Chronic kidney disease is a complex disease that impacts multiple organs and systems (including musculoskeletal and cardiorespiratory) leading to reduction of functional capacity.
ObjectiveThe aim of this study was to investigate the effect of a short period of high intensity inspiratory muscle training on maximum inspiratory pressure, functional capacity and endothelial function of chronic kidney disease patients on hemodialysis.
MethodsThis randomized controlled trial enrolled 25 patients who were allocated into two groups: intervention (IMTG=14) and control (CG=11) groups. Intervention patients received the exercise protocol over a period of 5 weeks, 6 times per week, with each session consisting of 5 sets of 10 repetitions with an initial load of 50% progressing to 70% of maximum inspiratory pressure , measured weekly. The primary outcome was inspiratory muscle strength and the secondary outcomes were functional capacity and endothelial function evaluated before and after the training protocol.
ResultsThe inspiratory muscle training induced a marked improvement in maximum inspiratory pressure which was evident after the training period (mean difference 19.0cmH2O – 95%CI 0.4–37.5; IMTG: 102±25.7cmH2O vs CG: 83±19.2; p=0.046). The magnitude of maximum inspiratory pressure improvement was 33.5% at the end of the protocol for the IMTG. Functional capacity and endothelial function did not vary between or within groups.
ConclusionA short period of high-intensity inspiratory muscle training for five weeks was able to improve inspiratory muscle strength of chronic kidney disease patients on hemodialysis (ClinicalTrials.gov registration NCT03082404).
Chronic kidney disease (CKD) is highly prevalent, reaching about 11 to 13% of the world population and it is considered a global health problem associated with high economic costs to health systems. CKD is characterized by irreversible renal injury, interfering directly with normal kidney function (i.e. hormonal, regulatory and excretory effects).1 Consequently, the most common substitutive renal therapy employed is hemodialysis (HD).2
Evidence suggests that CKD patients are at high risk of cardiovascular mortality and the condition is associated with age-related renal function decline, hypertension, diabetes and obesity.2 Moreover, endothelial cell dysfunction is a well-known mechanism associated with cardiovascular morbidity and its association with the atherosclerosis process.3 Peripheral endothelial dysfunction was considered a significant predictor of cardiovascular events in CKD patients.3 Furthermore, functional capacity impairment is one of the most important risk factor for cardiac death in this population which is 10–20 times higher than in the general population.4 Impairment of CKD patients’ functional capacity is associated with uremic myopathy and HD, with both conditions promoting protein breakdown and which can have an effect on the strength and endurance of both inspiratory and peripheral muscles.5 CKD patients usually demonstrate a reduction of 40–50% in their exercise capacity when compared to healthy individuals.6
A recent meta-analysis demonstrated that interventions that combine intradialytic aerobic and resistance exercise improve aerobic capacity, muscle performance, quality of life, and reduce depression symptoms as well as an improvement in the patients’ “toxin clearance”.7 However, there is no consensus in the literature about which exercise protocol would be more beneficial during HD.
CKD patients usually present with decreased inspiratory muscle strength or muscle weakness compared to predictive values.4,5,8 In order to improve the performance of the respiratory muscles, inspiratory muscle training (IMT) has been suggested as an alternative or complement to conventional exercise and, in the last few years, evidence in the literature have shown the efficacy of this therapy in CKD patients.4,5,9,10 Inspiratory muscles as skeletal muscles can physiologically remodel when stimulated with higher intensity exercise and frequency using the overload principle.11 Some studies have found an association between maximal inspiratory pressure (MIP) and functional capacity.4,8 However, studies to determine what load of respiratory training produces better results in CKD patients are inconclusive in the literature. There are studies that used overloads of 30%–60% of MIP in CKD patients.4,5,9,10,12 Another important issue is fact that there is no consensus about IMT prescription regarding: intervention time, how many times a day, number of sets and repetitions or session time.4,5,9,10,12
To the best of our knowledge, there are no studies in the literature demonstrating a short protocol of five weeks of high intensity IMT (70% of MIP) on patients who had CKD. Thus, the objective of the study was to investigate the effects of a short period of high-intensity IMT on the following health status parameters: inspiratory muscle strength, functional capacity and endothelial function in patients with CKD who were on HD.
