Insufficient sleep is common nowadays and it can be associated with chronic pain.
ObjectiveTo describe the main polysomnographic findings in patients with chronic musculoskeletal pain and to estimate the association between sleep quality, polysomnography variables and chronic musculoskeletal pain.
MethodsThis cross-sectional research analyzed a database from polysomnography type 1 exams results and then collected data via an electronic form from these patients. The form collected sociodemographic data and presented clinical questionnaires for measuring sleep quality, sleepiness, pain intensity and central sensitization signs. Pearson's correlation coefficient and odds ratio were used to estimate the associations.
ResultsThe mean age of the respondents was 55.1 (SD 13.4) years. The mean score of the Central Sensitization Inventory showed signs of central sensitization (50.1; SD 13.4) in the participants. Most patients (86%) had 1 or more nocturnal awakenings, 90% had one or more episodes of sleep apnea, 47% had Rapid Eye Movement sleep phase latency greater than 70–120 min and the mean sleep efficiency among all participants was 81.6%. The Pittsburgh Sleep Quality Index score was correlated with the CSI score (r = 0.55; 95% CI: 0.45, 0.61). People with central sensitization signs have 2.6 times more chance to present sleep episodes of blood oxygen saturation below 90% (OR = 2.62; 95% CI:1.23, 6.47).
ConclusionMost people with central sensitization signs had poor sleep quality, night waking episodes and specific disturbances in sleep phases. The findings showed association between central sensitization, sleep quality, nocturnal awakening, and changes in blood oxygen saturation during sleep.
Chronic pain affecting individuals of different age groups worldwide is currently a major challenge for clinicians.1 Due to the complexity of chronic pain and its impacts on quality of life, possible factors associated with this clinical condition have been widely discussed.2-10 Sleep disorders is one of the possible variables associated with chronic pain. The mechanisms for pain control and responses to sleep deprivation present overlapping neurophysiological components.3-5 Although a causal relationship between these variables is still unclear many findings support an association between them.3,5,10,11
Insufficient sleep is quite common and it can be associated with abnormalities in peripheral and central inflammatory responses.2,10,11 Central sensitization is a common feature of patients with chronic musculoskeletal pain and it can be associated with somatosensory brain cortex changes and pain distribution.2,7,11 The clinical management of these patients is complex and clinicians need to evaluate several aspects likely involved. Patients with chronic pain can present problems in sleep continuity, sleep architecture, and low scores in patient-related questionnaires for sleep quality.10-12 Insomnia is reported by 80 to 90% of individuals requesting treatment for chronic pain9,10 and the lack of restorative sleep can also lead to daytime sleepiness, fatigue, and concentration difficulties, ultimately causing major impact on an individual's work and daily living activities.9-12
Chronic pain and its impact on sleep needs further investigation. Studies about which sleep architecture variables can be associated with chronic musculoskeletal pain are scarce in the literature. The assessment of sleep quality can be performed using different measuring instruments, from observation by clinical questionnaires to accurate measurement of different physiological variables.13-17 Type 1 polysomnography (PSG) is currently considered the gold standard approach to diagnostic sleep disorders.13,14 The combination of this objective measurement instrument with clinical ones that can provide patient-centered assessment, such as validated clinical questionnaires, might combine to explain the impact of sleep architecture and sleep quality in daily life activities of patients with chronic musculoskeletal pain. Thus, this study aimed to describe the main polysomnographic findings in patients with chronic musculoskeletal pain and to estimate the association between sleep quality, polysomnography, and chronic musculoskeletal pain.
MethodsStudy designThe study was performed in two stages. In the first stage, we conducted a retrospective observational study with electronic medical records from a sleep medicine service of a University Hospital, in Rio de Janeiro, Brazil. In the second stage, we conducted a cross-sectional study using data provided directly by patients who completed an electronic form. The study was performed in accordance with the Helsinki Declaration. To access the patient's database the principal investigator requested authorization from the sleep medicine service chief and the study obtained approval from the Research Ethics Committee of Centro Universitário Augusto Motta (CAAE: 30075120.9.0000.5235). The informed consent form was included in the first part of the electronic form and all patients registered their consent before answering the questions. This research report followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
ParticipantsInclusion criteria were patients with pain complaint at the moment of the polysomnographic exam and collection of personal data; pain complain for more than three months; undergoing a polysomnographic type 1 exam within the three months prior to the moment of the database query; and age equal or above 18 years. Exclusion criteria were patients with history of musculoskeletal trauma in the last 3 months; positive test for COVID-19 in the last 6 months; arthritic disorders; diagnosed with or in cancer treatment; cognitive impairments or dementia (registered in clinical files or identified at the moment of the first screening, considering any difficulty to talk on the phone); severe respiratory or cardiovascular diseases; surgical intervention in the last 6 months.
