Supporting children and adolescents with cancer to be physically active can improve medium- and long-term health outcomes.
ObjectiveTo assess the feasibility of CanMOVE, a 10-week complex, theoretically-informed, behaviour change intervention to promote physical activity for children and adolescents undergoing acute cancer treatment.
MethodsA feasibility study using a single-group, repeated measures, mixed methods design. Participants completed CanMOVE, which included provision of a Fitbit (child/adolescent and carer) and structured support from a physical therapist. Feasibility domains of demand, acceptability, implementation, practicality, limited efficacy, and integration were evaluated. Data sources included service level data, objective assessment of physical activity, physical function, and health-related quality of life; and qualitative data collected via semi-structured interviews with participants and focus groups with staff.
ResultsTwenty children/adolescents (median age 13yrs, interquartile-range 9–14) with a mix of cancer diagnoses, 20 parents, and 16 clinicians participated. There was high demand with 95% enrolment rate. CanMOVE was acceptable for participants. All feasibility thresholds set for implementation were met. Under practicality, there were no serious adverse events related to the intervention. Limited efficacy data indicated CanMOVE showed positive estimates of effect in influencing child/adolescent physical activity behaviour, physical function, and health-related quality of life. Positive impacts were also seen in parent and staff attitudes towards physical activity promotion. To improve integration into the clinical setting, it was suggested the duration and scope of CanMOVE could be expanded.
ConclusionCanMOVE was feasible to implement in a paediatric cancer setting. CanMOVE is appropriate to be tested in a large-scale trial.
A child diagnosed with cancer has over 80% chance of long-term survival.1 Focus is shifting towards improving quality of survival through promoting healthy lifestyle behaviours that reduce chronic disease risk and increase health-related quality of life (HRQOL).2-5 Health interventions offered from diagnosis are required to minimise the impact of cancer treatment and improve immediate and long-term health.
Children and adolescents undergoing cancer treatment are less physically active compared to age-matched peers.6 In healthy and chronic disease populations reduced physical activity is associated with greater morbidity and mortality.4,7-9 Promoting physical activity for children/adolescents undergoing acute cancer treatment holds benefits including: minimising physical impacts of treatment, maximising independence, maintaining physical literacy skills, and managing fatigue.10,11 Even small changes in activity can be beneficial,9 however, the determinants of physical activity behaviour for children/adolescents with cancer are complex, making implementation difficult.12
To change complex behaviours such as physical activity, theoretically-informed, multifaceted interventions are required, especially in challenging environments such as hospitals.13-17 Undertaking a behavioural approach to physical activity promotion is supported widely in healthcare, including paediatric cancer settings.17,18 Studies employing behaviour change strategies to increase physical activity participation for children/adolescents with cancer exist.19-24 Yet, previous investigations focus on less intensive treatment phases, lack theory-based processes and are not explicit about the behaviour change techniques implemented.19-24
CanMOVE is a novel, 10-week complex behaviour change program designed specifically for children/adolescents undergoing acute cancer treatment. Design was informed by the Behaviour Change Wheel.25 Flexible, individualised intervention strategies aim to promote positive physical activity behaviours to mitigate declines in physical function and activity.26 Feasibility evaluation is a vital step within the complex intervention design process to determine suitability for large-scale trial and clinical implementation.27-29 This study therefore aims to assess CanMOVE feasibility across domains of demand, implementation, acceptability, practicality, limited efficacy, and integration.
