Photobiomodulation (PBM) and transcutaneous electrical nerve stimulation (TENS) are used to reduce neck pain.
ObjectiveTo investigate the immediate and 1-month post-treatment effects of 10 treatment sessions of PBM and TENS delivered over 2 weeks on pain intensity in individuals with neck pain.
MethodsIndividuals with neck pain were randomized into four groups: PBM+TENS, PBM, TENS, and Sham. Primary outcome: pain intensity at rest. Secondary outcomes: pain intensity during movement, pressure pain threshold (PPT), temporal summation (TS), conditioned pain modulation (CPM), cervical range of motion (ROM), psychosocial factors, drug intake for neck pain, and global perceived effect (GPE). All outcome assessments were made pre- and post-treatment. Mean differences and 95 % confidence intervals were calculated for between-group comparisons.
ResultsA total of 144 participants were recruited. No significant between-group difference was observed for pain intensity at rest, TS, CPM, ROM, psychosocial factors, and drug intake. The PBM+TENS showed a reduction in pain intensity during movement and GPE compared to the PBM (MD: 1.0 points; 95 % CI: 0.0, 2.0; MD: 2.0 points; 95 % CI: 1.0, 3.0) and Sham (MD: 2.0 points; 95 % CI: 1.0, 3.0; MD: 2.0 points; 95 % CI: 1.0, 3.0) groups. PBM+TENS presented a medium effect size to increase local PPT compared to PBM and Sham groups. TENS presented a medium effect size to increase local PPT compared to PBM and Sham groups. TENS presented a medium effect size to increase distant PPT compared to other groups.
ConclusionsThe use of PBM or TENS was not effective for reducing pain intensity at rest. The combination of PBM and TENS was effective in improving pain intensity during movement, local hyperalgesia, and the GPE. TENS reduced local and distant hyperalgesia.
Neck pain is a common public health problem leading to disability and resulting in high economic costs.1 Its incidence is higher in women and generally increases with age.1 The condition is considered chronic when it persists for >3 months,2 and nonspecific when the etiological factor is unknown. Photobiomodulation (PBM) and transcutaneous electrical nerve stimulation (TENS) are electrophysical agents commonly used to treat chronic neck pain.2–5 A systematic review from Chow et al.6 showed that PBM using low-level laser therapy reduced chronic neck pain up to 22 weeks post-treatment.6 Another systematic review by Kadhim-Saleh et al.7 demonstrated inconclusive evidence due to the heterogeneity of the studies and high risk of bias, in addition to the results, albeit significant, showing no minimally important clinical difference.7 These inconsistent findings on the effects of PBM in reducing chronic neck pain highlight the need for further research. Furthermore, there is a significant lack of studies comparing PBM to analgesic electrical currents. Therefore, investigations that both compare and combine PBM with TENS are particularly important and necessary.
In terms of TENS, the latest systematic reviews reported very low quality evidence for the use of TENS when compared to a sham treatment in individuals with neck pain.3,8 This finding was mainly due to the high heterogeneity of studies and high risk of bias. Hence, clinical trials with larger sample sizes with low risk of bias and adequate TENS application9 are still needed in this field.
PBM and TENS are both commonly used in clinical practice to reduce neck pain. However, to date, no studies have investigated whether PBM is superior to TENS, or if combining these electrophysical agents in the same session may enhance the analgesic effect. Given their different action pathways for analgesic production, it is possible that combining both interventions may result in a faster and/or longer-lasting analgesic effect. Therefore, this study investigated whether the effect of PBM and TENS alone or the combined effect of PBM and TENS on pain intensity, central sensitization (i.e., pressure pain threshold [PPT], temporal summation [TS] of pain, conditioned pain modulation [CPM]), cervical range of motion (ROM), psychosocial factors (i.e., depressive symptoms, neck disability, catastrophizing of pain, and quality of life), drug intake for neck pain, and global perceived effect compared with sham in individuals with nonspecific chronic neck pain.