The sample size calculation indicated a minimum of 46 participants, based on a power of 80%, a type-I error of 5%, and a moderate correlation between variables (r = 0.4).10 The calculation was performed using the GPower 3.1 software.
ProceduresStage 1 of the study compiled data extracted from an existing database containing biological signals of type 1 PSG exams from more than 200 patients. The equipment utilized was a BrainNet - BNT 36 with a 36-channel biological signal amplifier, compatible with electroencephalogram (EEG), brain mapping, video-EEG, and PSG.
The protocol utilized by the sleep medicine service followed the American Academy of Sleep Medicine standards and utilized the Rechtschafen and Kales criteria for performing and interpreting the exams.14 All PSG exams were read manually, reviewed, and approved by pulmonologists and cardiologists trained in sleep medicine. The protocol required the patients to arrive two hours before the exam to get used with the PSG environment and to allow exam preparation. During all exams a trained technician observed the patients through cameras and recorded the patient's behavioral variables and also any additional technical comments such as the patient's sleeping position.
The PSG exam consisted of simultaneously recording physiological variables during sleep. The protocol measured the following parameters: (1) four channels for the EEG, (2) two channels for the electro-oculogram (EOG), (3) five channels for the surface electromyography (EMG) of the digastricus, masseter and tibialis anterior muscles, (4) one channel for electrocardiogram (ECG), (5) two channels for airflow, (6) chest and abdominal ventilatory effort straps, (7) snoring sensor, (8) body position evaluation, and (9) peripheral oxyhemoglobin saturation and heart rate using digital pulse oximetry.13,14
The following PSG-related measurements were obtained: 1) percentage of sleep stages N1, N2, N3, and rapid eye movement (REM); 2) number of micro-awakening events; 3) presence or absence of snoring; 4) number of obstructive apnea or hypopnea events; 5) number referring to apnea and hypopnea index; 6) total sleep time (TTS); 7) sleep efficiency: TTS/ Total recording time (TTR); 8) sleep onset latency; 9) latency for REM sleep and for other sleep stages; 10) durations (minutes) and proportions of TTS sleep stages; 11) total number and index of apneas and hypopneas (AHI) per hour of sleep; 12) saturation values and oxyhemoglobin desaturation events (falls > 3 or 4%, within 10 s); 13) total number and index of periodic lower limb movements per hour of sleep; 14) total number and index of micro-awakenings per hour of sleep and their relationship with respiratory events or leg movements; and 15) rhythm and heart rate.14 The presence of systemic arterial hypertension, diabetes, heart failure, and any lung disease was also collected from the clinical files.
In stage 2, all patients from the database with no more than three months since the exam results were contacted by telephone for confirmation of eligibility criteria and explanations about the project. Patients who met the criteria and agreed to participate in the study received by email the questionnaires for additional data and consent statement. Clinical measurement instruments, previously validated in the Brazilian context, were compiled into a single electronic form, which used a free platform for submitting questions and recording responses (Google Forms™). The form also collected sociodemographic variables, such as: age, educational level, weight, and height.
To assess daytime sleepiness, the Brazilian version of the Epworth Sleepiness Scale (ESS) was applied. The ESS is a self-administered questionnaire with 8 questions. Respondents are asked to rate, on a 4-point scale (0–3), their usual chances of falling asleep while engaged in eight different activities. The ESS global score is the sum of 8 item scores, and it can range from 0 to 24. Higher scores indicate higher propensity of sleepiness in daily life activities.17
The Brazilian version of the Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality. The PSQI consists of 19 self-administered questions and five questions rated by the bedpartner or roommate. The latter five questions are used for clinical information only, and are not included in the scoring of the instrument. The 19 self-rated questions are grouped into seven dimensions (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction). Each item is weighted equally on a 0–3 scale and the seven dimensions scores are then summed to yield a global PSQI score. Higher scores indicate worse sleep quality.16 Both questionnaires had already passed through a cross-cultural validation process and also had already shown excellent psychometric measures considering construct validity, content validity, and reliability measures.15-17
The Numerical Pain Rating Scale (NPRS) was used to measure pain intensity. It requires the patient to rate their pain on a 0–10 points scale, considering 0 no pain and 10 the worst pain possible.18 To identify signs of central sensitization in the sample, the Brazilian version of the Central Sensitization Inventory (CSI) was used. The CSI is a self-report outcome measure designed to identify patients who have symptoms that may be related to central sensitization. The instrument is divided in two parts. Part A assesses 25 health-related symptoms that are common to central sensitization symptoms, with total scores ranging from 0 to 100. Part B (which is not scored) asks if one has previously been diagnosed with one or more specific disorders.19 The presentation of all instruments and the answer options in the online forms were identical to the original instruments.
