One of the sequalae of breast cancer treatments may be pelvic floor (PF) dysfunction such as urinary incontinence (UI), faecal incontinence (FI), and pelvic organ prolapse (POP).
ObjectiveThe aim of this study was to compare the occurrence and related distress and impact of PF dysfunction between women with and without breast cancer.
MethodsWomen with and without breast cancer participated in this cross-sectional study. The Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire were used to quantify the prevalence and related distress, and impact of PF dysfunction. Factors associated with PF outcomes were examined using logistic and linear regressions while controlling for known risk factors for PF dysfunction (age, body mass index, and parity).
Results120 women with breast cancer, and 170 women without breast cancer responded. The occurrence of any type of UI was higher in women with breast cancer than women without breast cancer (percentage difference=17%; 95% CI: 7, 29). Women with breast cancer experienced higher impact of urinary symptoms (mean difference=18.2; 95% CI: 8.9, 27.7) compared to those without. Multivariable analysis indicated that having breast cancer (β 0.33; 95%CI: 0.08, 0.51) was the strongest predictor of greater impact of urinary symptoms.
ConclusionWomen with breast cancer reported a higher occurrence and impact of urinary symptoms than women without breast cancer. While further studies are required to confirm our findings, routine screening and offering treatment for urinary symptoms may be indicated for women with breast cancer.
One of the sequalae of breast cancer treatments may be the onset of new, or aggravation of, pre-existing pelvic floor (PF) dysfunction.1 PF dysfunction in women following breast cancer treatment is presumed to be a result of ovarian suppression and failure secondary to chemotherapy in premenopausal women, and the use of endocrine therapy in both pre- and postmenopausal women.2 Prolonged hypoestrogenism may be an aetiological factor in the development of PF dysfunction.3
Emerging literature indicates that women treated for breast cancer may experience PF dysfunction at higher rates than women without breast cancer.1,4-7 However, little is known about the occurrence of specific types of PF dysfunction such as urinary incontinence (UI), faecal incontinence, and pelvic organ prolapse,8 and their burden in women with breast cancer. A systematic review reported that the pooled prevalence of UI in women with breast cancer was 38%.7 However, this systematic review did not include data from women without breast cancer. Such comparison is needed to understand whether having breast cancer is associated with the presence of UI and whether physical therapists working in women's health should be routinely screening, offering education on preventative strategies, and providing conservative management (such as PF muscle training) for UI in women with breast cancer.
Prior to screening or offering treatment for PF dysfunction, it is important to understand the symptom burden, including distress/bother and impact in women with breast cancer. In this study, we consider ‘distress/bother’ to be the perceived importance of a PF symptom when it worries, disturbs, or upsets women,9 and ‘impact’ to be the effects of PF symptoms on the activities of daily living of women.10 The aim of this study was to identify the magnitude of PF dysfunction in women with and without breast cancer. We compared the prevalence and the related distress and impact of PF dysfunction in both groups, and examined the association between breast cancer and the presence and related distress and impact of PF dysfunction.
MethodsThis study is reported according to the STROBE statement.11 Ethics approval was obtained from the Monash Health (HREC/46,186/MonH-2018–155,153) and Monash University (18,405) Human Research Ethics Committees.
Study design, setting, and participantsThis cross-sectional study included women with and without breast cancer recruited from outpatient clinics at a tertiary public hospital in metropolitan Melbourne, Australia. Data were collected between March - September 2019. For the group with breast cancer, we included women who had undergone primary treatment and/or adjuvant therapy for a histologically confirmed breast tumour and were ≥ 18 years of age. The inclusion criterion for the control group was women with no history of breast cancer. Women who were pregnant, unable to communicate in English, with neurological disorders, or with severe physical or psychiatric impairments were excluded from both groups.
Data collectionWomen with breast cancerTreating medical staff (breast surgeons, endocrinologists, and oncologists) identified eligible patients and registered their interest in the study using standardised instructions. Medical staff directed interested participants to the onsite primary researcher (UC) who further explained the study and gained signed consent. Following consent, participants were directed to independently complete either a paper-based or online-based questionnaire. The online questionnaire was administered using the Qualtrics program (Qualtrics, Provo, UT).