MethodsStudy designThis 4-arm double-blind randomized controlled trial with individuals with neck pain followed the “Consolidated Standards of Reporting Trials (CONSORT),”10 and was approved by the Federal University of São Carlos (UFSCar) Human Research Ethics Committee (CAAE: 81,711,417.0.0000.5504) and registered prospectively at Clinical Trials (NCT04020861). The protocol of this clinical trial was previously published11 and the study was conducted at the Physiotherapy Resources Research Laboratory (LAREF) of the UFSCar Department of Physical Therapy.
ParticipantsIndividuals with neck pain were recruited via electronic media, posters, and oral communication at UFSCar and city of São Carlos (São Paulo state, Brazil). Individuals of both sexes, aged between 18 and 65 years, with nonspecific neck pain for three months or more at rest, at intensity ≥ 3 out of 10 according to the numeric rating scale (NRS)12–15 were considered eligible for this study. Pain may be local and/or described for adjacent areas in the upper back region (unilateral or bilateral). In addition, individuals had to report a minimum score of 5 points on the Neck Disability Index (NDI),13,16 and all participants gave written informed consent. Exclusion criteria were previously described in detail in the protocol.11
Sample size calculationSample size was calculated based on pain intensity, measured by the NRS, with a difference of 2.3 points and standard deviation (SD) of 2.8.17 A statistical power of 80 %, 5 % alpha, and possible 15 % sample loss were considered. Thus, a total of 144 individuals were needed to perform the study (n = 36 per group). Sample size was calculated using Minitab software, version 17 (Minitab, Inc., PA).
RandomizationRandomization was performed by a researcher not involved in recruitment of participants or data collection from participants, using the website www.randomization.com, where participants were randomly (random block sizes - 144 individuals randomized into 18 blocks) distributed into 4 groups matched for sex (n = 36 per group): PBM + TENS, PBM, TENS, or Sham. Opaque sealed envelopes were used to maintain anonymous allocation and kept in a locked drawer to which only the researcher had access. The envelope was opened only after the participant was deemed eligible by the research team.
ProcedureThe area of electrophysical agent application was established daily according to the pain reported by the individuals and palpation by the physical therapist responsible for the treatment. Ten consecutive 60-minute treatment sessions were providing (excluding weekends) at the same time of day for two weeks.
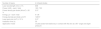
InterventionsPhotobiomodulation (PBM) was performed using low-level laser therapy (active or sham) with an Antares IBRAMED® device (Indústria Brasileira de Equipamentos Médicos, Amparo, São Paulo state, Brazil). The Cluster P1 applicator, which has 4 diode lasers emitting at a wavelength of 808 nm, was used. Table 1 presents the programmed parameters. PBM was applied as a sham with the same equipment. To simulate PBM application, the therapist turned on the device, simulated adjusting the parameters, placed the cluster on each painful area and 50 s was timed, with a sound emitted before and after the stipulated time, but no laser beam.
PBM parameters for cluster.