MethodsThis study was a randomized controlled trial approved by the Committee of Ethics in Research of Irmandade Santa Casa de Misericórdia de Porto Alegre (CEP/ISCMPA), Porto Alegre, Rio Grande do Sul, Brasil, according with judgment no. 832.142. The written informed consent was obtained from all participants according to the rules established by the Helsinki Declaration. The data collection was carried out from March to July of 2017 and the protocol was prospectively registered on ClinicalTrials.gov registration NCT03082404.
The primary outcome was inspiratory muscle strength (evaluated before and at the end of each week of IMT), and the secondary outcomes were functional capacity and endothelial function evaluated before and after the training protocol.
Study subjectsThe studied population consisted of patients with CKD who were on HD for at least three months with a frequency of three weekly sessions in the Hemodialysis Outpatient Clinic at Policlínica Santa Clara of Irmandade Santa Casa de Misericórdia de Porto Alegre. The participants were a convenience sample, by verbal invitation, regardless of ethnicity, sex or educational level, as long as they met the inclusion criteria. Patients of both sexes, over 18 years old, with a urea reduction ratio above 65% and having been at least three months in HD were included. Patients who had real difficulties with understanding and signing the written informed consent form and completing and interpreting the quality of life questionnaire were excluded. Furthermore, patients with recent sequelae of cerebrovascular accidents (i.e. in the last three months), osteoarticular or musculoskeletal incapacitating diseases, uncontrolled systemic hypertension (i.e. systolic and diastolic blood pressure above 200/110mmHg, respectively) at beginning of a dialysis session,13 grade III–IV on NYHA scale (New York Heart Association) or decompensated heart failure, uncontrolled diabetes (i.e. glycaemia >300mg/dL), unstable angina, fever and/or infectious disease, acute respiratory failure, recent acute myocardial infarction (i.e. in the last three months), peripheral vascular alterations or actively smoking were also excluded.
Patients were randomly allocated according to the data generated on www.random.org. One researcher who did not participate in the study generated the numerical sequence by off-site randomization after the inclusion of patients via eligibility criteria. The numerical sequence was kept confidential until the precise moment when intervention began. The sample was calculated based on the 5% alpha error and 80% beta error rates. The reference used to calculate the difference of values between the means and standard deviations were the MIP results found by Pellizzaro et al.9 The difference expected between the groups was 9%, and the standard deviations were 8cmH2O. The sample calculation result was ten individuals for each group.
Physical and biochemistry assessmentIn order to evaluate inspiratory muscle strength, the MIP was measured using the digital pressure transducer (MVD 300 Microhard System, Globalmed, Porto Alegre, Brazil) equipped with a 2mm diameter hole in the nozzle to compensate for the pressure change induced by the oropharynx muscles, using a nasal clip and the equipment had an interval of ±300cmH2O. MIP was evaluated based on residual volume while the participants were seated in a reclining chair (with knees and hips at 90) in a HD room connected to machine at the beginning of the HD session. Patients were encouraged by verbal stimuli during MIP evaluation such as “pull the strongest you can”, “harder”. The evaluation was carried out by the research team and the highest value of five valid measurements, with an interval of 1min between them, was retained.14 The measurements were considered satisfactory if variance between them was no more than 10%. MIP values are shown as both absolute and relative values based on the percentage achieved compared to the reference equations.15
Functional capacity was evaluated using the 6-minute walk test (6MWT)16 and the sit-to-stand test (STST). 6MWT was performed on a 30-m-long, 1.5m wide, according to American Thoracic Society guideline17 and the longest walked distance was computed. During the test, oxygen saturation (SpO2), heart rate, and blood pressure were monitored by the researcher team and standardized phrases of encouragement were used such as “you are doing well, keep up good work”.17 A Modified Borg Scale was used to assess the sensations of dyspnea and fatigue of the lower limbs during the walk. The 6MWT was performed before and after the training period and the absolute values were compared with the reference equation.18 The STST was performed to measure lower limb strength which typically measures the number of “stands” (i.e. repetitions) in 30seconds19 before and after the training period and the evaluation was performed by the research staff.
