Pelvic floor muscle training is the most commonly used physical therapy treatment for women with urinary incontinence.
ObjectivesTo assess the effects of Pelvic floor muscle training for women with urinary incontinence in comparison to a control treatment and to summarize relevant economic findings.
MethodsCochrane Incontinence Group Specialized Register (February 12, 2018). Selection criteria: Randomized or quasi-randomized trials in women with stress, urgency or mixed urinary incontinence (symptoms, signs, or urodynamic). Data collection and analysis: Trials were independently assessed by at least two reviewers authors and subgrouped by urinary incontinence type. Quality of evidence was assessed by adopting the Grading of Recommendations, Assessment, Development and Evaluation approach.
ResultsThe review included thirty-one trials involving 1817 women from 14 countries. Overall, trials were small to moderate size, and many were at moderate risk of bias. There was considerable variation in the intervention's content and duration. Based on data available, we can be confident that Pelvic floor muscle training can cure or improve symptoms of stress and all other types of urinary incontinence. It may reduce the number of leakage episodes and the quantity of leakage, while improving reported symptoms and quality of life. Women were more satisfied with Pelvic floor muscle training, while those in control groups were more likely to seek further treatment. Long-term effectiveness and cost-effectiveness of Pelvic floor muscle training needs to be further researched.
ConclusionsThe addition of ten new trials did not change the essential findings of the earlier review, suggesting that Pelvic floor muscle training could be included in first-line conservative management of women with urinary incontinence.
Urinary incontinence (UI) is a common problem amongst adults living in the community. It is more frequent in women, increasing with age, and is particularly common amongst those in residential care.1 Estimates of prevalence of UI in women vary between 25% and 45% in most studies,2 with a gradual increase in prevalence with age to an early peak prevalence around midlife (50–54 years) which coincides with menopause, followed by a slight decline or stabilization until about 70 years of age when the prevalence begins to rise steadily.3 Pregnancy, labour and vaginal delivery (versus caesarean section) are significant risk factors for later UI, but the strength of this association diminishes substantially with age.4
The type of urine leakage is classified according to what is reported by the woman (symptoms), what is observed by the clinician (signs), and on the basis of urodynamic studies.5 If a woman reports involuntary urine leakage with physical exertion (symptom) or a clinician observes urine leakage at the same time as the exertion (sign) this is called stress UI (SUI). The symptom of urgency UI (UUI) is present when a woman reports involuntary leakage associated with or immediately proceeded by a sudden compelling need to void. The sign of UUI is identified by the observation of involuntary urine leakage from the urethra synchronous with the sensation of a sudden, compelling desire to void that is difficult to defer. Many women have symptoms or signs of both stress and UUI, this is called mixed UI (MUI).
Isolated SUI accounts for half of all UI, with most studies reporting 10–39% prevalence. With few exceptions, MUI is found to be next most common, with most studies reporting 7.5–25% prevalence. Isolated UUI is uncommon, with 1–7% prevalence.4 Pelvic floor muscle training (PFMT) is defined as a programme of exercises to improve pelvic floor muscle (PFM) strength, endurance, power, relaxation or a combination of these parameters.6 It is the most commonly used physical therapy treatment for women with SUI. It is sometimes also recommended for MUI and, less commonly, UUI.
The biological rationale for PFMT in women with SUI is twofold. Firstly, an intentional, effective PFM contraction (lifting the PFMs in a cranial and forward direction) prior to and during effort or exertion clamps the urethra and increases the urethral pressure, preventing urine leakage.7 Secondly, the bladder neck receives support from strong, toned PFMs (resistant to stretching), thereby limiting its downward movement during effort and exertion, thus preventing urine leakage.8 PFMT could also potentially be used in the management of UUI. The biological rationale is based on Godec's et al.9 observation that a detrusor muscle contraction can be inhibited by a PFM contraction induced by electrical stimulation.9 After inhibiting the urgency to void, the woman can reach the toilet in time, to avoid urine leakage.
Earlier versions of this Cochrane systematic10–13 review are outdated with the publication of new trials. There is sufficient uncertainty about the effects of PFMT, particularly the size of effect, to suggest that continuing to update earlier Cochrane reviews is warranted. The present review is a short version of the 2018 Cochrane systematic review.14
Objectives- 1.
To assess the effects of PFMT for women with UI in comparison to no treatment, placebo or sham treatments, or other inactive control treatments.
- 2.
To summarize the availability and principal findings in terms of costs and cost-effectiveness of eligible economic evaluations in a Brief Economic Commentary.