MethodsStudy designA feasibility study30 using single-group, repeated measures, mixed methods design was completed. The protocol was prospectively registered (NCT04483362) and received ethics approval (61,709) with The Royal Children's Hospital Research Ethics and Governance. Reporting is consistent with Good Reporting of A Mixed Methods Study (GRAMMS) guidelines.31
Study population and environmentCanMOVE participants were recruited from a large tertiary paediatric hospital The Royal Children's Hospital, Melbourne, Australia. CanMOVE was implemented alongside existing referral-based physical therapy services. Participants were recruited 12 November 2020–16 June 2021. Eligible participants were children/adolescents aged ≥5 and ≤16 years, with new/relapse cancer diagnosis within >4 weeks and <6 months, receiving cancer treatment, and in-patient for ≥7 consecutive days. Exclusion criteria were those deemed unsafe by the medical team, unable to mobilise independently (carer assistance or gait aid acceptable), unable to follow simple instructions, and/or no suitable support person (≥18 years) available to participate. Following informed consent (adolescent assent), demographic data recorded age, sex, diagnosis, days since diagnosis/relapse, treatment modalities, and length-of-stay.
Staff participants were recruited to provide additional perspectives regarding CanMOVE feasibility. Staff across nursing, medical, and allied health disciplines were invited via email to provide informed consent and participate in focus-groups. Staff were identified based on their known involvement in the trial, as a ward-based clinician or trial assessor.
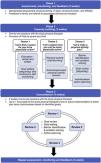
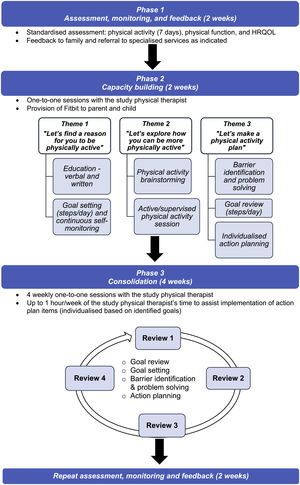
Intervention procedureCanMOVE is a 10-week intervention involving three phases (Figure 1). Full description of intervention design has been published.26 All sessions were administered one-to-one by study physical therapists with experience in acute paediatric oncology. If a child/adolescent was discharged home mid-trial, intervention sessions were continued as out-patient, or remotely via videoconference.
Phase 1: assessment, feedback, and monitoringObjective assessment of physical activity, physical function, and HRQOL was completed to build self-awareness of participants level of physical activity, understand factors contributing to physical activity, and guide goal setting (Supplementary material 1). Assessment tools included: Fitbit Inspire for physical activity; Movement ABC-2, Timed Up and Go, Timed Up and Down Stairs, 6 Minute walk Test, Timed Rise from the Floor, and 30 second Chair Stand for physical function; and Pediatric Quality of Life Inventory Core and Cancer Module for HRQOL. Six physical function tools were selected due to lack of consensus and available measurement properties for this population.32,33 Where indicated, referrals were made to specialised services, such as hospital or community-based physical therapy and mental health.
Phase 2: capacity buildingTheme 1Let's find a reason for you to be physically active
Individually tailored education about physical activity during cancer treatment was provided, along with an information booklet.34 The child/adolescent was asked to identify up to three reasons why being active was important for them. An initial daily step goal was set, noting baseline physical activity, ability, and current medical status. The Fitbit Dashboard was set up on a family device. A support person (e.g. parent) was given a Fitbit. No support person data were collected as their role was motivational. Steps could be monitored continuously via the watch and Fitbit Dashboard. The step goal was displayed visually in their hospital room and communicated to medical teams via their medical record.
Theme 2Let's explore how you can be more physically active
This involved brainstorming how physical activity could be maximised in their current environment. The child/adolescent planned and participated in a physical activity session with the study physical therapist. Activities were individually tailored to child/adolescent's interests, treatment, abilities, and safety restrictions. Only activities the child/adolescent could carry out independently (or carer assistance) were completed. Equipment, toys, or technology were used only if readily available for daily use.
Theme 3Let's make a physical activity plan
Progress towards the daily step goal was reviewed. Participants, support person, and study physical therapist collaboratively identified facilitators, barriers, and potential solutions to achieving their goal. The goal could be reduced to be more achievable or increased to create a challenge. An action plan of individualised strategies was formulated to work towards the step goal. Action planning tasks were implemented by child/adolescent, parent, and/or study physical therapist throughout the following week. Up to 60 min of physical therapist time could be used to support action plan implementation.
