Blood flow and brain ischaemia have been of interest to physical therapists for decades. Despite much debate, and multiple publications around risk assessment of the cervical spine, more work is required to achieve consensus on this vital, complex topic. In 2020, the International Federation of Orthopaedic Manipulative Physical Therapists (IFOMPT) Cervical Framework adopted the dubious terminology ‘vascular pathologies of the neck’, which is misleading, on the premise that 1) not all flow limitations leading to ischaemia, are associated with observable blood vessel pathology and 2) not all blood flow limitations leading to ischaemia, are in the anatomical region of the ‘neck'.
ObjectiveThis paper draws upon the full body of haemodynamic knowledge and science, to describe the variety of arterial flow limitations affecting the cervico-cranial region.
DiscussionIt is the authors’ contention that to apply clinical reasoning and appropriate risk assessment of the cervical spine, there is a requirement for clinicians to have a clear understanding of anatomy/anatomical relations, the haemodynamic science of vascular flow limitation, and related pathologies. This paper describes the wide range of presentations and haemodynamic mechanisms that clinicians may encounter in practice. In cases with a high index suspicion of vascular involvement or an adverse response to assessment/intervention, appropriate referral should be made for further investigations, using consistent terminology. The term ‘vascular flow limitation’ is proposed when considering the range of mechanisms at play. This fits the terminology used (in vascular literature) at other anatomical sites and is understood by medical colleagues.
Blood flow and ischaemia to the brain have been of interest to physical therapists for decades. The advent of manual therapy and manipulative techniques to the cervical spine in the 1960–70′s,1 brought with it the spectre of the risk of vascular ischaemia and potential harm to patients.2 Reports of traumatic injuries to the vertebral arteries soon began to appear in medical literature.3,4 The defensive response was for physical therapists to develop pre-manipulative guidelines outlining subjective questioning and physical tests, such as the vertebral artery (VA) test, purported to assess risk and contribute to patient safety.5–7
Decades later, there remains ongoing debate over the clinical utility of VA testing,8 and the optimal approach to risk assessment of the cervico-cranial region. The conflicting Australian Physiotherapy Association (APA) guidance,9 which advocates the ongoing use of VA positional testing and the International Federation of Orthopaedic Manipulative Physical Therapists (IFOMPT) Cervical Framework for risk assessment of the cervical spine,10 which suggests that VA testing should not form part of the clinical examination, illustrates that there is still work required to achieve consensus on this vital topic. Furthermore, the adoption in 2020 by the IFOMPT Cervical Framework10 of the dubious terminology ‘vascular pathologies of the neck’, is misleading, on the premise that:
- 1.
Not all flow limitations leading to ischaemia, are associated with observable blood vessel pathology.
- 2.
Not all blood flow limitations leading to ischaemia, are in the anatomical region of the ‘neck.’
This Masterclass unravels key issues with reference to contemporary haemodynamic science related to the cervico-cranial region and provides directions for future terminology and practice.
Understanding haemodynamics“Haemodynamics” means dynamics of blood flow. Haemodynamic control systems continuously monitor and adjust to changes within the body and its environment. It is known that both intrinsic and extrinsic factors can influence blood flow. Studies in the upper and lower limb illustrate clearly that flow limitations and resulting ischaemia can occur for numerous reasons, with or without internal vessel pathology (defined as observable changes in the structure of the vessels).11 It is known that external or mechanical mechanisms, can lead to flow limitations in the complete absence of vessel pathology.
In the upper limb, mechanical arterial flow limitations affecting the subclavian-axillary artery and posterior circumflex humeral artery are described. These commonly affect young, healthy individuals with no vascular risk factors and are classically link to anatomical anomalies of ribs, muscles, and other soft tissues.12 In the lower limb, external iliac artery flow limitations13 and popliteal arterial entrapment syndrome14 are rare but well known cause of lower limb ischaemia. Both conditions are known to occur in young, fit athletes due to abnormal haemodynamics (arterial looping and high demand) or mechanical compression of otherwise healthy arteries. It is important to note, that these flow limitations commonly occur initially, in the complete absence of underlying vascular pathology or risk factors.
The context of this haemodynamic knowledge is that these mechanisms are directly transferable to the cervical spine. The authors contend that to apply clinical reasoning and risk assessment in the cervico-cranial region, there is a requirement for clear understanding of anatomy/anatomical relations, the haemodynamic science of vascular flow limitation, and related pathologies.