This review drew on the search strategy developed by Cochrane Incontinence. We identified relevant trials from the Cochrane Incontinence Specialized Register. The date of the last search was February 12, 2018.
Selection of studiesWe included randomized and quasi-randomized controlled trials of PFMT for the treatment of UI. Two review authors (CD with LPC or JHS) independently screened the list of titles and abstracts generated by our search and further independently assessed the full-text articles or abstracts for eligibility. Any differences of opinion were resolved by discussion or involvement of a third party.
Data extraction and managementAll included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions.15 Data extraction was undertaken independently by two review authors (CD and LPC) and cross-checked by JHS. Any differences of opinion related to the data extraction were resolved by discussion. Where study data were possibly collected but not reported, or data were reported in a form that could not be used in the formal comparisons, further clarification was sought from the trialists.
Assessment of risk of bias in included studiesWe assessed the risk of bias using Cochrane's ‘Risk of bias’ assessment tool.15 Two review authors (CD with LPC) independently assessed these domains and they were cross-checked by JHS. Any differences of opinion were resolved by consensus.
Measures of treatment effectAnalyses were based on available data from all included trials relevant to the comparisons and outcomes of interest. For trials with multiple publications, only the most up-to-date or complete data for each outcome were included. Meta-analysis was undertaken where data were available from more than one study assessing the same outcome. For categorical outcomes, we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% confidence interval (CI). For continuous variables, we used means and standard deviations to calculate a mean difference (MD) with 95% CI.
Subgroup analysis and investigation of heterogeneityAnalysis within subgroups was used to address the effect of the type of incontinence on outcome. Because the rationale for PFMT is different for the two main types of UI (stress and urgency), it is plausible to expect a difference in the outcome of PFMT on the basis of the type of incontinence. The four pre-specified diagnostic subgroups were trials that recruited women with: SUI, UI, MUI, and UI all types (women could have stress, urgency or MUI, but data were not reported separately according to these subgroups). If heterogeneity between trials was sufficiently large, an investigation to identify its causes was conducted. If heterogeneity remained after appropriate investigation, and possible removal of outlying trials, a random-effects model rather than a fixed-effect model was used in the meta-analysis.
Quality of evidenceThe Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was employed to interpret findings16 and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.2 (Review Manager) to create ‘Summary of findings’ tables. These tables provide outcome-specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered. The following outcomes were included in the ‘Summary of findings’ tables: participant perceived cure; participant perceived cure or improvement; number of leakage episodes in 24h; short (up to one hour) pad test measured as grams of urine, and GRADE A UI-specific symptom measures; GRADE A UI-specific QoL measures.
Incorporating economics evidenceA Brief Economic Commentary was developed to summarize the availability and principal findings of the economic evaluations captured as part of this review. This included evaluations alongside trials and model based evaluations. This was carried out in accordance with current guidance. This commentary focused on the extent to which principal findings of eligible economic evaluations indicate that an intervention might be judged favourably (or unfavourably) from an economic perspective, when implemented in different settings. A supplementary search to identify economic studies was carried out according to the guidelines in Cochrane Economics Methods.17
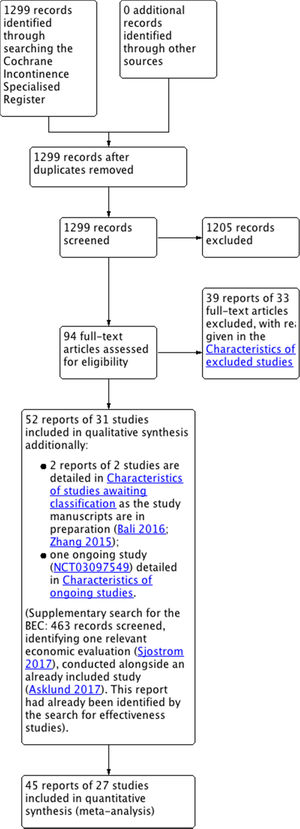
ResultsDescription of studiesThe search produced 1299 records, from which 94 potentially relevant full-text articles were retrieved. Fifty-two reports of 31 trials met the inclusion criteria. See Fig. 1.