Phase 3: consolidationFour weekly review sessions were conducted with the study physical therapist to evaluate and modify intervention strategies based on their success in changing physical activity behaviour. Daily steps data were reviewed, the goal could be altered to ensure it remained achievable in the context of changed circumstances (e.g. discharge home or medical status). New facilitators, barriers, and solutions were discussed. Success of action plan strategies were reviewed and altered. Positive reinforcement was provided in response to success, or attempts made towards goal achievement. Sixty minutes of physical therapist time could be used to support action plan implementation each week. Participants were disconnected from their study Fitbit account at their final visit. Families could opt to keep their Fitbits.
Feasibility assessmentFeasibility was assessed according to domains: demand, implementation, acceptability, practicality, limited efficacy, and integration (Table 1).35 Feasibility thresholds were pre-defined as: recruitment rate 75%, retention rate 90%, Fitbit adherence 90% (assessment) and 60% (monitoring), assessment completion 90%, and intervention attendance rate 90%.
Feasibility domains and analysis.
| Feasibility domain | Assessment item | Feasibility threshold | Type of data | Mode of analysis |
|---|---|---|---|---|
| DemandTo what extent is CanMOVE likely to be usedAcceptabilityTo what extent is CanMOVE considered suitable, satisfying, or attractive to participants and staffImplementationTo what extent was CanMOVE successfully delivered to the intended populationPracticalityTo what extent is CanMOVE able to be carried out with intended populationLimited efficacyDoes demonstrate positive estimates of effect?IntegrationThe extend that CanMOVE could be integrated into the existing healthcare setting | - Recruitment rate, reasons for non-participation- Reasons for participation- Perceived need for intervention participants/support person, staff- Intent to continue use- How suitable, satisfying, or attractive was CanMOVE according to participants/support person, staff- Retention rate- Adherence to wearing the Fitbit:- Phase 1: assessment- Phase 2/3: monitoring and goal setting- Intervention attendance rates- Phase 1: assessment- Phase 2/3- Description of intervention implementation- Description of flexible intervention components:- self- identified reasons to be physically active- common barriers and facilitators, action plan items- use of additional support from study physical therapist- Goal setting patterns and decisions- Factors impacting implementation according to participants/guardian, staff- Adverse events- Negative impacts of CanMOVE according to participants/guardian, staff- Patterns of physical activity across intervention- Physical activity⁎⁎- Physical function⁎⁎- HRQOL- Was CanMOVE described as efficacious according to participants/guardian, staff- Perceived sustainability and fit with current infrastructure of CanMOVE according to participants/guardian, staff | 75%90%90%60%90%90%No serious events | Descriptive statisticsQualitativeDescriptive statisticsQualitativeDescriptive statisticsQualitativeDescriptive statisticsQualitativeDescriptive statisticsQualitativeDescriptive statisticsInferential statisticsQualitativeQualitative | - %: n provided consent, out of all who received trial brief- Categorical: lack of time, not interested, intervention deemed inappropriate, other- Semi-structured interview and staff focus-group thematic analysis- Kept Fitbit (%): n decided to keep Fitbit, out of total participants- Semi-structured interview and staff focus-group thematic analysis- %: n completed trial, out of all who commenced- % valid assessments in Phase 1 (minimum of 4/7 valid days*)- Rate of adherence (mean, SD):% of valid days*, out of total intervention days- %: n of assessments where at least one physical function assessment tool was completed, out of total assessment- %: n completed HRQOL assessments, out of total assessments- %: n completed sessions, out of total intervention sessions- Sessions: duration (mean, SD), mode of delivery (%): in-person or remote, location (%): in-patient or out-patient status- Content analysis of notes taken by the study physical therapist within intervention sessions. Common concepts identified, collated, and synthesised descriptively- Goal setting:- pattern (mean, SD): weekly goal set across intervention- goal increased/decreased (%): n goal increased/decreased /remained unchanged- rate of change (median, 25–75th percentile): % of steps increased / decreased when goal altered- rate of attainment (mean, SD):% days goal attained, out of total valid intervention days- Semi-structured interview and staff focus-group thematic analysis- Event and action taken (description):- Number: how many adverse events- Frequency (%): n participant experiencing adverse event out of total participants commencing the trial- Severity (categorical): likelihood related to trial (possible, probably, definite); was it serious (yes, no); severity (mild, moderate, severe) (Definitions inSupplementary material 2)- Semi-structured interview and staff focus-group thematic analysis- Mean, SD: weekly average steps per day*- Mean change, paired t-test (95% CI): Fitbit- Mean change, paired-test (95% CI): Movement ABC-2, Timed Up and Go, Timed Up and Down Stairs, 6 Minute walk Test, Timed Rise from the Floor, 30 second Chair Stand- Mean change, paired t-test (95% CI): PedsQL Core, PedsQL Cancer Module- Semi-structured interview and staff focus-group thematic analysis- Semi-structured interview and staff focus-group thematic analysis |
CI, confidence interval; HRQOL, health-related quality of life; n, number; PedsQL, Pediatric Quality of Life Inventory; SD, standard deviation.
