The natural history of physical activity levels during and following gynaecological cancer treatment is not well understood. This is required in order to establish the time at which physical activity levels are lowest in order to target cancer rehabilitation or exercise interventions in gynaecological cancer population accordingly.
ObjectivesTo conduct a systematic review to evaluate the impact of gynaecological cancer treatments on physical activity levels and to summarise the pattern of changes in physical activity levels over time among patients with gynaecological cancer.
MethodsA comprehensive literature search was performed via MEDLINE (1946–2018), CINAHL (1982–2018), EMBASE (1947–2018), Ovid Emcare (1947–2018), PsycINFO (1806–2018) and the Cochrane Library (1991–2018). Studies were eligible for inclusion if they had assessed changes in physical activity levels during and after gynaecological cancer treatment. The methodological quality of the eligible studies was assessed by two independent reviewers using the Joanna Briggs Institute Critical Appraisal Tools.
ResultsIn total, six studies (three cohort studies and three cross-sectional studies) with 1607 participants were included. All studies used patient-reported physical activity measures. Two of the three cohort studies measured patient-recalled physical activity levels before diagnosis (baseline), and length of follow-up varied across all studies. The majority of participants were treated surgically±adjuvant therapy. Physical activity levels decreased at 6 months following surgery when compared with pre-treatment levels. Approximately 91% of participants did not meet physical activity guidelines 2 years following diagnosis, and 58% reported being less physically active 3 years after diagnosis, compared with the pre-diagnosis levels.
ConclusionsDespite the paucity of evidence and limitations in the current body of literature, this review demonstrated that compared to pre-diagnosis, levels of physical activity remain low in gynaecological cancer survivors up to 3 years after diagnosis. More research is warranted to better characterise the pattern of change of physical activity levels across the disease trajectory and identify changes in physical activity patterns by cancer treatments and gynaecological tumour streams in order to target interventions accordingly.
Worldwide, gynaecological cancer including cervical, ovarian, uterine, endometrial, vaginal, fallopian tube, placental, and vulval cancer accounts for 19% of all new female cancer cases.1 Cervical cancer is the most common gynaecological cancer diagnosed, followed by uterine and ovarian cancers.2 Treatment options for gynaecological cancer include surgery, chemotherapy, radiotherapy/brachytherapy, hormonal therapy, and/or targeted therapy.3–5 Although the aim of surgical and medical treatments for gynaecological cancer is to cure and to improve survival rates,6–8 the cancer and treatments themselves may contribute to impaired physical and psychosocial function,9–12 reduced physical activity (PA) levels13 and low health-related quality of life (HRQoL).14
International guidelines based on strong evidence have recommended regular PA for cancer survivors to aid the process of recovery and improve fitness.15,16 Exercise interventions are reported to improve PA levels,17 body mass index17 and HRQoL18 in patients with gynaecological cancer and are recommended to be integrated routinely into the delivery of optimal cancer care.19 However the PA recommendations for gynaecological cancer by Pennington and McTiernan19 are generic and not based on gynaecological cancer specific evidence.19 Despite the significant health benefits of exercise for cancer survivors, a previous study reports that approximately half of patients with gynaecological cancer do not meet the PA guidelines.20 Fatigue, feeling “too tired” and feeling “not well enough” are commonly reported barriers.20,21
There is emerging evidence of the potential benefits of PA on morbidity,19 risk of cancer occurrence22 and survival outcomes23 in patients with gynaecological cancer, however research identifying PA level changes at different time-points across the gynaecological cancer continuum, and thereby, optimal opportunities for exercise interventions, is limited.24 Although the long-term trends of PA levels after cancer diagnosis have been studied in several cancer populations,25,26 the characteristics of patients with gynaecological cancer are different to other cancer cohorts in terms of a range of different pelvic organs that may be involved,27 symptoms, surgical approaches, and side effects of adjuvant therapy.28–31 Women with gynaecological cancer also tend to feel differently from the general cancer community due to the influence of cancer type, cancer site, and treatment type on their experiences. This includes being embarrassed about their cancer site and marginalised if they have had an adjuvant treatment the community is unfamiliar with, such as brachytherapy.28 As PA, a modifiable lifestyle factor, plays a critical role in gynaecological cancer survivorship,19 a better understanding of the natural history of PA levels following gynaecological cancer treatment will potentially improve gynaecological cancer rehabilitation and prevent the sequelae of physical inactivity and non-communicable chronic diseases that may develop with ageing.32
Therefore, we aimed to conduct a systematic review to evaluate the impact of gynaecological cancer treatments on PA levels and to summarise the pattern of changes in PA levels at different time-points across the gynaecological cancer continuum. This review will assist health professionals and researchers better understand the natural history of PA levels in this population, identify times of low PA, and recommend strategies to address this problem.