Included studiesTen new trials were added in the update. In total, thirty-one trials involving 1817 women (933 PFMT, 884 controls) were included18–48; 27 trials contributed data to the meta-analysis (1570 women) with four trials containing no usable data.22,35,40,43 Twenty-one trials contributed to the analysis of primary outcomes.19–21,23,26–34,36,38,39,41,44,46–48 Further details on the characteristics of the included and excluded trials are provided in the full version of the Cochrane review.14
ParticipantsAll the women had UI. Based on diagnosis, the subgroups were: SUI (21 trials),18,19,22–30,32,34–36,41,43,45–48 MUI (one trial),38 UUI (one trial)23 and a combination of UI diagnoses, grouped together here as UI all types (ten trials).20,21,29,31,33,37,39,40,42,44
InterventionsThree trials gave no details of the PFMT programme used.25,26,43 Of the 28 remaining trials, 21 stated that a correct voluntary PFM maximal contraction was confirmed prior to training using either vaginal, rectal or physical examination.18,20,21,23,24,30,32–40,42,44–48 Five trials reported that participants were taught a voluntary PFM maximal contraction but did not say how.22,27–29,31 One trial reported that participants were instructed by a smartphone app to identify the correct voluntary PFM contraction, but without face-to-face interaction with health professionals.19
The individual characteristics of each exercise programme including the number of voluntary PFM contractions; duration of hold; duration of rest; number of sets per day; types of contraction (e.g., maximal, sustained, fast); body position; and adherence strategies are detailed in Appendix A (PFMT protocols).
Control interventions included no treatment,18–21,23–25,27,30,34–38,41–43,45,47 placebo drug44 and sham electrical stimulation.26 Inactive control treatments comprised use of an anti-incontinence device,46 advice on incontinence pads,32 motivational phone calls once per month,48 advice on simple lifestyle alterations,31,40 general education class (cognitive function, osteoporosis and oral hygiene),29,39 refraining from special exercises aiming to increase muscle strength, to reduce body mass index or to improve dietary habits28 and access to an educational pamphlet or advices on UI.22,33
OutcomesOverall there was no consistency in the choice of outcome measures, by trialists. This limited the possibilities for considering together the results from individual trials. Four eligible trials did not contribute any data to the main analyses because they did not report any pre-specified outcome of interest or they did not report their outcome data in a usable way (e.g., mean without a measure of dispersion, p values without raw data or only post-intervention minus pre-intervention data available).22,35,40,43 Communication with the authors was attempted but no responses were received.
Primary outcome measures: symptomatic cure and symptomatic cure or improvement of UI at the end of treatmentMany different scales were used to measure a participant's response to treatment, including Likert scales, visual analogue scales, and percent reduction in symptoms. Whatever the scale, data were included in the formal comparisons when the trialists stated the number of women who perceived they were cured or improved (as defined by the trialists) after treatment. Where more than one level of improvement was reported (e.g., much better and somewhat better), data for the greater degree of improvement was entered in the comparison. It was thought this was more likely to capture those who had improvement that was clinically important. As some trial reports did not differentiate cure from improvement, two measures (cure only, and cure or improvement) were used so that important data were not lost. The following definitions were used by the trialists.
Participant reported cure comprised:
- •
no urine loss or ‘dry’29,44;
- •
‘incontinence is now unproblematic’,46 and
- •
no leakage in a urinary diary.26,28,31
- •
Participant reported cure or improvement was defined as:
- •
much better and somewhat better19,21;
- •
‘75% or more perceived improvement’44;
- •
‘dry’ or ‘improved’,32 and
- •
‘continent’ or ‘almost continent’.46