PBM, photobiomodulation; nm, nanometers; mW, milliWatts; W/cm2, Watts per square centimeter; J, Joules; J/cm2, Joules per square centimeter; cm2, square centimeter; s, seconds
Transcutaneous electrical nerve stimulation (TENS) was applied using a portable Neurodyn IBRAMED® device (Indústria Brasileira de Equipamentos Médicos, Amparo, São Paulo state, Brazil), which emits a balanced asymmetrical biphasic pulsed current. Two or four self-adhesive electrodes (5 × 5 cm) (ValuTrode, Axelgaard, CA, USA) were placed on the painful area. The parameters selected were frequency of 100 Hz, pulse duration of 250 µs, and strong but comfortable intensity, as described by the individual. Application time lasted 30 min, and if necessary, intensity was adjusted every 5 min. A digital oscilloscope (TDS 430A, Tektronix Inc, Beaverton, OR) was used during the study to calculate the current amplitude (mA). The portable IBRAMED® Neurodyn device was used to apply TENS as a sham and adjusted to emit a balanced asymmetrical biphasic pulsed current for 30 s, followed by a gradual decrease in current emission for 15 s. Thus, the device was active for 45 s and the individuals were subjected to the current until their sensory threshold was reached within this period. However, even after 45 s, the device was kept operating with a light on to simulate current emission until 30 min of application time. Intensity was increased until the sensory threshold of individuals and every five minutes they were asked if they felt comfortable. This sham application of TENS was validated, does not reduce pain, and therefore provides a true sham treatment.18
Outcome measuresPain intensity at rest post-treatment (primary outcome) and pain intensity during movement were evaluated using the numeric rating scale (NRS).12 Local (12 points in the neck and shoulder girdle areas) and distant (1 point on the right tibialis anterior muscle) PPTs11 were measured using a Somedic Type II pressure algometer (Somedic®, Hӧrby, Sweden). The absolute mean of the PPT values at the 12 points assessed were used to generate topographical pressure pain sensitivity maps of the neck and shoulder girdle areas19 in Matlab (The Mathworks, Natick, MA, USA). TS of pain was assessed in the upper trapezius muscle on the more painful or dominant side. The higher the value, the greater the TS of pain.11 To assess CPM, the cold pressor test was applied as conditioning stimulus and the PPT of the upper trapezius on the less painful or non-dominant side was used. To analyze CPM effectiveness, the average value before immersion was subtracted from the post-immersion value. The lower the latter value, the lower the effectiveness of endogenous pain inhibition.11
A fleximeter20 (Sanny, São Paulo, SP, Brazil) was used to measure active cervical ROM. Neck disability was measured using the Neck Disability Index (NDI). Psychosocial factors were assessed considering the presence of depressive symptoms (Beck Depression Inventory [BDI], scores range from 0 to 63 points, and scores higher than 15 detect dysphoria and scores over 20 indicate depression),21 pain catastrophizing (Pain Catastrophizing Scale (PCS), total score ranges from 0 to 52 points, higher scores indicate greater catastrophizing of pain),22 and quality of life (12-Item Short-Form Health Survey – version 2 (SF-12v2). This is a self-report measure that assesses physical (physical component summary—PCS) and mental (mental component summary - MCS) health on a scale of 0 to 100. Higher scores represent better levels of quality of life).23 Global perceived effect (GPE) was assessed using the Global Perceived Effect Scale. It consists of an 11-point scale that ranges from –5 (vastly worse) through 0 (no change) to 5 (completely recovered). The patients were asked “Compared to when this episode first started, how would you describe your back these days?” A higher score represents a better condition.24 Drug Intake for Neck Pain: All individuals were asked to record all the neck pain medication taken during the week prior to treatment onset. Opioid analgesics were converted into morphine equivalent dose.25 Non-opioid analgesics were converted according to the equivalence table for acetaminophen.26 Information on intraexaminer reliability can be accessed in the Supplementary material online.
Outcomes were assessed by an independent assessor before and after the 10 treatment sessions. One month after the study, the examiner followed-up via telephone to reassess pain intensity at rest and GPE.
Blinding assessmentAfter reassessment, the assessor and the participants declared whether they thought that PBM and TENS were real, sham, or that they did not know. The answers were used to measure blinding effectiveness in participants with neck pain and the assessors. Therapists were not blinded in this study.
Statistical analysisStatistical analysis was conducted according to intention-to-treat analysis, meaning that all participants were analyzed in the group they were allocated. Data imputation was performed for the missing values using expected maximization.27 The Kolmogorov-Smirnov test was used to determine data normality. Descriptive data for the variables with non-normal distribution were presented as median, interquartile range, difference in medians and 95 % confidence interval (CI) and normally distributed data as mean, standard deviation, mean difference (MD), and a 95 % CI.