MethodsThis systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines33 and the Meta-analysis of Observational Studies in Epidemiology.34 The protocol for this review was registered prospectively on PROSPERO (registration number 2018 CRD42018091565) and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=91565
Search strategySix electronic databases, MEDLINE (1946–2018), CINAHL (1982–2018), EMBASE (1947–2018), Ovid Emcare (1947–2018), PsycINFO (1806–2018) and the Cochrane Library (1991–2018) were searched and accessed via Monash University library by one reviewer (KYL) in March 2018. A combination of the following search terms were used: gyn?ecolog*; uter*; endometrial; cervi*; ovar*; vagin*; vulva*; fallopian tube; placenta*; genital; cancer; carcinoma; neoplasm; tumo#r; radiotherapy; chemotherapy; surgery; brachytherapy; treatment; therap*; targeted; physical activit*. An example of a search strategy is presented in Annex 1. No restrictions on the publication date and language were imposed in the initial search. Additional references were identified by hand searching the reference lists of identified articles.
Study selectionStudies were eligible if they met the following inclusion criteria.
Types of studiesQuantitative study designs (randomised controlled trials [RCTs], pseudo-RCTs – alternate allocation of some other method, cohort studies, cross-sectional studies, case–control studies or case series)35 that provided PA data pre- and post-cancer treatment or reported changes in PA after cancer treatment were eligible for inclusion. Only studies published in English and in a peer reviewed journal were eligible.
Types of participantsAdult women (≥18 years of age) diagnosed with gynaecological cancer, at any stage of their illness, were eligible to be included in this review. Studies with mixed cancer cohorts that included at least 75% of patients with gynaecological cancer or provided separate data for patients with gynaecological cancer were included.
Types of exposureStudies that provided gynaecological cancer treatments as interventions or included patients who had received gynaecological cancer treatment including surgery, radiotherapy/brachytherapy, chemotherapy, targeted therapy, or hormonal therapies, either as single or combined modality treatments were eligible.
Types of outcomesStudies were eligible for inclusion if their outcomes included PA levels either measured objectively using pedometers or accelerometers, or subjectively measured by patient self-report.
Screening was undertaken using Covidence,36 an online software designed to facilitate the process of systematic reviews. Three reviewers (KYL, LE and CG) independently screened study titles and abstracts against the inclusion criteria. Two independent reviewers (KYL and LE) subsequently reviewed the full text of the potentially relevant studies to assess the eligibility. Any disagreements were resolved by discussion between the reviewers and if necessary by a fourth independent reviewer (HF). If more information was needed to screen an article, the study investigators were contacted by email.
Data collection processData including first author's name; year published; country; study design; number of participants; age; body mass index; cancer type; cancer stage; type of cancer treatment; PA assessment outcomes; and results of reviewed studies were extracted from the included studies and recorded in a spreadsheet by one reviewer (KYL). A second independent reviewer (LE) then crossed-checked the extracted data. Any discrepancies were resolved through discussion.
Quality assessmentAs cohort and cross-sectional studies were the study designs identified, the methodological quality of the eligible studies was assessed independently by two reviewers (KYL and LE) using standardised critical appraisal instruments, the Joanna Briggs Institute (JBI) Critical Appraisal Tools (one checklist per study design).37 The JBI includes comprehensive appraisal checklists for diverse study designs38 and the checklists have been used in previous gynaecological cancer systematic reviews.39,40 The checklists for cohort studies and cross-sectional studies consist of eleven and eight items, respectively, and require a yes, no, unclear or not applicable response for each item (more ‘yes’ responses represent higher quality). Any disagreements were resolved by discussion and a consensus response was assigned after discussion. Due to the limited number of studies available, no studies were excluded based on methodological quality, however quality was taken into consideration in the interpretation of findings.
StatisticsThe inter-reviewer agreement scores for study selection and quality assessment were calculated as kappa statistics and percentage agreement using SPSS for Windows statistical software package (SPSS Inc., Version 25, Chicago, IL). Values of kappa between 0.6 and 0.74 reflect “good” agreement and 0.75 or more reflect “excellent” agreement.41 Due to the heterogeneity of study populations, study designs and the method of PA level measurement and reporting, a meta-analysis was not possible and therefore data were summarised narratively in tables and figures. Physical activity level changes over time were grouped and reported according to short (≤6 months post-cancer treatment), medium (>6 months and ≤1 year post-cancer treatment), and long-term (>1 year post-cancer treatment).