Thirteen trials used GRADE A psychometrically robust questionnaires for assessment of incontinence symptoms (ICIQ-UI and UDI),19,20,27,30,34,38 the impact of these symptoms on QoL (ICIQ-LUTSqol, IIQ and I-QOL),19,20,33,34,37,39,48 or both (King's Health Questionnaire).36,41,47
Secondary outcomes- •
Longer-term symptomatic cure and improvement after stopping treatment (six months to one year after end of treatment; more than one year after end of treatment)24,31,39;
- •
Satisfaction44,46,48;
- •
Need for further treatment44,46;
- •
Self-efficacy39
- •
Number of urinary leakage episodes (per 24h)19,20,23,32–34,39,44–46,48;
- •
Number of micturitions during the day (frequency) or night (nocturia)20,21,23,42;
- •
Pad and paper towel testing short (up to one hour) or long (24h) urine loss (grams of urine lost) at the end of treatment20,21,34,36,39,42,46,48;
- •
Number cured or improved based on pad weights in short office-based pad test at the end of treatment18,24,25,46,48;
- •
Other pad or paper towel tests (e.g.: those not reported as cure, cure and improvement or grams, those reported at other time points after treatment)18,21,35,39,43;
- •
QoL (not condition specific)27,44,46;
- •
Sexual function or problems46;
- •
Adverse effects32,33,38,39,44,46,48;
- •
Socioeconomic measures19,49;
- •
Measures of PFM function,18,20,21,23,30,34–36,40–42,45–48 and
- •
Measures of adherence.19,28,31–33,38–40,43,45,46,48
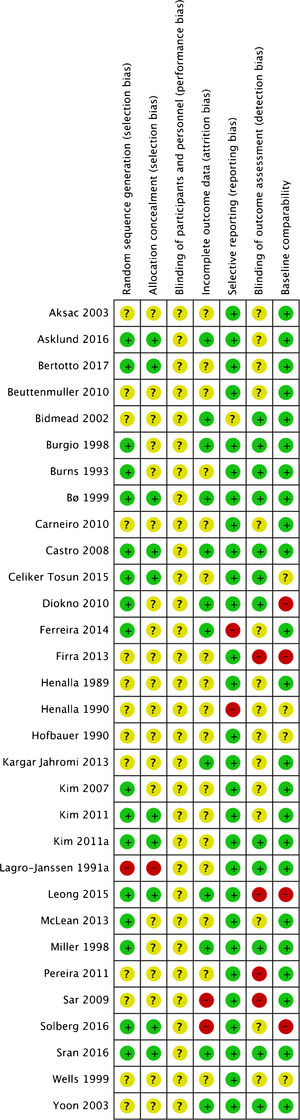
Due to brevity of reporting, it was difficult to assess the two trials that were published as conference abstracts.25,43 Fifteen of the trials were small, with fewer than 25 women per comparison group.18,21–23,25–27,30,34–39,42 Ten were of moderate size, with around 25–50 per group.24,28,31–33,41,45–48 The other five allocated more than 50 women per group.19,20,31,40,44 Bidmead et al.43 randomized participants in a 2:1 ratio, with 40 in the PFMT group and 20 as controls.43 Eleven trials, including five recent ones, reported on a priori power calculation.19,20,23,28,31,33,34,37,39,46,48 Risk of bias assessment is illustrated in Fig. 2 and fully described in the complete Cochrane review.
GRADE assessmentThe following factors were considered to downgrade the evidence: limitations in the study design; inconsistency of results; indirectness of evidence; imprecision, and publication bias.
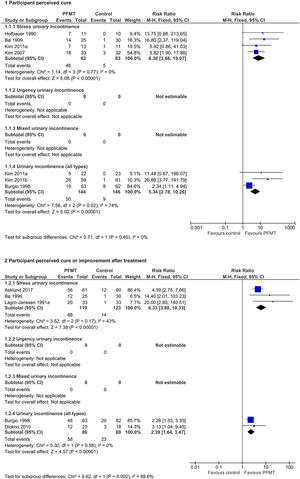
Effects of interventionsPrimary outcome measuresSymptomatic cure of UI at the end of treatmentSix trials reported data on cure only,26,28,29,31,44,46 all with wide confidence intervals. All trials found that PFMT women were more likely to report cure. From the pooled results, after a PFMT programme women with SUI alone were eight times more likely to report cure26,28,29,46 (56% versus 6%; RR 8.38, 95% CI 3.68–19.07; 4 trials, 165 women; high-quality evidence; I2=0%); and women with UI all types were five times more likely to report cure,29,31,44 although with substantial statistical heterogeneity (35% versus 6%; RR 5.34, 95% CI 2.78–10.26; 3 trials, 290 women; moderate-quality evidence; I2=74%). When using a more conservative random-effects model the results were maintained, still favouring PFMT (RR 7.50, 95% CI 1.03–54.63). Visual inspection of the forest plot suggested a smaller effect size in Burgio et al.,44 while the effect size appeared similar in the two remaining trials. A possible explanation of this difference in treatment effect may come from the percentage of women with urgency symptoms, which was higher in this trial. Refer to Fig. 3.
Symptomatic cure or improvement of UI at the end of treatmentFive trials contributed outcome data for cure or improvement.19,21,32,44,46 Similarly, all five reported that PFMT was better than control interventions. In trials which included women with SUI alone,19,32,46 PFMT women were six times more likely to report cure or improvement than controls (74% versus 11%; RR 6.33, 95% CI 3.88–10.33; 3 trials, 242 women; moderate-quality evidence; I2=43%); and in trials which included women with UI all types,21,44 PFMT women were twice as likely to report cure or improvement compared to controls (67% versus 29%; RR 2.39, 95% CI 1.64–3.47; 2 trials, 166 women; moderate-quality evidence; I2=0%). Refer to Fig. 3.