Quantitative data were collected via RedCAP36,37 and Fitbit Dashboard. Each session the child/adolescent was asked: have you had any falls, new injuries, felt distress due to the intervention, or experienced a change in medical or mobility status. Adverse events were categorised according to severity (mild/moderate/severe), where a severe event prevents usual activity or requires complex treatment; and relatedness to the intervention (unrelated/possible/probably/definite) (Supplementary material 2).
Qualitative data were collected via semi-structured interviews and focus-groups (Supplementary material 3), moderated by a physical therapist experienced in qualitative research. Semi-structured interviews were conducted with each participant (and support person) at final study visit. Three staff focus groups (allied health, nursing, doctors) took place at study completion.
AnalysisFeasibility analysis is presented in Table 1. Data were analysed using NVivo-12.6.1 and STATA-17.
Qualitative data were recorded, transcribed verbatim, deidentified (pseudonyms), and analysed using thematic analysis.38 Analysis began with deductive coding of transcripts line-by-line under each feasibility domain. Data were then analysed inductively to group emergent codes into subthemes. A second investigator (NS) independently deductively coded 50% of transcripts to ensure rigour. Both investigators completed inductive coding under each domain and met to discuss resultant subthemes. Member checking was undertaken whereby a summary of themes were sent to confirm interpretation. Strategies to ensure credibility, dependability, and transferability of results included: data triangulation (participant, support person, staff feedback), reflexive reporting, audit trail, peer examination, and clear description of participants, aims, and research processes.39
ResultsParticipantsTwenty children/adolescents and 20 parents completed CanMOVE (Table 2). All participants completed an interview; 18/20 by parent and child/adolescent, 2/20 parent only. Sixteen staff participated in focus groups (Table 2).
Characteristics of participants.
| Characteristics | ||
|---|---|---|
| Children and adolescents | Age (y) | 13 (9–14) |
| Sex (females)Diagnosis,- AML- ALL- Burkitt Lymphoma- Hodgkin Lymphoma- Neuroblastoma- OsteosarcomaDays since diagnosisRelapsed diseaseTreatment modality (prior to or during intervention)- Chemotherapy- Surgery- HSCT- Radiation- CAR T-cell therapyLength of stay at time of consent (days)Support person- Parent | 9 (45%)8 (40%)7 (35%)2 (10%)1 (5%)1 (5%)1 (5%)51 (31–83)6 (30%)20 (100%)2 (10%)3 (15%)3 (15%)2 (10%)19 (9–29)20 (100%) | |
| Staff | Profession- Nursing- Medical- Allied HealthYears of experience in paediatric oncology- 1–5 y- 6–10 y- 11–15 y- >15 y | 10 (63%)3 (19%)3 (19%)*11 (69%)3 (19%)1 (6%)1 (6%) |
Data are median (25th-75th percentiles) or frequency (proportion).