The variables difference in pain intensity at rest and during movement, TS of pain, cervical disability, depressive symptoms, drug intake for neck pain, and GPE showed non-normal distribution. As such, the Kruskal-Wallis test with post-hoc Mann-Whitney U test was applied for within-group comparison considering the score change between timepoints: pre and post-treatment, and pre and follow-up for pain intensity at rest and GPE; pre and post-treatment for pain intensity during movement, TS of pain, cervical disability, depressive symptoms, and drug intake for neck pain. Differences compared using with the Mann-Whitney U test were considered significant only for values below 0.008 (the Bonferroni correction: 0.05/6).
Given that the variables PPT, CPM, ROM, pain catastrophizing, and quality of life exhibited normal distribution, mixed factorial analysis of variance (ANOVA) with Tukey's post hoc was applied (between group factor: PBM+TENS, PBM, TENS, Sham; time factor: pre/post), with significance set at p < 0.05. Blinding success was analyzed using the chi-squared test. Data were double entered in Microsoft Excel 2016. IBM SPSS Statistics for Windows was used for data processing.
ResultsA total of 144 individuals with neck pain were considered eligible to participate in the study (Fig. 1). Clinical and demographic characteristics for each group are described in Table 2. A total of 360 treatment sessions per group were offered. There were 27 absences in the PBM + TENS group (92.5 % present); 69 in the PBM group (80.8 % present); 36 in the TENS group (90 % present), and 43 in the Sham group (88 % present). In the PBM group, four participants were lost at the post-treatment time point of assessment and three participants at the 1-month follow-up. The reasons for loss to follow-up were: moved to another city (n = 1), health problems not related to neck pain (n = 4), unable to contact (n = 1), and personal reasons (n = 2). Average PBM energy (J) and average current amplitude (mA) applied in the groups were 1836 J and 38.7 mA in the PBM + TENS group, 1692 J and 0 mA in the PBM group, 0 J and 40.1 mA in the TENS group, and 0 J and 0 mA in the Sham group. The laser diodes emitted 170, 172, 171, and 170 mW, respectively, prior to the beginning of the study, and 170, 164, 170, and 169 mW, respectively, at the end. A digital oscilloscope (TDS 430A, Tektronix Inc, Beaverton, OR) was used during the study to calculate the current amplitude (mA). Antares IBRAMED® device was calibrated by the IBRAMED® to verify diode output power before and at the end of the study.
Demographics and clinical characteristics of the patients for each group.
Data are mean (SD) or n ( %). BMI, body mass index; N, number of patients; NDI, neck disability index; NRS, numerical rating scale (0–10); PBMT, photobiomodulation therapy; SD, standard deviation; TENS, transcutaneous electrical nerve stimulation; %, percentage.
Table 3 summarizes median (interquartile range) of pain intensity at rest and during movement, TS of pain, neck disability, depressive symptoms, and GPE overtime according to group. There were no significant between-groups differences for changes in pain intensity at rest from baseline to end of intervention, and from baseline to one-month follow-up (Table 4). Pain intensity during movement declined significantly more in the PBM+TENS compared to the PBM (MD: 1.0 points; 95 % CI: 0.0, 2.0) and Sham (MD: 2.0 points; 95 %CI: 1.0, 3.0) groups (Table 4).
Median [IQR] of pain intensity at rest and during movement, temporal summation of pain, neck disability, depressive symptoms, and global perceived effect overtime according to group from an intervention-to-treat analysis.