Symptom and condition-specific QoL measuresA narrative summary of all Grade A UI symptoms and condition-specific QoL measures are presented in the summary of findings table (Tables 1 and 2). Eight out of nine different measures of QoL specific to the effect of UI were in favour of PFMT in women with SUI, MUI and UI all types. In the King's Health Questionnaire, which measures the impact of incontinence after treatment, there was considerable statistical heterogeneity (I2=76%). When a random-effects model was used there was no evidence of a difference between treatment groups, although all trials had the same direction of effect and their confidence intervals included clinically important differences favouring the PFMT groups.
Summary of findings for women with stress urinary incontinence.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with no treatment, placebo or control | Risk with PFMT | |||||
| Participant perceived cure after treatment Treatment duration: 3 to 6 months | 60 per 1000a | 505 per 1000 (222–1000) | RR 8.38 (3.68–19.07) | 165 (4 RCTs) | ⨁⨁⨁⨁ HIGH b | |
| Participant perceived cure or improvement after treatment Treatment duration: 3–6 months | 114 per 1000 a | 720 per 1000 (442–1000) | RR 6.33 (3.88–10.33) | 242 (3 RCTs) | ⨁⨁⨁◯ MODERATE c,d | |
| Number of leakage episodes in 24h assessed with: bladder diary Treatment duration: 8 weeks–6 months | The mean number of leakage episodes in 24h ranged from 1.07–3.61 episodes | The mean number of leakage episodes in 24h in the intervention group was 1.23 episodes lower (1.78 lower to 0.68 lower) | – | 432 (7 RCTs) | ⨁⨁⨁◯ MODERATE e,f,g,h,i | |
| Short (up to one hour) pad test measured as grams of urine Treatment duration: 6 weeks to 6 months | The mean short (up to one hour) pad test measured as grams of urine ranged from 3.64–38.70g | The mean short (up to one hour) pad test measured as grams of urine in the intervention group was 9.71g lower (18.92 lower to 0.5 lower) | – | 185 (4 RCTs) | ⨁⨁⨁◯ MODERATE i,j,k | |
| GRADE A UI-specific symptom measures Treatment duration: 4–12 weeks | Three different Grade A psychometrically robust symptom questionnaires were used by trialists including KHQ severity domain (3 trials; n=65), ICIQ-UI (3 trials; n=98) and UDI (1 trial; n=17). Patients in the PFMT group reported significant improvement in UI symptoms. | (7 RCTs) | ⨁⨁⨁◯ MODERATE l,m,n,o | |||
| GRADE A UI-specific QoL measures Treatment duration: 6 weeks to 6 months | Five different Grade A psychometrically robust QoL questionnaires were used by trialists including KHQ impact domain (3 trials; n=65), KHQ physical limitation domain (3 trials; n=65); ICIQ-Luts QoL (1 trial; n=60); IIQ (1 trial; n=17); IQOL (1 trial; n=24). Patients in the PFMT group reported significant improvement in UI specific QoL except for the KHQ impact after treatment, however with considerable heterogeneity (I2=76%). | (6 RCTs) | ⨁⨁◯◯ LOW i,l | |||
PFMT compared to no treatment, placebo or control for stress urinary incontinence in women (SUI).
Patient or population: stress urinary incontinence in women (SUI).
Setting: community-dwelling women.
Intervention: PFMT.
Comparison: no treatment, placebo or control.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI, confidence interval; RR, risk ratio; MD, mean difference.
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Explanations.