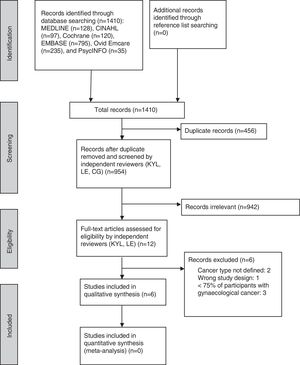
ResultsStudy selectionThe database search yielded a total of 1410 studies, of which 456 were duplicates. No additional studies were identified though searching reference lists. The screening of titles and abstracts resulted in 12 studies potentially meeting the inclusion criteria. The corresponding author of one of the potentially eligible studies was contacted to provide further details (pre-diagnosis PA data)20; this study was subsequently included. The study of Cho et al. was excluded because the baseline measure of PA was not pre-treatment (i.e. baseline assessment was undertaken the week before the second chemotherapy treatment and the number of participants with gynaecological cancer was not specified in their article).42 After searching the original randomised controlled trial, which Cho's study was part of, it was confirmed that only six of 119 (5%) participants were diagnosed with gynaecological (ovarian) cancer.43
After assessment of the eligibility of full-text articles, six studies were included in the final review (Fig. 1). The kappa statistics for agreement between the independent reviewers on title/abstracts (kappa=0.62, percentage agreement=98.7%) and full-text (kappa=0.68, percentage agreement=83.3%) were good.
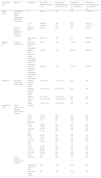
Study characteristicsMethodsThis review included three prospective cohort studies24,44,45 and three cross-sectional studies20,21,46 (Table 1).
Summary of included studies.
| Author and year | Country | Study design | Sample size | Age, years, mean (SD) | Body Mass Index, kg/m2, n (%) | Cancer type, n (%) | Cancer stage, n (%) | Cancer treatment, n (%) |
|---|---|---|---|---|---|---|---|---|
| Abbott 2018 | USA | Prospective cohort study | Total n=601, n=264 completed follow-up surveys | Group 1 (0 MET-hours/week): 58.9 (10.2) Group 2 (>0–9 MET-hours/week): 57.8 (9.6) Group 3 (>9 MET-hours/week): 57.4 (11.1) | Overweight/obese Group 1: 79 (87.8) Group 2: 81 (86.2) Group 3: 62 (77.5) | Ovarian | Stage I–II Group 1: 30 (33.3) Group 2: 32 (34) Group 3: 32 (40) Stage III–IV Group 1: 55 (61.1) Group 2: 58 (61.7) Group 3: 46 (57.5) Unstaged Group 1: 5 (5.6) Group 2: 4 (4.3) Group 3: 2 (2.5) | S±adjuvant CT |
| Beesley 2011 | Australia | Prospective cohort study | Total n=507, n=266 reported physical activity for all time points | 58 (10) | NR | Ovarian | Stage I–II: 169 (34.2) Stage III–IV: 325 (65.8) | S±adjuvant CT |
| Ryan 2012 | Australia | Prospective cohort study | Total n=40, n=31 completed second, n=23 completed third and final assessment | 57 (14.1) | NR | Ovarian 18 (45) Endometrial 11 (28) Cervical 9 (23) Other 2 (5) | NR | S±adjuvant CT or CT plus RT as well as S |
| Bifulco 2012 | Italy | Cross sectional study | Total n=263 Group A (18–45 years) n=106 Group B (46–65 years) n=157 | Group A: 42.8 (2.2) Group B: 55.7 (3.5) | NR | Uterine 126 (47.9) Ovarian 50 (19) Cervical 40 (15.2) Breast 47 (17.9) | Group A: Stage I: 92 (86.8); Stage II: 14 (13.2) Group B: Stage I: 122 (77.1); Stage II: 33 (22.3) | Group A: S 79 (74.5) S+RT 7 (6.6) S+CT 5 (4.7) S+HT 6 (5.6) S+CT+RT 5 (4.7) S+CT+HT 4 (3.8) Group B: S 108 (68.8) S+RT 19 (12.1) S+CT 10 (6.4) S+HT 4 (2.5) S+CT+RT 15 (9.5) S+CT+HT 1 (0.6) |
| Mizrahi 2015 | Australia | Cross sectional study | Total n=95 | n (%): 40–54: 28 (30); 55–69: 47 (49); ≥70: 20 (21) | 26.5 (6.8) Underweight/healthy: 43 (46); Overweight/obese: 47 (54) | Ovarian | Stage I: 11 (12) Stage II: 9 (9) Stage III: 31 (33) Stage IV: 31 (33) Unknown 12 (13) | CT (93%) S (97%) RT (14%) HT (10%) |
| Farrokhzadi 2016 | Australia | Cross sectional study | Total n=101 | 57.5 (range 19–87) | Mean 27.1 (range 18.7–54.9) | Endometrial 23 Ovarian 59 Cervical 5 Other 13 | NR | S, CT, RT, targeted therapy 95 |
Abbreviations: SD, standard deviation; n, number; %, percentage; USA, The United States of America; MET, metabolic equivalent; NR, not reported; S, surgery; RT, radiotherapy; CT, chemotherapy; HT, hormonal therapy; CRT, chemoradiotherapy.