| Outcomes | Pre | Post | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBM + TENS(n = 36) | PBM(n = 36) | TENS(n = 36) | SHAM(n = 36) | PBM + TENS(n = 36) | PBM(n = 36) | TENS(n = 36) | SHAM(n = 36) | PBM + TENS(n = 36) | PBM(n = 36) | TENS(n = 36) | SHAM(n = 36) | |
| Pain intensity at rest(NRS: 0 - 10) | 5.0[4.0, 6.0] | 5.0[4.0, 6.0] | 6.0[4.0, 7.0] | 4.5[3.3, 6.0] | 1.0[0.0, 2.0]a | 1.8[0.0, 3.0] a | 0.5[0.0, 2.0] a | 1.0[0.0, 2.0] a | 1.0[0.0, 3.0]b | 2.0[0.0, 3.0]b | 2.0[0.0, 3.8] b | 2.0[0.5, 4.0] b |
| Pain intensity during movement(NRS: 0 - 10) | 6.0[5.0, 8.0] | 7.5[5.0, 9.0] | 7.0[5.3. 8.0] | 6.0[5.0, 7.8] | 2.0[1.0, 3.0]a | 4.0[3.0, 6.0] a | 3.0[2.0, 5.0] a | 3.5[2.0, 6.8] a | NA | NA | NA | NA |
| Temporal Summation of Pain(NRS: 0 - 10) | 1.0[1.0, 2.0] | 1.0[1.0, 2.0] | 2.0[1.0, 3.0] | 1.0[1.0, 2.0] | 0.5[0.5, 1.0]a | 1.0[0.0, 1.0] | 1.0[0.0, 1.8] | 1.0[0.0, 2.0] | NA | NA | NA | NA |
| Neck Disability(NDI: 0 - 50) | 12.5[7.2, 15.0] | 13.0[10.0, 17.5] | 12.0[10.0, 15.7] | 11.0[7.3, 15.0] | 6.0[4.0, 10.0] a | 8.5[6.0, 12.0] a | 8.5[6.0, 12.0] a | 7.0[4.0, 11.0] a | NA | NA | NA | NA |
| Depressive symptoms(BDI: 0 - 63) | 7.5[5.0, 13.0] | 11.5[6.0, 18.8] | 9.5[6.0, 13.0] | 10.0[7.0, 13.0] | 7.0[2.3, 11.8] a | 10.0[5.3, 14.8] a | 7.0[4.0, 11.0] a | 6.0[3.0, 10.0] a | NA | NA | NA | NA |
| Global Perceived Effect(−5 a 5) | −2.0[−3.0, 0.0] | −1.0[−3.0, 0.0] | −2.0[−3.0, 0.0] | 0.0[−3.0, 0.0] | 4.0[3.0, 5.0]a | 3.0[1.0, 4.0]a | 4.0[3.0, 4.0]a | 3.0[1.0, 4.0]a | 3.0[2.0, 4.8]b | 2.0[0.3, 3.8]b | 3.0[1.3, 4.0]b | 2.0[0.0, 3.0]b |
BDI, beck depression inventory; IQR, interquartile range (Q1-Q3); Med, median; n, number of subjects; NA, not applicable; NDI, neck disability index; NRS, numerical rating scale; PBM, photobiomodulation; TENS, transcutaneous electrical nerve stimulation.
Estimated median differences (95 % CI) between groups for pain intensity at rest and during movement, temporal summation of pain, neck disability, depressive symptoms and global perceived effect for all timepoints from an intention-to-treat analysis.
Data highlighted in gray represent a significant difference between groups; For Mann-Whitney test, the data highlighted in gray represent a significant difference between group: p ≤ 0.008 (Bonferroni correction 0.05/6). CI, Confidence interval; NRS, numerical rating scale; PBM, photobiomodulation; TENS: transcutaneous electrical nerve stimulation; *Positive values indicate better mean in the first mentioned group.