Endothelial function was measured using flow mediated dilation (FDM) assessed by ultrasound Vivid-i (GE Healthcare, USA), equipped with a high resolution linear transducer 3–12MHz, L12-3 (Vivid-i, GE Healthcare, USA), with the arm-cuff placed above the eco probe at the brachial artery of the contralateral limb to the arteriovenous fistula.20 After endothelial dependent FMD, the response of the vascular smooth muscle to a nitroglycerin vasodilator (endothelium-independent dilatation after oral administration of 0.4mg of nitroglycerin (Nitrolingual Pumpspray, KG, Germany,) was also tested. The evaluations were carried out by an experienced researcher in this technique.
All evaluations were performed by a trained research team and the outcome assessors were not blinded to either group. Biochemical data of the routine exams were collected from the medical records of the patients.
High-intensity IMTThe protocol for high intensity IMT was executed using a linear pressure loading device (POWERbreathe Plus Light Resistance®, England), which allowed loading up to −90cmH2O. IMT was performed for five weeks, with a weekly frequency of six times (three times a week with supervision during the hemodialysis session and three other times at home monitored by a home-based diary). The IMT protocol consisted of five series with 10 repetitions, each series with a two-minute interval or according to the patient's feedback, using a Modified Borg Scale corresponding to moderate effort. Patients performed high-intensity IMT in the same position that inspiratory muscle strength assessment were performed as described before, receiving guidance from the team regarding the maneuver execution. At home, patients were instructed to follow the same procedures. IMT prescription was based on a pilot study regarding the physical condition and feedback from the patients.
Overload was adjusted weekly, beginning with 50% of the MIP during the first week, 60% of MIP in the second and third weeks, and 70% of MIP in the fourth and fifth weeks. MIP was evaluated before the training (i.e. baseline) and reassessed weekly to adjust the loads measured each week using a digital manovacuometer, in conformity with the methodology proposed by the American Thoracic Society. The control group did not receive the protocol training, however the MIP was evaluated before the training (i.e. baseline) and reassessed weekly for comparison purposes. Therefore, there was no masking of patients.
Statistical analysisThe descriptive variables were presented as mean±standard deviation or median (interquartile interval), and the categorical variables as relative and absolute frequency. The Shapiro–Wilk test was used to verify data distribution. One-factor analysis of variance (ANOVA) was performed for repeated measures to analyze the intra-groups differences and another t-test (or the Mann–Whitney test when necessary, was used) for independent samples in order to compare the means between the groups. A statistical significance of 5% was adopted (p<0.05) and the SPSS 21.0 software was used to analyze the data and intention-to-treat analysis was performed.