A total of 1607 participants were included in the studies (Table 1). Half of the studies (n=3/6) included only participants with ovarian cancer21,24,44; the other half included mixed gynaecological cancer cohorts.20,45,46 Overall, this review included 1330 (83%) participants with ovarian cancer, 160 (10%) uterine/endometrial cancer, 54 (3%) cervical cancer, 62 (4%) other cancer and 1 unspecified. Four studies reported the cancer stage of participants, and the total number of participants diagnosed with early (stage I–II, n=544) and advanced (stage III–IV, n=546) cancer stage was similar.21,24,44,46 Due to the varying methods of data presentation in included studies, it was not possible to present the tumour frequency by stages. The mean age of the participants across all studies ranged from 40 to 70 years.
ExposureThe types of cancer treatment varied among all studies (Table 1). All studies included participants who had undergone surgery, and either chemotherapy, radiotherapy and/or hormonal therapy. Only one study provided details regarding the proportion of participants receiving each treatment modality combination and ‘surgery only’ was the common treatment type (71%).46 Three studies included some participants currently undergoing cancer treatment (Beesley 2011, 19%; Farrokhzadi 2016, 61%; Mizrahi 2015, 39%).20,21,24
Outcome measuresAll included studies evaluated PA levels using self-reported measures. Three studies assessed PA levels using validated questionnaires (International PA Questionnaire [IPAQ],44,45 Godin Leisure-Time Exercise Questionnaire20 and Active Australia Questionnaire20). The IPAQ measures frequency (days per week) and duration (minutes per day) of PA (vigorous, moderate, and walking) in the last seven days and has acceptable reliability and validity.47 The Godin Leisure-Time Exercise Questionnaire, with established validity and reliability, consists of 4 questions on the weekly frequencies of strenuous, moderate, and mild activities and the frequency of weekly leisure-time activities (long enough to work up a sweat) in a typical 7-day period.48,49 The Active Australia Questionnaire measures the duration of three activity types (walking, moderate-intensity leisure-time PA and vigorous-intensity leisure-time PA) in the last week.50 One44 of the three studies also examined whether the participants had met the Physical Activity Guidelines (PAG) for Americans, which recommend adults to perform at least 150min of moderate-intensity, or 75min of vigorous-intensity aerobic PA per week, plus muscle-strengthening on at least 2 days each week.51 One study assessed PA by asking participants two questions on weekly frequency and intensity of strenuous and moderate PA, which were used to form a physical activity index (PAI) (low=activity less than once/week, moderate=strenuous activity once/week or moderate activity 1–3 times/week, high=strenuous activity ≥2 times/week or moderate activity ≥4 times/week).24 Two studies asked participants a single question on changes in PA after cancer treatment.21,46 No studies measured PA using objective measures such as pedometers or accelerometers.
Methodological qualityThe agreement between the two independent reviewers on quality assessment was excellent (kappa=0.927, percentage agreement=96.5%). Some JBI checklist items on the exposures were not applicable to the studies included in this review. All studies scored a minimum of four ‘yes’ responses out of seven or eight applicable items. All cohort studies were limited by the participants not being free of the outcome (PA) at baseline, and 67% of the cross-sectional studies were limited by the lack of valid and reliable outcome measures (Table 2).
Methodological quality of included studies.