Blinding of outcome assessor judged to be unclear in 1/3 trial, for which the participants filled web-based questionnaires with no face-to-face interaction with the researcher group.19
Baseline comparability judged to be high risk for not for this outcome and in a different sub-group in 1/7 trial (urinary frequency for the urge incontinent subgroup).23
Random sequence generation is unclear, and blinding of outcome assessment judge to be high risk in 1/4 trial.36
Random sequence generation, allocation concealment, incomplete data and blinding of outcome assessor unclear for one trial.47
Summary of findings for women with combined urinary incontinence types.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with no treatment, placebo or control | Risk with PFMT | |||||
| Participant perceived cure after treatment Treatment duration: 8 weeks to 12 weeks | 62 per 1000 a | 329 per 1000 (171–632) | RR 5.34 (2.78–10.26) | 290 (3 RCTs) | ⨁⨁⨁◯ MODERATE b,c,d | |
| Participant perceived cure or improvement after treatment Treatment duration: 6 weeks to 8 weeks | 288 per 1000 a | 687 per 1000 (471–998) | RR 2.39 (1.64–3.47) | 166 (2 RCTs) | ⨁⨁⨁◯ MODERATE e,f,g | |
| Number of leakage episodes in 24h assessed with: bladder diary Treatment duration: 8 weeks to 12 weeks | The mean number of leakage episodes in 24h ranged from 1.06–2.50 episodes | The mean number of leakage episodes in 24h in the intervention group was 1 episodes lower (1.37 lower to 0.64 lower) | – | 349 (4 RCTs) | ⨁⨁⨁◯ MODERATE h,i,j | |
| Short (up to one hour) pad test measured as grams of urine Treatment duration: 6 weeks to 6 months | The mean short (up to one hour) pad test measured as grams of urine ranged from 5.10–8.40g | The mean short (up to one hour) pad test measured as grams of urine in the intervention group was 3.72g lower (5.46 lower to 1.98 lower) | – | 146 (2 RCTs) | ⨁⨁⨁◯ MODERATE i,k | |
| GRADE A UI-specific symptom measures Treatment duration: 12 weeks | One Grade A psychometrically robust symptom questionnaire was used by one trial (n=63); the UDI. Patient in the PFMT group reported significant improvement in UI specific symptoms. | (1 RCT) | ⨁⨁⨁◯ MODERATE g,i,l | |||
| GRADE A UI-specific QoL measures Treatment duration: 6–12 weeks | Four different Grade A psychometrically robust QoL questionnaires were used by trialists including the IIQ short form (2 trials; n=91), the IIQ long form (1 trial; n=24); IQOL (1 trial; n=17). Patient in the PFMT group reported significant improvement in UI specific QoL. | (4 RCTs) | ⨁⨁◯◯ LOW g,i,j,m | |||
PFMT compared to no treatment, placebo or control for urinary incontinence in women (all types).
Patient or population: urinary incontinence in women (all types).
Setting: community-dwelling women.
Intervention: PFMT.
Comparison: no treatment, placebo or control.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, confidence interval; RR, risk ratio; MD, mean difference.
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Explanations
Baseline comparability judged to be high risk for 1/2 trial, with older participants in the PFMT group.21
Incomplete outcome data, blinding of participant and personnel, baseline comparability for a different outcome judged to be unclear in one trial.20
(PFMT group presenting lower impact on quality of life and higher night-time urinary frequency).
Blinding of outcome assessor and baseline comparability judged to be high risk in 1 trial (PFMT group older (p=0.06) and presenting higher impact on quality of life (p=0.06)).33
The main secondary outcomes are presented below. More details and forest plots (when applicable) are presented in the complete Cochrane review.14
Longer-term symptomatic cure and improvement after stopping treatmentThere was limited information from two low to moderate quality trials which indicated that UI cure or improvement after PFMT seemed to persist (after treatment stopped) for up to a year in both women with SUI only (54% versus 0%; RR 27.93, 95% CI 1.75–444.45; 1 trial, 51 women)24 and those with UI all types (39% versus 2%; RR 23.78, 95% CI 3.32–170.49; 1 trial, 82 women).31 The CIs in both trials were wide and hence these results need further confirmation. One new small trial of good quality indicated that the benefit of PFMT seemed to persist (after treatment stopped) for up to a year in women with UI all types in regards to symptoms (MD 38.58 lower, 95% CI 67.61 lower to 9.55 lower; 48 women) and UI-specific QoL measures (IIQ long form, MD 41.91 lower, 95% CI 83.20 lower to 0.62 lower; 48 women)39 Another new trial19 published one year50 and two year51 follow-up reports with data on symptoms (ICIQ-UI SF) and UI-specific QoL (ICIQ-LUTS), however with no control group comparison.