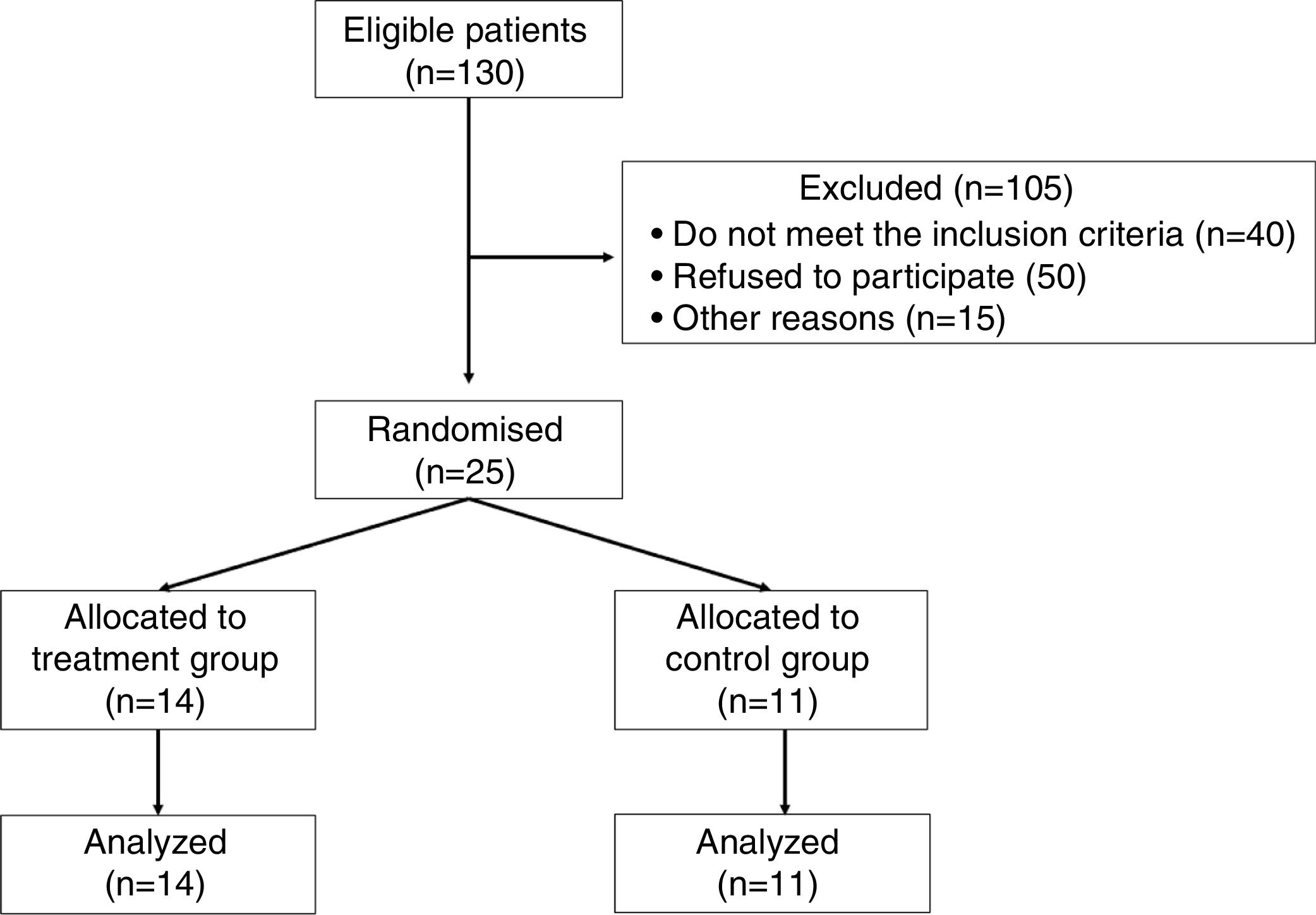
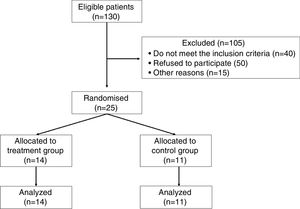
ResultsFrom 130 patients with CKD under dialysis in the unit, 25 agreed to participate and fulfilled the inclusion criteria to participate in the trial as shown in Fig. 1.