Data analysisAll numeric variables presented a normal distribution according to the Kolmogorov-Smirnov test. The numeric variables were described using means and standard deviations and categorical variables by absolute and relative frequencies. To identify association between sleep quality scores, sleepiness scores, main PSG findings, and pain variables, the Pearson's correlation coefficient was used for numerical variables and odds ratio (OR) for dichotomous variables, both reported with 95% confidence intervals. The analyses were performed using IBM SPSS Statistics for Windows, Version 23.0, with a significance level of 5% (two-sided).
ResultsBased on the number of eligible PSG exams, a total of 80 individuals were contacted to participate in the study. Among these individuals, three refused and 12 did not meet the eligibility criteria. Out of the 65 participants who agreed to participate answering the survey, 47 returned the electronic forms, representing a return rate of 72%. None of these participants presented missing data.
The mean age of the respondents was 55.1 (SD 13.4) years, 62% were females and 26% were more than 60 years old. The mean body mass index (BMI) was 31.3 kg/m2 (SD 8.1); 70% of the participants were overweight and obese and 6% were severely obese. Concerning presence of comorbidities, 74% of the participants had systemic arterial hypertension and 53% had diabetes. Most of the population presented low educational level at school (from 6 to 9 years of study).
The mean pain intensity was 6.7 (SD 2.3). The sample had an average of 163 (SD 13) days pain duration. The mean score on the CSI was 50.1 (SD 13.4) points and 72% of participants presented a score of more than 40 points, which is the cutoff point for central sensitization signs. The PSQI had a mean score of 10.9 (SD 2.8) points and the ESS a mean score of 10.2 (SD 7.3) points. It was determined that 53.1% of the participants had ESS abnormal scores and possible pathological sleepiness. Table 1 presents the sample characteristics.
Sample characteristics.
Abbreviations: CSI, Central Sensitization Inventory; ESS, Epworth Sleepiness Scale; NPRS, numeric pain rating scale; PSQI, Pittsburgh Sleep Quality Index.
Based on the main PSG variables, 90% of the sample had one or more episodes of sleep apnea. The mean frequency of apneas was 71.4 (SD 13.2) times in one night, the mean time of each apnea was 17.3 (SD 3.1) seconds, and 59.6% of these individuals had oxygen blood saturation values below 90% during apnea episodes. The apnea and hypopnea index per hour of sleep showed that most individuals (65%) fell into a moderate or severe sleep apnea category.
Most patients (86%) had 1 or more nocturnal micro-awakenings (sleep interruptions lasting from 3 to 15 s), and 46.51% had REM sleep phase latency (time elapsed from sleep onset to first record of REM sleep) greater than 70–120 min. The mean sleep efficiency of the sample was 81.6%. Table 2 shows the mean values of the main PSG findings.
Polysomnography findings.
| Variables | Mean (SD) | Reference values |
|---|---|---|
| Total sleep time (min) | 382 (27.2) | 420 - 540 |
| REM sleep phase latency (min) | 112 (21.3) | 70–120 |
| Sleep beginning latency (min) | 16 (6.9) | >5 |
| Sleep efficiency (%) | 81.7* (9.4) | >85 |
| Sleep Phase I (%) | 20.7* (8.2) | 2–5 |
| Sleep Phase II (%) | 51.9 (9.3) | 45–55 |
| Sleep Phases III and IV (%) | 16.8 (7.2) | 13 −23 |
| Blood oxygen saturation during sleep (%) | 93.5 (6.4) | >90% |
| Number of micro-awakenings (in an hour) | 25* (11.4) | 10 |
| AHI | 27.3* (10.8) | Light: until 5Moderate: from 6 to 15Severe: >30 |
| REM sleep phase (%) | 10.4* (6.2) | 20–25 |
Abbreviations: AHI, apnea and hypopnea index (per hour of sleep); REM, rapid eye movement.
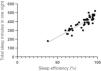
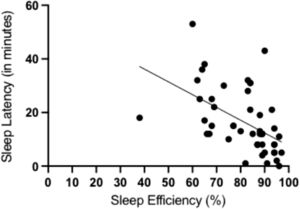
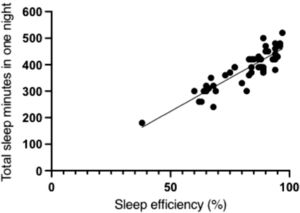
A negative correlation was found between sleep efficiency and sleep latency (r = −0.51; 95% CI: −0.57, −0.43) (Fig. 1), and there was a positive correlation between total sleep time and sleep efficiency (r = 0.88; 95% CI: 0.74, 0.93) (Fig. 2). A positive correlation was also found between the number of nocturnal micro- awakenings and the number of nocturnal apneas (r = 0.65; 95% CI: 0.52, 0.69) (Fig. 3). In addition, a positive correlation was identified between the total score on the PSQI and the total score on the CSI (r = 0.55; 95% CI: 0.45, 0.61).