PBM + TENS increased PPT significantly at most points in the neck and shoulder girdle areas compared to PBM (MD: 33.3 kPa; 95 %CI: 10.5, 56.0); and Sham (MD: 34.5 kPa; 95 %CI: 11.7, 57.3) groups (Tables 5 and 6 - Supplementary material online). TENS increased local PPT compared to PBM (MD: 35.7 kPa; 95 %CI: 12.9, 58.5); and Sham (MD: 36.9 kPa; 95 %CI: 14.2, 59.7) groups. TENS increased distant PPT compared to PBM+TENS (MD: 41.8 kPa; 95 %CI: 4.5, 79.1), PBM (MD: 57.1 kPa; 95 %CI: 19.7, 94.4), and SHAM (MD: 73.4 kPa; 95 %CI: 36.1, 110.8) groups (Tables 5 and 6 – Supplementary material online). Fig. 2 shows the topographical maps of individuals with neck pain of each group pre- and post-treatment.
Pressure pain sensitivity maps of the neck and shoulder girdles areas for each group pre- and post-treatment. The maps show higher PPTs in PBM+TENS and TENS groups compared to the PBM and Sham groups post treatment.
PBM, Photobiomodulation; PPT, Pressure Pain Threshold; TENS, Transcutaneous electrical nerve stimulation.
No significant between-group differences were observed for TS of pain, neck disability, depressive symptoms (Table 4), CPM, ROM, pain catastrophizing, and quality of life (Tables 7 and 8 - Supplementary material online).
No significant between-group difference was observed for drug intake for neck pain (Morphine equivalence, acetaminophen equivalence). The drugs that did not allow morphine or acetaminophen equivalence were segmented according to each component for analysis. No significant between-group difference was observed (carisoprodol, ketoprofen, lysine clonixinate and meloxicam (Mobic), adiphenine hydrochloride, promethazine hydrochloride, nimesulide, ketorolac trometamol, diclofenac, dipyrone, orphenadrine, isometheptene, and cyclobenzaprine).
The difference in GPE post-treatment in relation to treatment onset was significantly higher in the PBM+TENS compared to the PBM (MD: 2.0 points; 95 %CI: 1.0, 3.0) and Sham (MD: 2.0 points; 95 %CI: 1.0, 3.0) groups (Table 4). At 1 month follow-up the improvement was kept in the PBM+TENS compared to the Sham (median difference: 2.0 points; 95 %CI: 1.0, 4.0) (Table 4).
The examiner correctly identified the subjects who received active PBM treatment in 47.2 % of the cases (34 of 72). In the group that received sham PBM, the examiner correctly identified the treatment in 11.1 % of the cases (8 of 72). In other words, the examiner was blind 70.9 % of the time, which indicated successful blinding (p < 0.001). The examiner correctly identified that the subjects received active TENS treatment in 47.2 % of the cases (34 of 72). In the group that received sham TENS, the examiner correctly identified treatment in 30.6 % of the cases (22 of 72). In other words, the examiner was blind 61.1 % of the time, demonstrating successful blinding (p = 0.007).
The individuals were blind to the treatment 63.9 % (46 of 72) of the time in the active PBM group and 86.1 % (62 of 72) of the time in the sham PBM showing successful blinding (p < 0.001). The active TENS individuals were blind to treatment 6.9 % (5 of 72) of the time, and the sham TENS group 61.1 % (44 of 72) of the time. In the former group, the treatment was more frequently identified as correct than chance guesses (p < 0.001), showing that the individuals were not blind to the treatment. By contrast, the sham TENS group was not different from randomness (50:50 random probability), suggesting adequate blinding (p = 0.059). No severe adverse effects were observed, and more details can be accessed in the Supplementary material online.
DiscussionTo the best of our knowledge, this is the first randomized controlled trial to investigate the efficacy of PBM and TENS in chronic neck pain. Our findings show that there was no significant intergroup difference for self-reported pain intensity at rest. Interestingly, for pain intensity during movement, the combined use of PBM and TENS was found more effective than PBM alone and Sham. In addition, the combined use of PBM and TENS improved local hyperalgesia and GPE compared to PBM alone and Sham post-treatment and compared to Sham at one month follow-up. TENS reduced local hyperalgesia compared to PBM and Sham, and distant hyperalgesia compared to all groups.