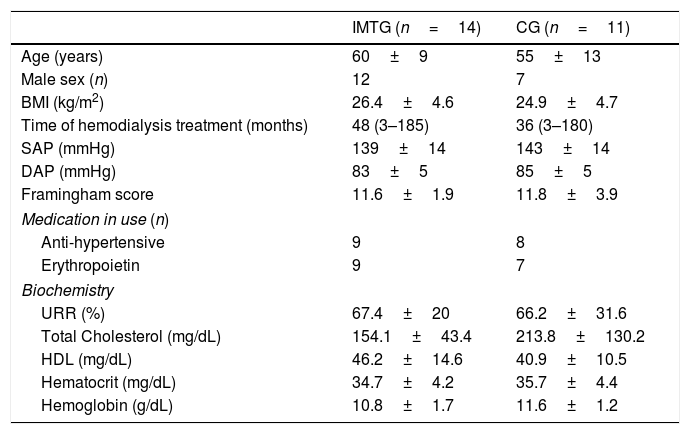
These patients were then randomized into two groups: inspiratory muscle training group (IMTG=14) and control group (CG=11). The sample consisted predominantly of men (80%), with twelve men in IMTG group and eight men in CG (Table 1). The most prevalent base pathologies were systemic arterial hypertension and diabetes mellitus in both groups, followed by other causes (e.g. polycystic kidneys, necrotizing vasculitis and unknown causes) (data not shown).
Clinical characteristics of 2 groups of patients with CKD who were on HD who took part in a study involving a short period of high intensity IMT.
| IMTG (n=14) | CG (n=11) | |
|---|---|---|
| Age (years) | 60±9 | 55±13 |
| Male sex (n) | 12 | 7 |
| BMI (kg/m2) | 26.4±4.6 | 24.9±4.7 |
| Time of hemodialysis treatment (months) | 48 (3–185) | 36 (3–180) |
| SAP (mmHg) | 139±14 | 143±14 |
| DAP (mmHg) | 83±5 | 85±5 |
| Framingham score | 11.6±1.9 | 11.8±3.9 |
| Medication in use (n) | ||
| Anti-hypertensive | 9 | 8 |
| Erythropoietin | 9 | 7 |
| Biochemistry | ||