An association was found between the occurrence of central sensitization signs and blood oxygen saturation below 90%. People with score more than 40 points on the CSI have 2.6 times more chance to present sleep episodes of blood oxygen saturation below 90% (OR = 2.62; 95% CI: 1.23, 6.47). The other PSG variables did not show statistical evidence of association with pain intensity and other patient-reported outcomes measures.
DiscussionThe majority of participants included in the study showed signs of central sensitization and the PSG variables showed that most of them had one or more episodes of sleep apnea, nocturnal awakening, sleep efficiency reduction, and changes in sleep architecture (sleep phases). The study also found correlation between sleep quality scores and central sensitization scores, as well as an association between presence of central sensitization signs with changes in blood oxygen saturation.
Patients with chronic pain and signs of central sensitization can present changes in motor patterns, cerebral cortex activation, and endogenous pain modulation.20,21 However, our findings also draw attention to other possible aspects that may be associated with chronic pain. The association between changes in blood oxygen saturation (below 90%) and the number of nocturnal awakenings emphasizes the need for in-depth analysis by clinicians and researchers on respiratory aspects and sleep disorders in patients with chronic pain and signs of central sensitization.
Similar findings have already been reported by other studies.10 A meta-analysis conducted by Matias et al13 compiled results of 503 PSG studies from individuals with chronic pain and controls and found that people with chronic pain present problems in sleep continuity, sleep architecture, and sleep fragmentation.
Different studies have already used clinical questionnaires to evaluate sleep disorders and also found association between sleep quality, acute pain, and chronic pain.21-24 Such findings have already been shown in patients with fibromyalgia25 and temporomandibular joint disorders.26 However, the association between PSQI scores and CSI scores found here reinforce that sleep quality is a relevant clinical measure for patients with signs of central sensitization, independently of the clinical diagnosis.
The results of the study also highlight the relevance of improving sleep quality for patients with chronic pain. Sleep disorders can also present association with emotional and occupational disorders, increasing analgesic and opioids intake.27-29 In their systematic review, Whibley et al.30 investigated variables that could act as mediator on pathways between sleep and pain intensity. The included studies showed mediators as mood, depression and/or anxiety, attention to pain, pain helplessness, stress, fatigue, and physical activity. Even considering some methodological limitations, the evidence supported a mediating role for psychological and physiological aspects of emotional experiences. Considering the complexity of the construct “sleep - chronic pain” and the clinical impact of these two health conditions, an in-depth investigation of possible associated variables should be performed.31
Even though PSG is a gold standard method for studies of sleep disorders,10,30 due to the cost and complexity of performing the examination, it is not commonly used in studies on sleep disorders and pain. The utilization of patient-reported outcome measurement and PSG type 1 exam in the present study gives a unique contribution to the results.
Considering that chronic pain and sleep quality are health conditions usually managed by multidisciplinary professionals,32 it is important to highlight that rehabilitation programs for patients with chronic pain with sleep problem complains should include a biopsychosocial approach with moderate-to-vigorous physical activity33 and cognitive behavioral therapy.34 The findings of the present study also bring an important message to all rehabilitation sciences clinicians: sleep quality should be assessed as part of the clinical evaluation of patients with chronic pain and central sensitization.
LimitationsThis study presents some limitations that we should acknowledge. The population heterogeneity, with different comorbidities, makes it difficult to build a homogeneous sample and control all variables that may also influence pain and sleep. The absence of a control group and the retrospective scope of data collection do not allow us to extrapolate the results and also to conclude if the central sensitization precedes the sleep disturbance. Another limitation is the remote application, via electronic form, of the questionnaires utilized for data collection. Although the instruments have previous psychometric validations, an assessment of operational equivalence of the clinical instruments, with validation of the remote format was not performed. It is noteworthy that other variables can influence the occurrence of an association between sleep disorders and pain (such as psychological disorders, financial difficulties, family conflicts, diet, physical activity, and leisure) which were not analyzed in this study and requires further investigation.
ConclusionMost participants with central sensitization signs had poor sleep quality, night waking episodes, sleep apnea, and specific disturbances in sleep phases. The findings showed association between central sensitization scores and sleep quality scores. Polysomnographic records also revealed a negative correlation between sleep efficiency and sleep latency, a positive correlation between total sleep time and sleep efficiency, a positive correlation between the number of nocturnal micro-awakenings episodes and number of nocturnal apneas. There was also an association between central sensitization signs and changes in blood oxygen saturation during sleep.
This study was supported by the Fundação Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ, No. E-26/211.104/2021) and Coordenação de Aperfeiçoamento de Pessoal (CAPES, Finance Code 001; No. 88881.708719/2022-01, and No. 88887.708718/2022-00).