This paper draws upon the full body of haemodynamic knowledge and science, to describe the variety of causes of arterial flow limitations in the cervico-cranial region. A summarizing overview of vascular flow limitations in the cervico-cranial region is presented in Table 1.
Vascular flow limitations in the cervico-cranial region.
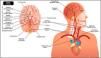
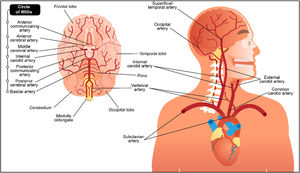
It is helpful to consider the vascular anatomy as a system.15 That way it becomes easier to visualise the arteries as a series of interconnected pipes which provide blood flow to the brain. The key elements of this system are illustrated in Fig. 1.16
Cervico-cranial vasculature, including the circle of Willis.16Reprinted and adapted with permission from 123RF ©123RF.com.
Any single part of this system can impact on blood flow to the brain, with any one element or vessel impacting on another. Disruptions of circulation to the brain can have major neurological consequences. A thorough understanding of anatomy and clinical significance of the cranio-cervical vascular system is critical for risk assessment prior to physical therapy interactions of any sort. Each element of the system will be discussed in line with emerging knowledge of known mechanisms of vascular flow limitation and associated clinical presentations.
Vascular flow limitations affecting the posterior circulationThe posterior circulation supplies around 20% of the blood supply to the brain, specifically the brainstem, the cerebellum, and the posterior cortex.
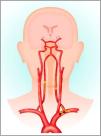
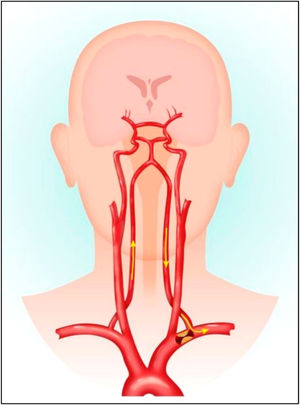
Subclavian steal syndromeHaemodynamic flow mechanisms are highlighted exquisitely by the Subclavian Steal Syndrome (SSS), which falls outside the anatomical region of the neck and illustrates the subtle haemodynamic relations between the vertebral, subclavian and carotid arteries and circle of Willis. SSS is a rare vascular disorder in which occlusion or stenosis of the subclavian artery proximal to the VA origin, leads to an alteration of vascular haemodynamics. This results in retrograde blood flow in the VA of the same side during upper limb efforts or activity. The upper limb ‘steals’ blood from the VA at times of high demand (Fig. 2).17 These lesions only manifest in vertebrobasilar insufficiency (VBI) symptoms when compensatory flow diverts blood flow toward the upper limb and away from intra-cranial structures.18 The systems’ collateral circulation to the posterior fossa is through the circle of Willis, principally through the posterior communicating artery. This communication may be absent or inadequate, due to co-existing extracranial carotid stenosis. VBI symptoms become manifest, usually during activity involving the upper limb.
Pathology of subclavian steal syndrome. Reprinted from Konda et al.,17copyright 2015, with permission from Elsevier.
Subclavian artery stenosis is commonly linked to atherosclerosis and more rarely, giant cell arteritis.19 It is important to note that the VA itself may be entirely non-pathological, investigations such as Duplex ultrasound or magnetic resonance arteriography should observe the whole system for optimal impact, and interventions target the site of the lesion.20 Of note, positional VA testing - which continues to be recommended by the APA9 would be normal in people with SSS, despite the subjective and possibly objective (functional upper limb activity), reproduction of classical VBI symptoms such as dizziness, visual disturbances, or drop attacks.
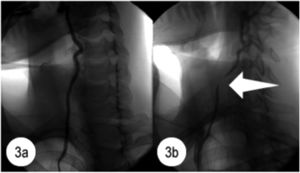
Bow hunters syndromeBow Hunters Syndrome (BHS) or rotational VA occlusion syndrome is an example of dynamic VA compression which leads to VBI during rotational movements of the neck. Arterial compression leads to partial or complete blood flow limitation in the affected vessel (Fig. 3).21 Resulting ischaemia, can occur with or without the presence of underlying arterial pathology and has been reported in children and older adults.22 Dynamic stenosis or compression of the VA by abnormal bony structures occurs during neck rotation/extension. External compression may be linked to a range of associated pathologies such as cervical spondylosis, osteophytes, disc herniation, fibrous/tendinous bands, or tumours. Vessel wall injury may eventually occur due to endothelial damage caused by repetitive shear stresses. Thrombus formation may occur due to stasis in the VA.23
Angiogram of the right vertebral artery. Fig. 3a shows the normal appearance of the right vertebral artery during the angiogram performed in neutral head position. Fig. 3b shows evidence of flow disruption (level of C7) during the angiogram performed in right head rotation. Reprinted from Lee et al.,21copyright 2011, with permission from Elsevier.