| URR (%) | 67.4±20 | 66.2±31.6 |
| Total Cholesterol (mg/dL) | 154.1±43.4 | 213.8±130.2 |
| HDL (mg/dL) | 46.2±14.6 | 40.9±10.5 |
| Hematocrit (mg/dL) | 34.7±4.2 | 35.7±4.4 |
| Hemoglobin (g/dL) | 10.8±1.7 | 11.6±1.2 |
BMI, body mass index; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; URR, urea reduction ratio; HDL, high-density lipoprotein; IMTG, inspiratory muscle training group; CG, control group. Data are expressed as mean ±SD, median and interquartile range (P25–P75) or frequency. No significant difference between groups.
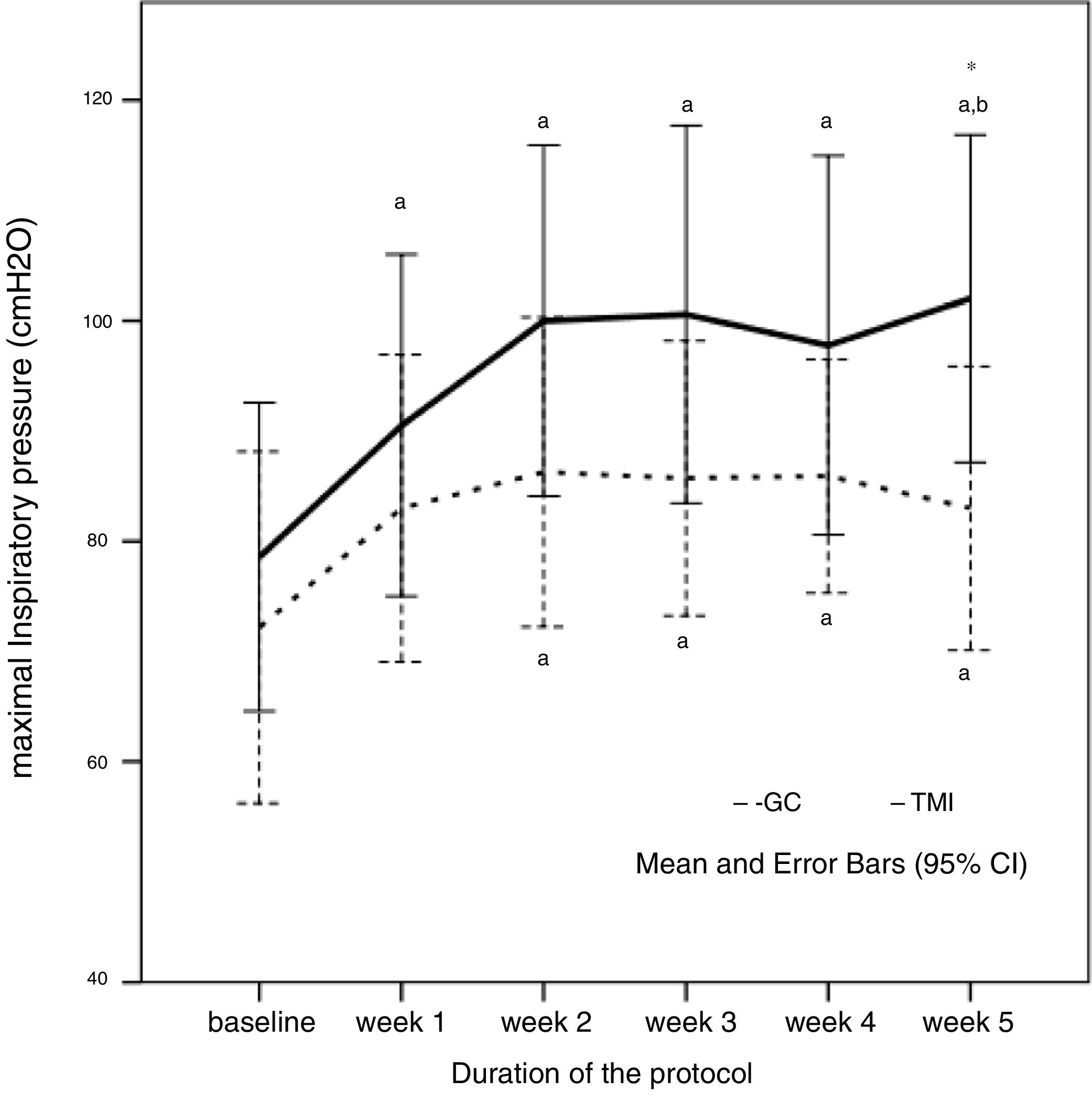
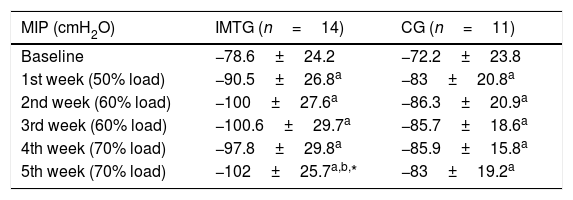
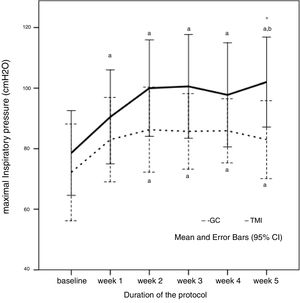
Both groups showed lower MIP values than predicted (IMTG: −78.6±24.2 vs −103±10.8cmH2O, 76.3% of predicted – p=0.002; CG: −72.2±23.8 vs −101.5±16.5cmH2O, 71.1% of predicted – p=0.003). MIP at baseline did not differ between the groups. After five weeks of training, IMT showed a significant improvement in the MIP in IMTG compared with the CG (IMTG: 102±25.7 vs CG: 83±19.2cmH2O; p=0.046), as shown in Fig. 2.