| Joanna Briggs Institute checklists | Ryan et al., 2012 | Beesley et al., 2011 | Abbott et al., 2018 | Bifulco et al., 2012 | Mizrahi et al., 2015 | Farrokhzadi et al., 2016 |
|---|---|---|---|---|---|---|
| Cohort studies | ||||||
| 1. Were the two groups similar and recruited from the same population? | Not applicable | Not applicable | Not applicable | |||
| 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Not applicable | Not applicable | Not applicable | |||
| 3. Was the exposure measured in a valid and reliable way? | Not applicable | Not applicable | Not applicable | |||
| 4. Were confounding factors identified? | No | Yes | Yes | |||
| 5. Were strategies to deal with confounding factors stated? | No | Yes | Yes | |||
| 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | No | No | No | |||
| 7. Were the outcomes measured in a valid and reliable way? | Yes | No | Yes | |||
| 8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? | Yes | Yes | Yes | |||
| 9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? | Yes | No | Yes | |||
| 10. Were strategies to address incomplete follow up utilised? | Yes | No | Yes | |||
| 11. Was appropriate statistical analysis used? | Yes | Yes | Yes | |||
| Cross-sectional studies | ||||||
| 1. Were the criteria for inclusion in the sample clearly defined? | Yes | Yes | Yes | |||
| 2. Were the study subjects and the setting described in detail? | Yes | Yes | Yes | |||
| 3. Was the exposure measured in a valid and reliable way? | Not applicable | Not applicable | Not applicable | |||
| 4. Were objective, standard criteria used for measurement of the condition? | Yes | Yes | Yes | |||
| 5. Were confounding factors identified? | Yes | Yes | Yes | |||
| 6. Were strategies to deal with confounding factors stated? | Yes | No | Yes | |||
| 7. Were the outcomes measured in a valid and reliable way? | No | No | Yes | |||
| 8. Was appropriate statistical analysis used? | Yes | Yes | Yes | |||
Physical activity level study results are presented in Table 3. Two of the three cohort studies measured PA levels before diagnosis (baseline) retrospectively, with a recall period of 5 months44,52 and 19 months.24 All studies had different lengths of follow-up. The short-term results showed a decrease in median vigorous, moderate, and total PA levels at 3 months post-adjuvant treatment/6 months post-surgery, compared to pre-treatment PA levels.45 The medium-term results showed a decrease in the number of participants engaged in moderate and high PA 1 year after diagnosis.24 The study by Farrokhzadi et al.20 measured pre-diagnosis PA levels with the Godin Leisure-Time Exercise Questionnaire and used a different measure, the Active Australia Questionnaire, to assess post-diagnosis PA levels.20 Farrokhzadi et al.20 reported that 38% of participants engaged in less than 120min per week of PA before diagnosis, and approximately 47% of participants were inactive or insufficiently active at a mean time of 10 months since diagnosis.20
Results: patient-reported physical activity levels pre- and post-cancer treatment.
| Author and year | Measure | Categories | Pre-cancer treatment/diagnosis, n (%) | Post-cancer treatment/diagnosis (<6 months), n (%) | Post-cancer treatment/diagnosis (6–12 months), n (%) | Post-cancer treatment/diagnosis (>12 months), n (%) |
|---|---|---|---|---|---|---|
| Abbott 2018 | Recreational Physical Activity (adapted from IPAQ), MET-hours/week | 0 | 90 (34.1) | N/A | N/A | 130 (49.2) |
| >0–9 | 94 (35.6) | N/A | N/A | 90 (34.1) | ||
| >9 | 80 (30.3) | N/A | N/A | 44 (16.7) | ||
| Physical Activity Guidelines (PAG) for Americans | Meeting PAG | 65 (24.6) | N/A | N/A | 24 (9.1) | |
| Not meeting PAG | 199 (75.4) | N/A | N/A | 240 (90.9) | ||
| Beesley 2011 | Physical Activity Index | Low level (activity less than once/week) | 59 (22) | N/A | 109 (41) | 64 (24) |
| Medium level (strenuous activity once/week or moderate activity 1–3 times/week) | 101 (38) | N/A | 85 (32) | 109 (41) | ||
| High level (strenuous activity 2+ times/week or moderate activity 4+ times/week) | 106 (40) | N/A | 72 (27) | 93 (35) | ||
| Ryan 2012 | IPAQ, total hours/week, median (IQR) | Vigorous physical activity levels | 4 (2.8–9.5) | 3.0 (1.5–7.5) | N/A | N/A |
| Moderate physical activity levels | 5.5 (2.9–11.0) | 3.0 (1.0–9.0) | N/A | N/A | ||
| Walking | 3 (1.4–8.0) | 3.8 (2.5–8.3) | N/A | N/A | ||
| Total activity | 24.3 (15.6–49.5) | 14.8 (9.0–25.5) | N/A | N/A | ||
| Sitting | 4.0 (3.0–7.0) | 4.0 (3.0–5.0) | N/A | N/A | ||
| Farrokhzadi 2016 | Godin Leisure-Time Exercise Questionnaire, min/week | Vigorous | ||||
| None | 74 (76) | N/A | N/A | N/A | ||
| 1–120 | 14 (14) | N/A | N/A | N/A | ||
| >120 | 9 (9) | N/A | N/A | N/A | ||
| Moderate | ||||||
| None | 44 (45) | N/A | N/A | N/A | ||
| 1–120 | 23 (24) | N/A | N/A | N/A | ||
| 121–240 | 16 (16) | N/A | N/A | N/A | ||
| >240 | 14 (14) | N/A | N/A | N/A | ||
| Light | ||||||
| None | 45 (45) | N/A | N/A | N/A | ||
| 1–120 | 21 (21) | N/A | N/A | N/A | ||
| 121–240 | 17 (17) | N/A | N/A | N/A | ||
| >240 | 16 (16) | N/A | N/A | N/A | ||
| Total | ||||||
| None | 15 (16) | N/A | N/A | N/A | ||
| 1–120 | 21 (22) | N/A | N/A | N/A | ||
| 121–240 | 20 (21) | N/A | N/A | N/A | ||
| 241–480 | 23 (24) | N/A | N/A | N/A | ||