The hypothesis of this study was confirmed for pain intensity during movement and GPE. We believe that this additional analgesic effect is related to the different mechanism of action to achieve analgesia between these resources. The analgesic efficacy of TENS, primarily high-frequency TENS (> 50 Hz) ,28 is considered related to: δ-opioid receptor activation in the rostroventromedial medulla,29,30 M1M3 muscarinic receptor activation,31 α2A-adrenergic receptor activation,32 increased GABA neurotransmitter release and GABA receptor activation,33 increased dynorphin A concentration34 and β-endorphins,35,36 a decline in aspartate and glutamate release,37 and a decrease in substance P in the dorsal root ganglion of the spinal cord.38
Unlike TENS, the analgesic efficacy of PBM seems to have a relation with opioid release,39 decreasing oxidative stress and fatigue,40,41; increasing serotonin42,43 and inhibiting transmission in the neuromuscular junction.44 The fact that no studies that investigated the efficacy of these resources in combination or the superiority of one were found, precluded making comparisons.
According to our results, isolated PBM with the protocol used, was not effective for patients with chronic neck pain. Our findings corroborate those of studies that reported no difference between PBM and Sham for the following outcomes: pain intensity at rest,45–47 local hyperalgesia,46 active ROM,45,46 quality of life,48,49 and drug use for neck pain.47 By contrast, some studies have observed benefits of PBM compared to Sham in improving pain intensity at rest5,17,49–51 or during movement,17,51 local hyperalgesia, active neck ROM51 and neck disability,50,51 depressive symptoms,17 and quality of life.5,17
With respect to TENS, our findings agree with other studies that reported no differences between TENS and Sham for pain intensity at rest.48,52 Moreover, our findings agree with those of other studies that found TENS to be superior to Sham in decreasing local hyperalgesia.52–54 However, the literature is inconsistent, given that some studies reported differences for pain intensity at rest53 or during movement,52 and no differences for local hyperalgesia48,54 between TENS and Sham. Although TENS alone was better than PBM in decreasing local hyperalgesia, it was not possible to compare these findings with those of other studies, because none have compared these therapies. A significant decrease in distant hyperalgesia was also observed after TENS application compared to the other groups. This suggests that TENS could have a central effect on pressure pain sensitivity.
It is important to emphasize that in this study, over 60 % of the time the examiner was blind to the type of treatment (active or sham) that patients received during PBM and TENS application. Individuals with neck pain were also blind to PBM (active or sham) and for TENS (sham) 60 % of the time. These data show the blinding success of the study, consistent with earlier investigations.18,55–57
The present study exhibited strong points, such as being the first to our knowledge to investigate the efficacy of combined use of PBM and TENS in individuals with nonspecific chronic neck pain, and to compare these electrophysical agents. The adherence of individuals with chronic pain was high in relation to the proposed treatment. Limiting factors were the lack of blinding for therapy due to the type of interventions applied, the lack of evaluating psychosocial factors in the follow-up period, and PBM and TENS are not in the first line of recommendations for the treatment of neck pain. Although, we have found few statistically significant results, we believe that this well-designed clinical trial and according to the proposed protocol, makes an important contribution to science-based rehabilitation practice.
ConclusionPBM and TENS were not effective to alter resting pain. However, the combination of PBM and TENS improved pain during movement, local hyperalgesia, and GPE. In addition, TENS reduced local and distant hyperalgesia. Future studies using different protocols are recommended, mainly for PBM and the additional effect of these therapies on exercises or pain education.
The authors gratefully acknowledge the individuals who participated in this study, “Indústria Brasileira de Equipamentos Médicos – IBRAMED®, Amparo, São Paulo, Brazil, which provided the ANTARES and portable Neurodyn devices and technical assistance.
This work was supported by the “Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2017/26447–2)”. FAPESP had no role in study design, collection, management, analysis, and interpretation of data, writing of the report, or the decision to submit the report for publication, nor will they have ultimate authority over any of these activities.