ALL, Acute lymphoblastic leukaemia; AML, Acute myeloid leukaemia; HSCT, hematopoietic stem cell transplantation; y, years.
A recruitment rate of 96% (21/22) exceeded feasibility threshold and indicated strong demand for CanMOVE (Supplementary material 4). Demand was evident from participant interviews. For many, the decision to participate was easy. Most parents said they were concerned their child/adolescent was not physically active yet didn't know how to help. Some families hesitated to consent due to their child/adolescent being medically unstable.
“We could see he wasn't doing anything. He wasn't being his active self and whatever we could do to get him back to semi-normal was going to happen”. (Parent-P8)
Staff could see the need for physical activity promotion, which they said was generally a responsibility left to parents, with professional support offered only once a child/adolescent displayed a large physical decline.
“We talk about it but maybe it's a useless conversation….because we don't necessarily follow through with any substance.” (Medical Doctor)
AcceptabilityFamilies and staff liked CanMOVE because it was fun, motivating, gave purpose, and brought a sense of normality. They also thought CanMOVE was well suited to the setting, describing it as non-intrusive and achievable. Families said the technology was simple and engaging for children/adolescents of all ages. All families opted to keep their Fitbits at trial completion, and many said they intended to continue applying the concepts they had learnt.
“It feels weird not to wear it. I want to know how many steps I've done still, and it's been a routine now.” (P20)
ImplementationRetention rate exceeded feasibility threshold at 95% (20/21) (Supplementary material 4). Mean intervention duration was 11 weeks. In Phase 1, assessment completion rate was above feasibility threshold. Valid Fitbit data were available for 98% (39/40) assessments, 96% (154/160) HRQOL assessments were completed, and at least one physical function assessment tool was completed in 90% (36/40) of assessments. Overall, 74% physical function assessment tools (177/240) were completed (Supplementary material 5). Analysis of assessment tool psychometric properties and feasibility will be reported elsewhere.
Phase 2/3 intervention completion rate was 95% (20/21), above feasibility threshold. Mean (standard deviation [SD]) session duration for each theme in Phase 2 was 40 (20) minutes (content often split over two sessions) and 17 (5) minutes for Phase 3. Participants were in-patients for 49%, and out-patients for 51% of sessions; 74% were in-person and 26% conducted remotely. The mean adherence rate to wearing the Fitbit for monitoring and goal setting was 79% (SD 17), exceeding the feasibility threshold. Table 3 summarises implementation of Phase 2/3. Despite many unmodifiable barriers, participants achieved their step goals at a mean rate of 85% (SD, 17). Common facilitators included having a goal, time at home, and professional support. Action plans centred around creating everyday opportunities for physical activity, and facilitating access to space, equipment, and social interaction. All participants were referred to hospital physical therapy services for management of specific impairments.
Implementation: flexible intervention components.