| >480 | 16 (17) | N/A | N/A | N/A | ||
| Active Australia Questionnaire, min/week | Inactive (0) | N/A | N/A | 12% | N/A | |
| Insufficiently active (1–149) | N/A | N/A | 35% | N/A | ||
| Sufficiently active (≥150) | N/A | N/A | 53% | N/A |
| Author and year | Measure | Categories | Changes in physical activity after cancer treatment, n (%) |
|---|---|---|---|
| Bifulco 2012 | Physical activity measured considering its frequency per year | GROUP A (young adults, between the ages of 18–45 years) | |
| No | 81 (76.4) | ||
| Reduction | 15 (14.1) | ||
| Increase | 10 (9.4) | ||
| Stop | 0 (0) | ||
| Start | 0 (0) | ||
| GROUP B (midlife adults, between the ages of 46–65 years) | |||
| No | 128 (81.5) | ||
| Reduction | 11 (7.0) | ||
| Increase | 7 (4.5) | ||
| Stop | 0 (0) | ||
| Start | 11 (7.0) | ||
| Mizrahi 2014 | A single item consisting of whether they were less, more, or similarly physically active compared with before their initial diagnosis | Less | 55 (58) |
| More | 15 (16) | ||
| Similar | 25 (26) |
Abbreviation: n, number of participants; IQR, inter-quartile range; %, percentage; N/A, not available; MET, metabolic equivalents; IPAQ, International Physical Activity Questionnaire; PAG, Physical Activity Guidelines for Americans.
Two cohort studies assessed long-term changes (>1 year) in PA levels following cancer treatment.24,44 Abbott et al. reported an increase in the number of ‘inactive’ participants and a corresponding increase in the number of participants not meeting PAG at a mean time of 22.4 months since diagnosis (75% pre- versus 91% post-diagnosis).44 Beesley et al.24 reported a slight increase in the number of participants engaged in low and medium levels of PA 2–3 years after diagnosis, compared to the number before diagnosis.24
Two cross-sectional studies also assessed long term changes in PA after cancer treatment using retrospective recall.21,46 Bifulco et al.46 found that the majority of participants (79%) reported no changes in PA 33–34 months after cancer treatment, and only approximately 10% of participants reported a reduction in PA after treatment.46 In contrast, the study by Mizrahi found that at a mean time of 37 months post diagnosis, more than half (58%) of participants reported that they were less physically active compared with before their initial diagnosis.21 Only 26% reported similar PA levels before and after cancer treatment.21
The study by Beesley et al.24 reported PA levels at 4 or more years after diagnosis and found that the number of participants engaged in both medium and high PA levels decreased slightly, from 38% to 30% and 40% to 39% respectively, compared to pre-diagnosis level.24
DiscussionThis study summarises changes in PA levels before and after gynaecological cancer treatment, a topic that was under-investigated in the literature. No studies assessing PA levels at more than three time-points post cancer diagnosis were identified in this review. This highlights the need for longitudinal observational studies in this field to provide information on patterns of change in PA levels to identify times of low PA levels. Despite the paucity of studies and the low to moderate methodological quality of the included studies, this systematic review found that PA levels of participants decreased over time up to 2 years after diagnosis.45
However, the findings for changes in PA levels >2 years following diagnosis from three studies21,24,46 were inconsistent. One study included overweight/obese participants (54%) and reported persistent low PA levels 3 years following dignosis.21 As high body mass index is a strong determinant of a sedentary lifestyle,53,54 further research is needed to investigate the risk factors for low PA levels in gynaecological cancer survivors, including body mass index. Furthermore, the studies by Beesley et al.24 and Mizrahi et al.21 included participants diagnosed with ovarian cancer only, and there is insufficient evidence regarding whether this cohort differs from other gynaecological cancer cohorts with respect to PA levels. The study by Bifulco et al.46 measured PA by asking participants to consider frequency per year, which may artificially inflate the reported PA levels. Nevertheless, the findings on reduced PA 33–37 months after cancer treatment compared with the pre-diagnosis level21,46 are in line with previous studies in colorectal and breast cancer survivors, which showed that 15–40% of participants were inactive or not meeting PA guidelines >3 years after diagnosis.25,55
The reference point for changes in PA levels following diagnosis/treatment is the pre-diagnosis level, therefore interpretation of the change is only as accurate as the pre-diagnosis data. The pre-diagnosis PA levels of participants with gynaecological cancer (38%-40% reporting moderate/high levels of activity) are comparable to 34% of women with breast cancer meeting PA guidelines before diagnosis25 and data from the general population where only 41% meet the recommended PA levels (data from women aged 45–74 years in Australia).56 As most of the included studies were conducted in Australia, the findings were compared with the Australian general population. However, the pre-diagnosis PA levels reported in this review may be subject to recall bias, given they involve retrospective patient self-report, in contrast with the Australian general population estimate which is based on prospective data with fewer potential sources of bias. Although the number of participants (53%)20 who were sufficiently active 10 months following diagnosis was higher than the general population, this number24 fell below that of the general population ≥4 years after diagnosis (30%). The decreased PA levels may be associated with the late effects of cancer treatments57 and ageing.58,59