Satisfaction and need for further treatmentIn trials which included women with SUI alone,46,48 PFMT women were five times more likely to be satisfied with the intervention than controls (71% versus 13%; RR 5.32, 95% CI 2.63–10.74; 2 trials, 105 women; I2=74%). In the one trial with women with UUI or MUI, PFMT women were three times more likely to be satisfied with the intervention than controls (78% versus 28%; RR 2.77, 95% CI 1.74–4.41; 108 women).44 In contrast, two trials reported that more women needed further treatment in the control groups; one trial in women with SUI (16% versus 93%; RR 0.17, 95% CI 0.07–0.42; 1 trial, 55 women),46 and one in women with UI all types (14% versus 76%; RR 0.19, 95% CI 0.10–0.36; 1 trial; 106 women).44
Number of urinary leakage episodes (per 24h)Women with SUI doing PFMT experienced one fewer leakage episodes in 24h compared to controls (MD 1.23 lower, 95% CI 1.78 lower to 0.68 lower; 7 trials, 432 women; moderate-quality evidence; I2=73%).19,23,32,34,45,46,48 Similarly, those with UUI (MD 1.83 lower, 95% CI 2.65 lower to 1.01 lower; 1 trials, 12 women; low quality evidence)23 and UI all types (MD 1.00 lower, 95% CI 1.37 lower to 0.64 lower; 4 trials, 349 women; moderate-quality evidence; I2=28%)20,33,39,44 experienced about one fewer leakage episode per 24h compared to controls. For women with UUI one recent trial23 reported a greater reduction in the number of leakage episodes with PFMT in comparison with inactive control (MD 1.83 lower, 95% CI 2.65 lower to 1.01 lower; 12 women; low-quality evidence).
Number of micturitions during the day (frequency)One small trial reported on number of micturitions per day for SUI (21 women) and UUI (12 women) separately, without evidence of a difference between groups.23 In three trials, PFMT women with all types of UI reported about two fewer micturitions per day than controls (MD 2.32 lower, 95% CI 3.21 lower to 1.43 lower; 3 trials, 187 women; I2=0%).20,21,42
Short pad tests (up to one hour)Women with SUI in the PFMT groups lost significantly less urine in short (up to 1h) pad tests; the comparison showed considerable heterogeneity but the finding still favoured PFMT if a random-effects model was used (MD 9.71 grams lower, 95% CI 18.92 lower to 0.50 lower; 4 trials, 185 women; I2=78%).34,36,46,48 For women with UI all types, PFMT groups also reported less urine loss on short pad tests than controls (MD 3.72 grams lower, 95% CI 5.46 lower to 1.98 lower; 2 trials, 146 women; I2=0%).20,42
Sexual function or problemsOne trial46 in women with SUI suggested that sexual function was improved by PFMT, specifically in reduction of urine leakage during intercourse (RR 0.25, 95% CI 0.6–1.01; 45 women).
Adverse effectsSeven trials specifically mentioned adverse events, with five not reporting any in the PFMT groups.33,39,44,46,48 Two trials reported adverse events with PFMT,32,38 which were: worsening of incontinence symptoms after the first two treatments that disappeared as treatment continued (one participant),38 or pain (one participant), uncomfortable feeling during exercise (three participants) and ‘not wanting to be continuously bothered with the problem’ (two participants).32
Socioeconomic measuresAt this time, we are only starting to gather data on whether PFMT is cost-effective. Up until now, only one recent report19 documented cost-effectiveness through annual costs per group49 (please refer to the brief economic commentary below).
Measures of adherenceTwelve trials attempted to measure adherence to home PFMT using either exercise or training diaries,19,28,31,33,38–40,43,45,46,48 or self-reported adherence.32 Four trials reported very good to excellent attendance rates at clinic appointments (70–98%)28,33,39,48 with only one study reporting attendance on the control group (for education sessions).39
Grading of recommendations assessment, development and evaluation (GRADE) quality of evidenceGRADE summary of findings tables were prepared separately for women with SUI, for women with UUI and for women with all types of UI (SUI, UUI, MUI) at baseline. Refer to Tables 1 and 2 for women with SUI and UI all types respectively and to the full in the complete Cochrane review for women with UUI as up until now, only one study contributed to this table. Only ‘Participant perceived cure – SUI’ was rated as high quality evidence using the GRADE approach, and the strength of all other findings was downgraded based on evidence quality.
Brief economic commentaryTo supplement the main systematic review of the efficacy of PFM exercises in the treatment of UI, we identified economic evaluations comparing the intervention to a placebo or a sham control. One cost utility analysis was identified.49 The analysis claimed to adopt a societal perspective over a one year time horizon. The costs included administration costs for running the app and lost earnings for the women while doing the exercises. The development costs for the application were excluded. The outcomes were expressed as ICIQ-UI SF and ICIQ-LUTSqol scores. These scores were mapped to utility values using a preference-based index. The authors of the evaluation reported that the application providing instructions for PFMT was a cost-effective first-line treatment alternative.
DiscussionThirty-one trials involving 1817 women were included; 27 trials (1570 women) contributed data to the meta-analysis. The results were consistent for most of the outcomes, favouring PFMT over control. The only outcome that was consistently not different between the experimental and control conditions was generic QoL (data not reported here – see full Cochrane review); such measures may not be sensitive enough to pick up changes due to improvement in UI.