| Type of session | Variable component | Outcome / common themes | |
|---|---|---|---|
| Theme 1: Let's find a reason for you to be physically activeTheme 2: Let's explore how you can be more physically activeTheme 3: Let's make a physical activity planANDPhase 3: Consolidation | Self-determined reasons to be physically activeSupervised physical activity sessionGoal settingBarrier and facilitator identificationAction planningExtra study physical therapist time | Commonly recorded reasonsCompletion rate, reason for non-completionTypes of activities recordedDirection of change (/96)Rate of change:Rate of goal attainment:Factors considered in goal decision making:Barriers reported: themesFacilitators reported: themesAction plan items implemented: themesRate of useDuration if time usedTime spent: themesReferrals or re-referral to other services*, (/20 participants) | - Improve current physical function and ability- Get back to the things they love after treatment- Improve motivation and participation in daily life during treatment40% (8/20), reasons- Already independent with chosen activity (6/8)- Already set up with physical therapy program (1/8)- Medically unstable (1/8)- Adapted sporting and play activities- Independent exercise plan- Seek hospital spaces outside of their hospital room- Active gaming using technology- Goal increased: 52/96 (54%)- Goal decreased: 12/96 (13%)- Goal unchanged: 32/96 (33%)- When increased, goal increased by median 60% (40–113)- When decreased, goal decreased by median: 39% (19–50)Mean 85% (17)- Participant location: in-patient or out-patient- Upcoming treatment and medical status- Patterns of activity and goal attainment: are they achieving goal easily? Do they achieve it every day?- Preference: do they want goal to be achievable / set high to motivate a challenge- Physical symptoms (reduced function, fatigue, treatment side effects)- Restricted access to people, activities, equipment, and spaces (small spaces, COVID lockdown, infection risk, attachments, can't do things for themselves)- Reduced mental health (low motivation, fear, anxiety, family stress)- Medical appointments and procedures- Faulty equipment/technology (Fitbit issues, broken sporting equipment)- Having a goal- Time spent at home- Access to achievable physical activity options (routines, interests, equipment)- Access to physical space (larger open spaces, unrestricted movement)- Feeling better (reduced physical symptoms and improved function)- Social interaction- Professional support- Planning daily routines (walks, incidental activities, ADLs, routines, exercise program, leisure activities, outings, planning for upcoming admissions)- Increase healthcare professional support (see below- extra physical therapy time)- Organising social interactions (friends, family, school visits, community sport visits)- Creating reminders to get up and move (visual reminders, Fitbit reminders, physical activity diary)- Sourcing equipment (technology, sports equipment)45% (36/80 available weeks)Mean 28 (16) minutes- Building confidence and capacity (reminders, positive feedback, loaning equipment, reviewing exercise program, additional brainstorming)- Engaging other healthcare professionals (referrals, line-free time, time outside/off ward, engage other staff members in motivating family)- Symptom management education (fatigue, pain, neuropathy)- PT (20/20): strength impairments, mobility issues, vincristine neuropathy- OT (3/20): hand function, ADL support- Mental health (2/20): family stress, deteriorating mood |
Data are mean (standard deviation) or median (25 %−75 % percentiles).
ADL, activities of daily living; HCP, healthcare professional; OT, occupational therapist; PT, physical therapist; SD, standard deviation.
Technology issues impacted implementation. Some children/adolescents forgot to wear the device, didn't keep it charged, or found it uncomfortable to wear when unwell, others said it would sometimes not sync. Some families found CanMOVE challenging when their child/adolescent was acutely unwell.
“…it was just probably the worst timing for him…that first little bit he didn't really even remember wearing the band because he was so sick. And we were in bed most of the time.” (Parent-P14)
PracticalityThere were no serious adverse events related to the intervention. Nineteen mild adverse events were reported from 15 participants (Supplementary material 2). Despite using the highest security settings, ‘friend requests’ from unknown accounts cannot be blocked on the Fitbit Dashboard. Unsolicited ‘friend requests’ containing an explicit photo were sent to five Fitbit Dashboards (content not seen by participants) (Supplementary material 2).
Qualitatively, few negative comments were raised. Some children/adolescents described ‘getting discouraged’ when a goal was not achieved, or their physical function performance was worse than expected.
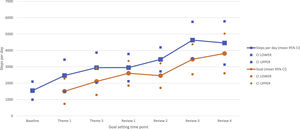
Limited efficacyResults suggest CanMOVE influenced physical activity behaviour. Mean increase in average steps per day was 2980 (95% Confidence Interval: 1569, 4391) (Supplementary material 5). A steady increase in goal setting and average daily steps was achieved during the intervention (Figure 2). Families said CanMOVE helped them see physical activity as achievable and beneficial. Consequently, they modified everyday routines by optimising activities of daily living, and scheduling daily walks, exercise, or play. Parents reported a shift away from doing everything for their child/adolescent. This made physical activity feel automatic and encouraged children/adolescents to re-engage with interests. Intervention components identified as facilitating behaviour change were flexible goals, real-time monitoring, and weekly support. Families felt in control, motivated, and accountable for their behaviour. Families valued the collaborative and individualised approach and felt a sense of pride in achievements.