The sedentary lifestyle of cancer survivors is well documented.60–62 The results of this review showed that many patients only engaged in light-intensity activity following gynaecological cancer diagnosis and treatment. These results are consistent with previous studies in mixed cancer,63 breast cancer64,65 and colorectal cancer cohorts,66 showing that cancer survivors significantly reduce their total, vigorous and moderate intensity PA 6 months and 1 year after diagnosis or treatment. The present review adds further evidence to demonstrate persistent low PA levels among patients following gynaecological cancer diagnosis and suggests that exercise recommendations for other cancer cohorts may also be applicable to this population.67
The findings of this systematic review may have important clinical implications. Given the potential benefits of exercise on many patient-centred outcomes including physical functioning, PA levels, fatigue, weight loss and survivorship in gynaecological cancer survivors,17,23,68 it is crucial for healthcare professionals including surgeons, oncologists, oncology nurses and physical therapists to identify the times of low PA across the disease trajectory in order to refer to a physical therapy programme or provide appropriate interventions tailored to the characteristics, capabilities, needs and preferences of individual patients.16 Furthermore, at the time of diagnosis healthcare professionals should emphasise the need for ongoing regular physical exercise across the lifespan,67 as sedentary behaviour is significantly related to all-cause and cardiovascular mortality.69 As women with gynaecological cancer report high levels of varied and complex social distress which impact on their management,70 behaviour change techniques and psychological supports should be included in PA counselling sessions to provide a holistic care to this population.71–75
The important role of physical therapists in exercise prescription and PA promotion (the core skills of physical therapists)76 may benefit the oncology patients by maintaining patient optimal health and healthy lifestyle behaviour throughout the cancer survivorship. In addition to the persistent low PA levels, pelvic floor dysfunction is common among women with gynaecological cancer.77 Therefore, the integration of a women's health physical therapist into the oncology team may assist in early detection and management of pelvic floor dysfunction throughout the gynaecological cancer trajectory.
Recommendations for future research include prospective longitudinal observational studies to investigate the natural history of PA before and after gynaecological cancer treatment, which would contribute to a more thorough understanding of how different cancer types and cancer treatments impact on these outcomes. We recommend that the authors of future studies provide details of cancer types, cancer stage, cancer treatments and PA assessment time-points, and use optimal outcome measures for PA in order to identify changes in PA patterns and to determine subgroups at higher risk of low PA in gynaecological cancer populations. As women would like to be introduced to a lifestyle programme at diagnosis or during treatment, but prefer to participate in such programme after the cancer treatment has completed,78 future studies need to determine the optimal timing for exercise interventions in gynaecological cancer populations.
Given that all studies included in this review assessed PA levels with self-reported measures, PA levels may be over- or under-reported due to recall bias, social desirability or social approval.79 Although a recent systematic review reported that objective measures of PA, particularly accelerometers/activity monitors, demonstrate more consistent results than self-reported measures,80 accelerometers may not be as feasible and easily accessible as questionnaires. Therefore, it is recommended that future studies use both self-reported and objective measures to obtain more comprehensive PA data.
From our literature search, four published abstracts that assessed PA levels in patients with gynaecological cancer were found.81–84 This emerging research will add to the evidence base in the future. Although previous studies have identified barriers and enablers to PA in gynaecological cancer survivors,20,21,78,85,86 future studies may consider conducting qualitative interviews to investigate factors influencing self-motivation for PA and exercise adherence,87,88 which may inform the optimal design of interventions to promote the uptake and maintenance of regular PA across the gynaecological cancer continuum. These interventions then require testing for clinical and cost-effectiveness in robust trials.