Maximal inspiratory pressure (MIP) behavior during the five weeks of protocol in control (CG) and intervention group (IMTG). a One-way ANOVA for repeated measures, p<0.05 vs. baseline; b one-way ANOVA for repeated measures, p<0.05 vs. week 1; *t-test for independent sample p<0.05 vs. CG. One-way ANOVA of repeated measures was significant different in IMTG group (p=0.007), but not in CG group (p=0.258).
At the end of the training, IMTG presented a mean MIP similar to the predicted values15 (IMTG: 102±25.7 vs 103±10.8cmH2O, p=0.889). The MIP of the CG did not change throughout of the study protocol, differing from the predicted values at the final of protocol (83±19.2 vs 101.5±16.5cmH2O, p=0.025) as shown in Table 2. Although the groups did not have ventilatory muscle weakness (<70% of predicted),21 short periods of high intensity IMT were able to increase the ventilatory muscle strength reduction in the trained group. After five weeks of IMT, MIP raised 23.4cmH2O at the end of the protocol when compared to baseline values in the IMTG, an improvement of 33.5%.
Maximal inspiratory pressure (MIP) in absolute values during the training period in 2 groups of patients in a study looking at the effect of a short period of high intensity inspiratory muscle training.
| MIP (cmH2O) | IMTG (n=14) | CG (n=11) |
|---|---|---|
| Baseline | −78.6±24.2 | −72.2±23.8 |
| 1st week (50% load) | −90.5±26.8a | −83±20.8a |
| 2nd week (60% load) | −100±27.6a | −86.3±20.9a |
| 3rd week (60% load) | −100.6±29.7a | −85.7±18.6a |
| 4th week (70% load) | −97.8±29.8a | −85.9±15.8a |
| 5th week (70% load) | −102±25.7a,b,* | −83±19.2a |
MIP, maximal inspiratory pressure in absolute values; IMTG, inspiratory muscle training group; CG, control group; mean ±SD of CG and IMTG.
Functional capacity did not change between or within groups in 6MWT (IMTG: 413.7±66.5 vs 422.1±78.1m, p=0.537; CG: 443.2±128.3 vs 456.3±123.2m, p=0.456) and STST (IMTG: 11±3 vs 11±3 repetitions, p=0.743; CG: 12±5 vs 13±6 repetitions, p=0.514). Both groups showed lower values than predicted on 6MWT (IMTG: 413.7±66.6 vs 533.8±67.5m, p=0.001–77.5% of predicted; CG: 443.2±128.3 vs 552.5±87.7m, p=0.019–80.2% of predicted). Endothelial function data did not show significant changes after the follow-up period in the IMTG (7.4±3.5 vs 9.1±3.1%; p=0.127) and CG (8.2±5.4 vs 7.2±4.7%; p=0.624). Funcional capacity and vascular function data are presented in Table 3.
Physical assessment of 2 groups of CKD patients on HD following high intensity IMT.
| IMTG | CG | Comparison between groups | p value | |||
|---|---|---|---|---|---|---|
| Before IMT | After IMT | Before IMT | After IMT | Mean difference (95% CI)After IMT | ||
| 6MWT (m) | 413.7±66.5 | 422.1±78.1 | 443.2±128.3 | 456.3±123.2 | −34.2 (−124.4, 56.0) | 0.434 |
| STST | 11±3 | 11±2 | 11±4 | 13±6 | 2.1 (−6.4, 2.2) | 0.320 |
| FMD (%) | 7.4±3.5 | 9.1±3.1 | 8.2±5.4 | 7.2±4.7 | 1.9 (−1.7, 5.6) | 0.287 |
6MWT, six-minute walk test; STST, sit-to-stand-test; FMD, flow-mediated dilation; IMT, inspiratory muscle training; CKD, chronic kidney disease; HD, hemodialysis; IMTG, inspiratory muscle training group; CG, control group; data are expressed as mean (SD).
More than 90% of the patients had hemoglobin values below the recommended values for the general population, while 71% and 54% of the patients in IMTG and CG, respectively, presented values below normal for the hematocrit.