Presenting symptoms include headache, vertigo, and blurred vision (occasionally associated with syncope) during partial head rotation (the ‘bow hunter's position), or extreme rotation. The ‘systems’ element is illustrated by its association with a poor collateralisation: either a hypoplastic or absent contralateral VA or a deficient circle of Willis. BHS is most commonly caused by degenerative changes in adults and congenital anomalies of the craniocervical junction in paediatric patients.23
The role of the physical therapist is to identify the haemodynamic mechanisms, in this case transient positional ischaemia and promptly refer on for appropriate vascular testing. Dynamic imaging studies may reveal the side and localisation of the stenosis and the status of the contralateral VA and circle of Willis.24 Surgical options generally have an excellent prognosis.23
In BHS, the ‘vertebral artery test’9 would be more likely to record a true positive result with reproduction of classical VBI symptoms such as dizziness, visual disturbances, or drop attack/syncope. However, it remains questionable what further information would be gained, or what risks are associated with the application of this test in such patients.
Vertebral artery insufficiencyVBI results from inadequate blood flow through the basilar artery. For VBI to manifest, there must be significant occlusion in both VAs, or within the basilar artery.25 Compensatory mechanisms dictate that there must also be a diminished contribution from the internal carotid circulation via the posterior communicating artery in the circle of Willis. VBI should be thought of as a manifestation of dysfunction of a combination of elements of the system, rather than just a product of VA flow limitation in isolation. The VA test,9 is most likely a test of the flow capabilities and compensatory abilities of the whole circulatory system (to the brain), not the VA in isolation.
Vertebral artery atherosclerosisFlow limitations of the posterior circulation due to insufficiency (narrowing) of the vessels are commonly linked to atherosclerotic disease. Atherosclerotic occlusive disease of the posterior circulation or vertebro-basilar system is an important aetiology, responsible for approximately a third of cases of posterior circulation strokes, which account for 20–40% of all ischaemic strokes.26 VBI symptoms, namely, headache, nausea, vomiting, vertigo, nystagmus, diplopia, dysphagia, dysarthria, dysphonia, and/or cerebellar ischaemic symptoms, such as ataxia, vertigo, and/or nystagmus, should be considered as an early warning sign of systemic posterior circulation disease and potential transient ischaemic attack and stroke until proven otherwise.27
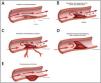
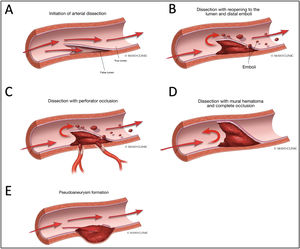
Vertebral artery dissectionThe term ‘dissection’ relates to a tear in the wall of an artery leading to the intrusion of blood within the layers of an arterial wall (intramural haematoma). This causes stenosis of the vessel lumen when blood collects between the tunica intima and media (Fig. 4).28
Various Manifestations of Intracranial Dissections. A. During initiation of the arterial dissection blood dissects into the subintimal space to create a false lumen to create an intramural haematoma. B. If there is an exit site for the dissection then a true and false lumen appear. There can be emboli from the intramural haematoma in the false lumen that result in embolic stroke. C. If the intramural haematoma involves the origin of perforator vessels, perforator occlusion can occur. D. If the intramural haematoma lacks an exit site, it can build up to eventually cause occlusion of the parent artery. E. If the haematoma breaks through the adventitia then pseudoaneurysm formation can occur. Reproduced with authorization from Bond et al.,28copyright © Elsevier Masson SAS.
Headache and/or neck pain are the most common early symptoms associated with VA dissection, and may precede the development of neurological symptoms by as long as 14 days.29 These patients may be misdiagnosed, potentially delaying appropriate management.