“We didn't need to baby him; we didn't need to make him be sick. We treated him a bit more normal and tried to get him to do more.” (Parent-P8) “You're not having to get psyched up to do it. It becomes an automatic thing. It makes it easier then”. (Parent-P6)
Results indicate that CanMOVE can positively influence physical function and HRQOL (Supplementary material 5). Families and staff noted benefits including improved physical ability, energy, appetite, sleep, and mood. Families said they saw their child/adolescent grow in confidence and independence, which led to a renewed sense of self.
“It just helped me build my muscles and everything and get me stronger, because my muscles used to be really weak and everything.” (P10)
Parents liked that CanMOVE was something positive they could do with their child/adolescent and provided an opportunity to bond. They said CanMOVE took the effort out of trying to get their child/adolescent moving. In some cases, parents felt a greater sense of hope and positivity towards the future. Doctors said families were more receptive to physical activity advice. Some parents reported feeling more motivated to improve their own health, and this translated to positive changes across the whole family.
“Cancer was trying to steal as much as it could from us, and that's when we said, it can't steal this as well. So, I guess it gave us the ability to take some of that power back.” (Parent-P1)
Staff and families described positive changes in daily interactions with children/adolescents. Nurses said it encouraged them to help children/adolescents achieve their goal and described a flow-on effect to other families. Doctors said having a visible steps goal acted as a prompt to discuss physical activity and was a useful way to monitor how a child/adolescent was coping physically.
“… the nurses and the doctors really took it on board. It was really good for me to see the nurses interact with Luke. Not just like, all right, we're here to take your blood, or we're here to give you this medicine, we're here to do this test… It was like, (child's name) how many steps have you got? How many have you got left? It was a more positive interaction.” (Parent-P7)
IntegrationStaff identified the benefit of a multidisciplinary team approach to physical activity promotion. Nurses suggested their role could be formally integrated into the program, indicating the necessity of education and training. “Maybe if we all have a strong front and thinking that they all have to do it, so we all just agree and you do it…it's just an expectation.” (Nurse)
Many families noted the value in offering the program to all children/adolescents close to diagnosis. Physical therapists agreed, yet highlighted the importance of timing to suit individual circumstances. They also recommended extending the program to help maintain behaviour change.
Families and staff suggested changes to the physical hospital environment could promote physical activity. Examples included: providing spaces to be physically active, facilitating outdoor access, and making equipment readily available. This was emphasised for adolescents. They also recommended additional opportunities for social and supportive interaction between families. “… having an outdoor space that they can access with all of their attachments because you know getting outside can make such a difference. Whether that is during our sessions…or there's a nurse allocated specifically to be able to take kids off the ward.” (Physical therapist)
Maximising a child/adolescent's physical activity participation during acute cancer treatment has potential to improve immediate and long-term health outcomes. Increasing importance is placed on theory-driven behaviour change programs.17,18,40 CanMOVE is a feasible physical activity behaviour change intervention for children/adolescents undergoing acute treatment. There was high demand, high acceptability from participants and staff, and no serious adverse events related to the intervention. Results indicate CanMOVE showed positive estimates of effect in changing child/adolescent's physical activity behaviour, and supportive behaviours of parents and staff. Data showed a positive effect on physical and mental health outcomes. This study identifies key intervention features that were effective in supporting positive physical activity behaviour. These strategies can be used to inform future intervention design initiatives, and by healthcare professionals in the clinical setting.
Individualised goal-setting and real-time monitoring were highlighted as key features of CanMOVE. Implementing a tailored approach to activity promotion and applying clustered self-regulatory techniques has been associated with effective physical activity interventions.41 By employing these strategies, CanMOVE gave control and accountability back to children/adolescents and provided a tangible avenue to monitor improvement. This facilitated independence and encouraged families to acknowledge improvements and celebrate success. Results suggest a commercial activity monitor is effective for implementing these strategies.42 In addition to being engaging and easy to use, this technology gives an opportunity to implement cost-effective programs across in-patient and out-patient settings. There are risks associated with using on-line technology that must be mitigated to ensure safe use.