Although we pre-specified four clinical subgroups for baseline type of UI (SUI, UUI, MUI, UI all types) in the analysis, most of the trials reported data on two of them (SUI, UI all types). Two small trials included in this update investigated the effect of PFMT versus control in the two remaining subgroups, one in women with UUI only, and another on women with MUI only.
As noted in prior version of this systematic review, some limitations remain. The trials were generally of small or moderate size, with insufficient detail of participant selection and a lack of clear description of the PFMT programmes. There was considerable variation in interventions used, study populations, and outcome measures. Further, the exercise regimen in both the clinic-based and home PFMT programmes was often incompletely reported. It was difficult to make judgements about the similarities and differences between the training programmes, and hence their potential relative effectiveness. Nevertheless, the more recent trials reported PFMT exercise regimens, more in line with the literature on skeletal muscle training theory and PFM dysfunction, with supervised progressive training protocols.
Attendance at treatment sessions was generally good, and women were also motivated to practice their pelvic floor exercises during the intervention period. However, adherence was mainly reported in the short-term (during the intervention) and mainly for the PFMT groups. It was, therefore, not possible to assess the interactions between the effect size and the adherence to treatment.
The information about persistence of benefit in the long-term was only presented in three trials, and the need for further treatment such as incontinence surgery or drugs was scant. Maintaining the effects of randomization in longer-term follow-up is problematic because it is often confounded by the offer of treatment to women in the control arms; however, longer-term follow-up of the whole cohort would potentially yield some useful data about duration of treatment effect after supervised treatment ends.
We did not critically appraise the economic evaluation and we do not attempt to draw any firm or general conclusions regarding the relative costs or efficiency of the PFMT interventions. However, this evaluation does provide some evidence that application based PFMT is a promising strategy for the management of UI. End users of this review will need to assess the extent to which methods and results of the economic evaluation may be applicable or transferable to their own setting.
ConclusionImplications for practiceThe addition of ten new trials did not change the essential findings of the prior review. The wider range of populations, countries and secondary outcomes within these new trials emphasized the strength of recommendation of PFMT for women with UI. For women with UUI treated with PFMT there is now one report of reduction of urinary leakage episodes, and for women with MUI treated with PFMT, there is now one report of better QoL. Of note, in almost all new included trials, the PFMT protocols were described in more details, with progressive training based on exercise physiology. Moreover, Grade A patient reported symptoms and QoL outcomes, were used more often in line with recent recommendations.52 Finally, we are starting to gather data supporting PFMT cost-effectiveness. However, the limited nature of follow-up beyond the end of treatment in the majority of the trials means that the long-term outcomes and cost-effectivess of use of PFMT remain uncertain.
Implications for researchThere is a need for a pragmatic, well-conducted and explicitly reported trial comparing PFMT with control to investigate the longer-term clinical effectiveness and cost-effectiveness of PFMT for women with symptoms of stress, urgency, or MUI. Although the quality of recent trials has improved (choice of outcome, duration of follow-up, reporting method and data), most of the data in this review comes from small to moderate sized trials of moderate methodological quality. In planning future research, trialists are encouraged to consider the following.
- •
The choice of primary outcomes important to women (urinary outcomes and QoL), the size of a clinically important effect, and subsequent estimation of sample size.
- •
Choice and reporting of PFMT exercise programmes, including details of the number of voluntary PFM contractions per set, duration of hold, duration of rest, number of sets per day, body position, types of contractions, and other recommended exercises.
- •
The reporting on adherence outcomes and adherence strategies, including practice of PFM exercises in both the intervention and control groups.
- •
The need for further treatment, such as with pessaries, surgery or drugs.
- •
The choice and reporting of secondary outcome measures, e.g. sexual function.
- •
The duration of follow-up, especially long-term.
- •
The reporting of formal economic analysis (for example cost-effectiveness, cost utility).
The authors declare no conflicts of interest.
This work was supported by the National Institutes of Health, UK via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane Incontinence Group; University of Montreal, Canada; Canadian Research Chair of the Canadian Institute of Health Research; Centre de recherche de l’Institut de Gériatrie de Montréal, Canada; Sao Paulo Research Foundation. We would like to acknowledge the Cochrane Incontinence Group for their support.
This paper is part of a Special Issue on Women's Health Physical Therapy.
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) doi:10.1002/14651858.CD005654.pub4 (see www.cochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the CDSR should be consulted for the most recent version of the review.