Converging evidence suggests patients may have predisposing arterial wall weakness.30 This may be linked to a range of associated factors including larger aortic root diameter, endothelial dysfunction, and arterial malformations such as kinks, coils, or loops.31 Arterial dissection is a common feature of certain rare inherited connective tissue disorders, such as vascular Ehlers–Danlos syndrome and Marfan syndrome, suggestive of underlying vessel wall weakness.32
VA dissection was the most common finding in major adverse events reported after manual therapy interventions (spinal manipulation) in a systematic review of 227 cases.33 Arterial dissection was reported in 57% of the cases, only 45.8% had immediate onset symptoms, suggestive of a delay to a fully developed neurological presentation. These findings are consistent with a review of large hospital cohorts which suggested that ‘cervical pain and headache are the most common symptoms of impending dissection related stroke, and are therefore very suggestive of cervical arterial dissection’.34
A causal link between spinal manipulation and arterial dissection has not been established.35 These data suggest that patients may attend physical therapy treatment with a pre-existing arterial dissection (neck pain and headache being the pre-ischaemic symptoms) and that interventions were not the cause of neurovascular symptoms that would have developed independent of the intervention.35 This raises a wider debate over appropriate assessment, clinical reasoning, and heightened levels of index of suspicion of potential vascular mechanisms in first contact clinical encounters.
Vascular flow limitations affecting the anterior circulationThe anterior circulation (AC) provides around 80% of the blood flow to the brain.
Carotid artery atherosclerosisThe internal carotid artery (ICA) is a terminal branch of the common carotid artery, arising around the level of the C4 vertebra, where it ascends intracranially to join the circle of Willis. The ICAs provide around 80% of the blood supply to the brain, specifically the anterior portion, including the retina. The external carotid artery supplies the face, scalp, skull, and meninges. Flow limitations of the anterior circulation can lead to retinal and/or cerebral ischaemic symptoms, namely, hemiparesis, hemisensory loss, neglect, aphasia, gaze deviation, dysarthria, and monocular visual loss.
Atherosclerotic plaques and resultant vessel narrowing, have been found to be most severe within 2 cm of the bifurcation of the common carotid artery. The plaque encroaches on the lumen of the ICA and also extends caudally into the common carotid artery.36 Additionally, thrombus can develop on the atheroma which further decreases the vessel lumen.
The link between carotid artery (CA) disease and stroke is well established. Stroke is ranked the second leading cause of death worldwide with an annual mortality rate of about 5.5 million.37 This high morbidity also results in up to 50% of survivors being chronically disabled.38 Several factors have been identified which associate CA disease as a strong predictor of subsequent stroke. At least two factors are universally accepted as being associated with an increased risk of stroke: the severity of arterial stenosis/flow limitation and a history of neurological events.39 Physical therapists managing patients who present with neck pain or headache with vascular risk profiles, need to be cognisant of this and retain a high index of suspicion for underlying carotid disease and target questioning toward neurological manifestations.
Carotid artery aneurysmExtracranial CA aneurysms are uncommon, representing less than 1% of all peripheral artery aneurysms, but they may exhibit severe clinical manifestations due to complications. Cases of rupture can be fatal and there is a risk of distal embolisation and stroke in thrombosed cases.40 Neck pain, a pulsatile mass, and murmur at auscultation are the most common symptoms.41
Carotid artery aneurysms are classified as either pseudo or true aneurysms.42 Arterial pseudoaneurysms are pulsatile, expandable masses, associated with localised neck swelling and pain. Atherosclerosis is the most common aetiological factor, leading to pseudoaneurysm formation through a process of vessel wall weakening and eventual ulceration. True aneurysms are the localised permanent enlargement of the arterial diameter, affecting the integrity of all vascular layers. Symptoms and signs are commonly a pulsatile mass, murmur on auscultation, and neck pain. Early recognition is vital to prevent potential complications.40
Carotid artery dissectionArterial dissections in the cervico-cranial region have been increasingly recognised as a cause of stroke, particularly in young people.34 Dissection (previously described), refers to a tear of the internal layers of the ICA, resulting in an intramural haematoma and/or an aneurysmal dilatation, which may result in minor blood flow limitation, sub arachnoid haemorrhage, embolic stroke, or at worst, death.43 The pathophysiology of dissection is not completely understood. Although multiple risk factors have been described: cervical trauma, recent infection, hypertension, and migraine. Paradoxically, some protective factors (hypercholesterolaemia, overweight) have also emerged.34
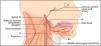
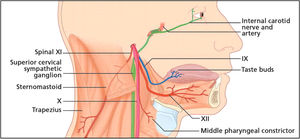
Carotid artery dissection is more frequent than VA dissection in the general population. The incidence rate of CA dissection is relatively low, and has been estimated at 2.9/100,000 individuals per year in a European population.44 Neck pain and headache are the most common early presenting symptoms, and notably, precede the onset of cerebral ischaemia from a few days to more than a month. Horner's syndrome can occur in patients with CA dissection, because of the compression of sympathetic fibres by the dilatation of the artery within the carotid sheath (Fig. 5).45
The lowest four cranial nerves are shown emerging from the jugular and hypoglossal foramina, where they join the sympathetic plexus within the carotid sheath. Here, these structures are vulnerable to the compressive effects of the dilatation of the artery resulting from a carotid artery dissection. Reprinted from Fitzerald et al.,45copyright 2007, with permission from Elsevier.