LimitationsThere are several limitations of this review which should be outlined including the small number of studies eligible for inclusion; heterogeneity in the study designs, populations, assessment time-points and methodological quality; self-reported outcome measures; publication bias (peer reviewed journals); and language bias (English).89 As there is no “gold standard’ critical appraisal tools for the study designs included in this review,90 the use of the JBI critical appraisal tools might impact the interpretation of the quality of the research, which shows an overall low to moderate methodological quality of the studies due to the inherent methodological shortcomings of the included studies. All studies included were observational studies and all used different outcome measurement tools for PA. The findings were predominantly presented as the number of participants engaging in different levels/intensities of PA, making comparisons of changes of PA levels in MET-hour/week between studies difficult. Moreover, ovarian cancer was the most common cancer in the included studies; therefore, the results may not be generalisable to all gynaecological cancer types. The paucity of data in other gynaecological tumour streams which are more frequent (cervical and uterine cancers), limits the interpretation of results, and the knowledge of natural history in those cohorts remains deficient. Furthermore, none of the included studies reported PA levels separately for participants who had undergone different cancer treatments. Due to the insufficient details regarding cancer treatment, it was difficult to comment on which treatment has the most impact on PA levels; the impacts of different cancer treatment modalities on PA levels remains unknown. As the follow-up assessment time-points of four of the included studies were undertaken at a time-point identified from diagnosis, not from the completion of the cancer treatment, and three of these studies also included some participants currently undergoing treatment, ongoing treatment may have had an impact on PA outcomes.21 Likewise, varying baseline assessment time-points (pre-diagnosis vs. pre-treatment) may impact changes in PA levels at follow-ups as cancer diagnosis has been shown to act as a trigger to health behaviour change,91 and cancer treatment and medications have been reported as a barrier to implementing behaviour change.92 Finally, none of the studies specified whether the participants had received advice on or been encouraged to implement health behaviours from health care professionals, which may also impact on our interpretation of the natural history of PA levels.
ConclusionThis study has summarised the trends of changes in PA levels among patients across the gynaecological cancer continuum. The results provide further evidence that many patients are still inactive or engage in low PA levels in the long-term after diagnosis and completion of treatment. Regular PA as a lifestyle should be encouraged by healthcare professionals working with this population. However, firm conclusions regarding the impact of gynaecological cancer treatment on PA levels cannot be drawn due to the paucity of evidence. Furthermore, the findings of this systematic review should be read with caution due to the heterogeneity of types of gynaecological cancer, cancer stage, treatment modalities, assessment time-points and methodological quality; and self-reported measures of PA in the included studies. Further research is necessary to inform the optimal timing for personalised/individualised exercise interventions following gynaecological cancer diagnosis.
Conflicts of interestThe authors declare no conflicts of interest.
MEDLINE was searched using the Ovid interface on 16th March 2018 for the period 1946 to February 2018
1. Genital Diseases, Female/ or Gynecology/ or gyn?ecolog*.mp. or Obstetrics/ or Vagina/ (141173)
2. Uterine Neoplasms/ or Uterine Cervical Neoplasms/ or uter*.mp. or Uterus/ (282652)
3. Endometrium/ or Endometrial Neoplasms/ or Uterine Neoplasms/ or endometrial.mp. (90871)
4. cervi*.mp. (245639)
5. Ovarian Neoplasms/ or ovar*.mp. (260965)
6. Vaginal Neoplasms/ or Vagina/ or Vulvar Neoplasms/ or vagin*.mp. or Cervix Uteri/ (152457)
7. Vulva/ or vulva*.mp. (18827)
8. fallopian tube.mp. or Fallopian Tubes/ (17854)
9. placenta*.mp. or Placenta/ (102100)
10. genital.mp. or Genital Neoplasms, Female/ (79191)
11. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (948409)
12. cancer.mp. or Neoplasms/ (1454788)
13. Carcinoma/ or carcinoma.mp. (687918)
14. tumo?r.mp. or Neoplasms/ (1753525)
15. 12 or 13 or 14 (2594666)
16. 11 and 15 (245725)
17. physical activit*.mp. (77155)
18. RADIOTHERAPY/ or RADIOTHERAPY, ADJUVANT/ (60002)
19. Chemotherapy.mp. or Drug Therapy/ (377639)
20. surgery.mp. or General Surgery/ (1016832)
21. brachytherapy.mp. or BRACHYTHERAPY/ (20733)
22. Treatment.mp. or Therapeutics/ (3808056)
23. Therap*.mp. (2778307)
24. Targeted.mp. (230893)
25. 18 or 19 or 20 or 21 or 22 or 23 or 24 (6094547)
26. 16 and 17 and 25 (128)
This paper is part of a Special Issue on Women's Health Physical Therapy.