from the response shift across patients and over time, recall bias, or patient's impaired self-awareness.9,11,33 The use of an objective anchor in this study may have overcome these challenges posed by subjective anchors. However, in the absence of a gold standard for estimating the anchor-based MID, different studies may use various clinically important objective anchors to estimate the MID of a particular outcome assessment. This can lead to multiple MID values for that outcome assessment. In such instances, clinicians can utilize the range of MID values to interpret the change scores.
A prior study showed that patients admitted to a rehabilitation ward about 34.4 days after a stroke had a PASS score threshold of 12.5 points upon admission. This score was indicative of whether these patients with subacute stroke could walk more than 10 m unassisted by the time of discharge.34 Combined with the MID of 3.0 points identified in the current study, both findings can aid clinicians in clinical reasoning. For example, when setting the discharge goal for a patient approximately a month post-stroke with an admission PASS score of less than 12 points, it suggests that they would be less likely to achieve independent walking at discharge, potentially necessitating assistive devices for ambulation and environmental modifications. Meanwhile, when evaluating the clinical meaningfulness of the improvement in postural control during rehabilitation, clinicians can determine whether the MID of 3.0 points on the PASS change score has been achieved.
Three limitations are of concern in this study. First, the participants were at the subacute stage, which restricts the generalization of the results to the persons with acute or chronic stroke. Second, the MID estimates were estimated from participants with improvement in mobility, which may be different from those based on deterioration. Thus, the MID estimates in this study may apply only to improvement and not to deterioration. Last, this study was the first to determine the MID of the PASS. Because of the heterogeneity of persons with stroke, further studies cross-validating our findings are needed.
ConclusionsWe found that a 3.0 point improvement was the MID for the PASS in patients with subacute stroke. The MID of the PASS can be used as the threshold to determine whether an individual patient with stroke reaches an important change in posture control. The findings of this study can aid clinicians and researchers in interpreting change scores, making decisions to continue or alter treatment, and demonstrating individual-level treatment effects.
This work was funded by the Chung Shan Medical Universityand Chi Mei Medical Center (CSMU-CMMC-108–04). The funders did not have a role in designing the study or writing the manuscript.