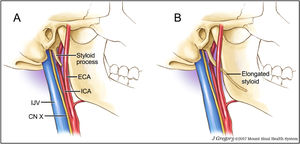
Eagle syndrome also referred to as stylocarotid syndrome46 is a rare, under-recognised disorder which has been implicated in more than 30 documented cases of CA dissection. The syndrome relates to the unusual anatomical variant of an elongated styloid process.47 The styloid processes’ proximity to the ICA has been implicated as a contributor to CA dissection due to repeated compression (Fig. 6).48 Styloid anomalies can be identified via head and neck computed tomography scans (requesting stylohyoid complex measurements), three-dimensional computed tomography scans, lateral cervical radiographs, and panoramic dental radiographs.49 It is important for clinicians to be able to detect such patients.
A: Normal length styloid process and associated vascular and neural structures. Fig. 7B: Elongated styloid process travelling just proximal to the carotid bifurcation as seen in patients with Eagle syndrome. ICA = internal carotid artery. ECA = external carotid artery. IJV = internal jugular vein. CN X = cranial nerve 10 – vagus nerve. Image by Jill Gregory,48 reprinted with permission from ©Mount Sinai Health System.
Symptom production in Eagle syndrome is commonly reported with neck and head rotation.50 Investigative studies of the carotid bifurcation and ICA show that as the head turns, evident changes of the morphology of the vessels occur, which impact upon vessel haemodynamics and stress on various aspects of the arterial wall.51 It has been suggested that the styloid process may impact on the wall of the ICA like being ‘stabbed with a pointed object’.52 Left undetected or inappropriately treated, the condition may lead to major adverse events such as transient ischaemic attack or stroke.47 The only definitive treatment for symptomatic Eagle syndrome once identified, is appropriate referral for surgical shortening of the styloid.50 It is suggested that otolaryngologist or ear, nose, and throat specialists, should be the triage route for styloidectomy.47
Other mechanisms of vascular flow limitationArteritisGiant cell arteritis is a vasculitis of large-sized and medium-sized vessels which causes blood flow limitation and potential critical ischaemia. As such it is considered a medical emergency, with permanent visual loss reported in around 20% of patients.53 It is the most common vasculitis in Caucasians.53 Acute visual loss in one or both eyes is by far the most serious and irreversible complication.54 Temporal arteritis presents as acute onset temporal headache and is linked to polymyalgia rheumatica. Both conditions occur in age groups above 50 years and are associated with constitutional symptoms and systemic inflammatory response. Older age patients who have developed or are developing ischaemic symptoms, such as diplopia, transient visual loss, or jaw/tongue claudication, are at particularly high risk.
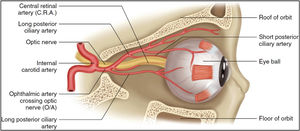
Giant cell arteritis causes inflammatory blood flow limitation to the anterior optic nerve head via the short posterior ciliary arteries and that of the retina via the central retinal artery (Fig. 7).55 Studies have reported that patients presented initially with transient ischaemic events including jaw claudication, blurred vision, and amaurosis fugax (temporary visual loss), before going on to permanent vision loss, and/or ischaemic stroke.56,57
Arterial supply to the retina. Reprinted from Harris et al.,55copyright 2019, with permission from Springer Nature Customer Service Centre GmbH.