Another key feature of CanMOVE was parental engagement. Parents play an integral role in promoting healthy lifestyle behaviours,18 and literature supports their involvement in physical activity interventions.43 Results indicate CanMOVE was helpful in overcoming parental overprotection, a behaviour commonly seen in this setting.44 Re-framing physical activity as an essential and achievable aspect of daily routines appeared to challenge their child/adolescent's perceived vulnerability and may have contributed to a shift in perception. Collaborating to devise individualised and flexible solutions gave families control of the strategies implemented, fostered motivation, and built confidence in their child/adolescent's ability to safely move.
Our results highlight the importance of supportive networks, social engagement, and the environment in promoting physical activity. Successful behaviour change interventions rely on addressing all aspects of the social ecological model, including individual, environmental, and organisational factors.17 The role of the multidisciplinary team is crucial. A concerted effort to involve medical, nursing, and allied health teams could lead to long-standing changes to clinical practice and organisational values. To achieve this, appropriate training is needed to build understanding of the importance of physical activity and effective ways to promote it in day-to-day care.45 Changes to clinical processes, practices, and policy could lead to environmental changes. For example, policies and services that facilitate safe and routine access to open spaces and equipment, opportunity for social interactions, and time out of the hospital environment.
Promoting physical activity is only one aspect of a multi-faceted approach required to improve health outcomes for children/adolescents with cancer. Understanding how programs such as CanMOVE can sit within broader clinical services is essential. Services such as physical therapy are vital to treat specific impairments to avoid costly and chronic musculoskeletal complications. CanMOVE could be the first step in a layered approach to service delivery, similar to the stepped-care model in adult cancer care,46 where intervention intensity is proportional to need. For example, all children/adolescents receive an intensive block of physical active support through CanMOVE, then continue with routine monitoring and be triaged to more intensive services as needed. This ensures impairment level interventions are targeted and timed appropriately.
Main strengths of the study included the use of a theoretically designed behaviour change intervention, and a mixed methods study design to assess feasibility. The broad eligibility criteria gave insight into CanMOVE's suitability for diverse participants. There were some limitations, participant preparedness for behaviour change and cost-effectiveness were not evaluated. Study findings will inform further CanMOVE development; including a defined role for nursing staff through co-design, streamlining assessment tools, and additional assessment time-points to evaluate behaviour change maintenance. Future challenges include determining an appropriate study design for large-scale trial, evaluating costs involved in using technology, and managing large quantities of physical activity data.
ConclusionPhysical activity promotion should be a prioritised aspect of clinical care for children/adolescents with cancer. CanMOVE is a feasible physical activity program where individualised goal setting, real-time monitoring, professional support, and parental input were important intervention characteristics. The benefit of re-framing physical activity as an achievable and essential part of everyday routines and social interactions were also highlighted. Key intervention strategies can be applied to the clinical setting and to future intervention design. Future studies will develop CanMOVE for large-scale trial and work towards integrating the program into a multidisciplinary clinical model. Applying these findings to clinical care will improve access to evidence-based services that maximise physical activity participation and future health outcomes.
This work was supported by an Australian Government Research Training Program Scholarship through La Trobe University, a PhD top-up scholarship from the Murdoch Children's Research Institute, and a project grant from the charity organisation Little Big Steps. These funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in writing the report, nor the decision to submit the article for publishing.
Supplementary files
Supplementary material 1: Objective measures
Supplementary material 2: Practicality results
Supplementary material 3: Interview and focus group interview schedules
Supplementary material 4: Flow diagram of participants (children/adolescents aged ≥5 and ≤16 years, with new/relapse diagnosis of cancer; and staff participants)
Supplementary material 5: Limited efficacy