DiscussionThe results obtained in this randomized controlled trial demonstrated that five weeks of high-intensity IMT was able to increase inspiratory muscle strength of patients with CKD on HD. However, no differences were found regarding exercise tolerance and endothelial function after protocol training.
Patients with CKD on HD showed a reduction of inspiratory muscle strength, as already demonstrated by our group8 and other studies.5,9,22 According to the literature, IMT increases MIP and improves inspiratory muscle weakness (<70% of predicted).4,21 In CKD patients, 40% of MIP after eight weeks was not able to improve inspiratory muscle strength,12 50% of MIP was able to generate an increase of 22.5cmH2O (an increase of 25%)9 and 36.5cmH2O (an increase of 70%)5 when trained for 8 and 10 weeks, respectively. The magnitude of MIP improvement in our study was 23.4cmH2O (33.5%). Our study demonstrated that high intensity training associated with reduced intervention time (i.e. 5 weeks) presented similar effects to those studies described in the literature. In heart failure patients, high-intensity IMT (i.e. 60–70% of MIP) can produce better results than low-intensity training.23 Most of studies with CKD patients used intervention time ranging from six weeks to six months with light to moderate intensity exercise.4,5,9,10,12
Some authors suggest that there is a physiological plateau in strength and power development in response to IMT at 6 weeks in healthy subjects.24 We can speculate that in patients with CKD in HD, due of the complex pathophysiological mechanisms, there was an early plateau (2 weeks) as demonstrated by our work. Our results could be related to specific factors: the form of training used, which began with high loads (i.e. 50% of MIP), with weekly increments (principle of overload), reaching values of up to 70% of MIP in a short period of time; specificity of the training leading to changes in muscle structure, and function determined by the nature of the applied stimulus; and the patients’ clinical situation, which could involve reduced inspiratory muscle strength compared to predicted values, and, at the same time, the patients willingness to perform the proposed activity. Recently, a randomized controlled trial showed that MIP was an independent predictor of functional capacity and inspiratory muscle weakness increased the odds of poor functional capacity (<16mL/kg/min) by 5.7 times.4
Studies using IMT showed positive effects on exercise tolerance assessed by 6MWT in chronic diseases.10,21 Data from a cohort study of 52 patients with CKD on HD published by our group illustrated that survival increased approximately 5% for every 100m walked in the 6MWT.25 In our work, exercise capacity did not change after five weeks assessed by 6MWT and STST. Some factors that may explain the lack of positive outcomes regarding physical capacity in the IMTG of the present study include: short time for peripheral effects to occur, IMTG patients were more sedentary compared to CG group (86% vs 63%) and 71% of patients on IMTG had hematocrit values below normal values compared to 54% of CG. Improved exercise tolerance after inspiratory muscle training could be partly explained by the attenuation of the inspiratory muscle metaboreflex described in patients with heart failure.26 Thus, the conditioning of inspiratory muscles could attenuate inspiratory metaboreflex and sympatethic efferent modulation to vascular bed improving the perfusion of peripheral muscles21,26 with possible benefits on exercise tolerance in CKD patients.
Although we did not manage to identify changes in endothelial function, studies have already shown that patients with CKD present with a reduced bioavailability of nitric oxide, with impairment of the endothelial function.27 Recently, 41 CKD patients underwent 8 weeks of respiratory muscle training in a randomized clinical trial and no changes in vascular parameters were demonstrated.28 The authors felt that the results were due to the small sample size and short intervention period since effects on vascular function have been described with longer periods of physical training in CKD patients.29
The limitations of the present study are the facts that no other physical or physiological variables were evaluated that allowed for the establishment of a causal relationship between the results found, and absence of follow-up of these patients to investigate a prolonged effect of short period training with high-intensity load. In conclusion, five weeks of high intensity IMT (70% of MIP) improved inspiratory muscle strength in patients with CKD on HD.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to acknowledgment Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES by grant of scholarship and hemodialysis unit staff.