Reversible cerebral vasoconstriction syndrome (RCVS) refers to a group of disorders characterised by clinical manifestations typically including thunderclap headaches, and much less commonly, focal neurological deficits related to brain oedema, stroke, or seizure. Angiographic findings may be normal58 or reveal, temporary, reversible multifocal narrowing of the cerebral arteries.59
The pathophysiology of the abrupt-onset headache and of the prolonged but reversible vasoconstriction remains unknown and there is no apparent vessel disease or pathology. Rather, reversible angiographic narrowing suggests an abnormality of the control of cerebrovascular tone.60
RCVS is being reported with increasing frequency, perhaps due to greater awareness of the syndrome, higher detection rates due to the widespread use of tests such as computed tomography angiography and magnetic resonance angiography.61 RCVS occurs worldwide in individuals of all races. RCVS in adults, has been reported to predominantly affect women, with female to male ratios ranging from 2:1 to 10:1. However, a 2017 review of paediatric RCVS found that most cases affected boys aged 11–13 years.62
A range of individual risk factors, triggers, and conditions have been associated with RCVS (including pregnancy, migraine, use of vasoconstrictive drugs, other drug reactions, neurosurgical procedures, hypercalcemia, unruptured saccular aneurysms, cervical artery dissection, cerebral venous thrombosis, and others.60 The clinical presentation of RCVS is described as ‘dramatic’, with sudden onset, excruciating headaches which reach peak intensity within seconds, meeting the definition for "thunderclap headache".63 The character of these headaches is reported to differ from the patient's prior history of migraine headaches, if any.63
Though most patients suffer headaches only, some develop focal neurological deficits such as ischaemic stroke, intracerebral haemorrhage, or cerebral oedema.64 Thunderclap headache with or without vasospasm is known to be precipitated by sexual activity, intensive exertion, the Valsalva manoeuvre, acute hypertensive crisis, or ingestion of sympathomimetic drugs65 suggestive of excessive sympathetic activity. Considering the lack of consensus on the topic, physical therapists may be wise to consider RCVS as a potential source of acute onset thunderclap headache (of vascular origin) and triage on appropriately for further testing.
Implications for practiceThis Masterclass details the wide range of potential vascular flow limitations which may present to musculoskeletal clinicians who manage people with neck pain/headache/orofacial symptoms and associated conditions such as visual or balance disturbances. Much of the evidence is drawn from a literature base outside of physical therapy and this gives a broader overview of the topic and related science.
The ongoing traditional physical therapy focus on “VA dissection” related to manual therapy in particular, fails to incorporate wider knowledge of potential flow limitations necessary for cervico-cranial risk assessment, and may lead to errors of clinical reasoning and potential mismanagement.
Implications for practice for the assessment of people with cervico-cranial pain and dysfunction:
- 1.
Clinicians who manage people presenting with neck pain, headache, orofacial pain, and/or associated symptoms, require awareness of the range of haemodynamic mechanisms leading to vascular flow limitations.
- 2.
Clinicians can play an important role in identifying flow limitations, potentially leading to better patient outcomes.
- 3.
Flow limitations may present, in the presence of observable vascular pathology (e.g. VA/CA atherosclerosis or vessel dissection).
- 4.
Equally, flow limitations may present in the absence of observable vascular pathology e.g. Bow Hunters syndrome, Eagle Syndrome.
- 5.
Flow limitations also occur outside the anatomical confines of ‘the neck’ e.g. sub-clavian steal syndrome, RCVS, and temporal arteritis.
- 6.
There are no known reliable clinical tests to identify the range of the described clinical presentations.
- 7.
Haemodynamic knowledge, targeted history taking, and sound clinical reasoning skills are required to follow the guiding principle of do-no-harm.66–68
- 8.
History taking has been suggested as the single most important factor for detecting subtle symptoms of cervico-cranial blood flow limitations.
- 9.
Unusual onset of new/unusual headache, in combination with associated ischaemic symptoms, raises the index of suspicion of vascular flow limitation.
- 10.
Where there is a high index of suspicion of vascular involvement or adverse response to assessment/intervention, appropriate referral should be made for further investigations using consistent and easily understood terminology.
- 11.
The terms ‘vascular flow limitation’ and ‘ischaemia’ are consistent with those used by medical colleagues, and as such are recommended for the communication of suspected cases.
- 12.
Future research should focus on the efficacy of the current risk assessment strategies advocated by Rushton et al.10 and consider whether further modifications are recommended.