Women without breast cancerA link to the online-based questionnaire was advertised on flyers posted on targeted women's health groups on Facebook™. A wide recruitment strategy was used to gain a representative sample of women without breast cancer. A larger number of women without breast cancer were also recruited to minimise selection bias. The consent form was outlined at the start of the online-based questionnaire to allow participants to provide consent electronically. Following consent, participants were asked to answer a screening question to determine their eligibility. Those who responded affirmatively were directed to complete the e-questionnaire on the Qualtrics program.
Study variablesSocio-demographic and medical variables collected included age, body mass index (BMI), parity, relationship status, social situation, educational level achieved, employment, smoking, menopausal status, Charlson Comorbidity Index,12 and medications. Menopausal status for the control group was categorised according to the description outlined by the Australasian Menopause Society.13 As breast cancer treatments may interfere with ovarian function, menopausal status fluctuates in women with breast cancer.14 Therefore, menopausal status in women with breast cancer was categorised according to the description within breast cancer literature15 in consultation with an endocrinologist (AV). Additional clinical data including breast cancer stage, treatment status, and current medications for cancer treatment were extracted from the medical records of consenting participants.
Measurement instrumentsThe occurrence of and distress related to PF dysfunction were assessed using the Pelvic Floor Distress Inventory (PFDI-20).10 This instrument consists of 20 questions in three domains: urinary (6 questions), pelvic organ prolapse (6 questions), and colorectal-anal (8 questions).10 The PFDI-20 has been shown to have good face, content, and construct validity16 and test-retest reliability.17 The impact of PF dysfunction was assessed using the Pelvic Floor Impact Questionnaire (PFIQ-7).10 This questionnaire consists of seven questions in three domains (urinary, pelvic organ prolapse, and colorectal-anal)10 and has established good face, content, and construct validity16 and test-retest reliability.17
Outcome of interestThe primary outcome of this study was the presence of UI which was established from a positive response to either question 16 or 17 on the PFDI-20.10 An affirmative response to questions 9 or 10 of the PFDI-2010 was used to quantify the presence of faecal incontinence while question 3 was used to quantify the presence of pelvic organ prolapse.10 The PFDI-2010 was also used to quantify the severity of bother (distress) of PF dysfunction as per the scoring algorithm.10 Summary scores ranged from 0 (least distress) to 300 (greatest distress).10 The impact of PF dysfunction on the daily lives of women was quantified using summary scores of the PFIQ-7.10 The PFIQ-7 summary score was calculated according to the scoring algorithm and ranged from 0 (least impact) to 300 (greatest impact).10
Sample size calculationThe sample size for this study was based on our primary outcome which was the occurrence of UI. The occurrence of UI within the last four weeks has been found to be 45–55% in women receiving endocrine therapy for breast cancer18,19 compared to 25–36% in women without breast cancer20,21; a 20% difference in occurrence rates. To achieve a between-group difference in occurrence of UI of 20%, with 80% power and 95% confidence intervals, we calculated a sample size of 240 participants would be required i.e. 120 in each group.
Statistical analysisDescriptive statistics were used to summarise participant demographics and the magnitude of PF dysfunction in women with and without breast cancer. Independent t–tests for continuous data and χ2 tests for categorical variables were used to compare the differences in participant demographics and prevalence, distress, and impact of PF dysfunction between groups.
Logistic regression models were used to examine the association between breast cancer and the presence of PF dysfunction. A two-step modelling approach was applied. Firstly, univariable regression models for potential predictors and the presence of PF dysfunction were computed. Secondly, factors with a moderate association (p ≤ 0.1)22 on univariable analysis were entered into a multivariable model. Logistic regression models were adjusted for known covariates of the occurrence of PF dysfunction: age, BMI, and parity.23 The final model included the variables associated with the occurrence of PF dysfunction, variables of interest to the study (eg. presence of breast cancer), and the variables arising from sample differences (eg. age, BMI, and parity). Model findings were reported with odds ratios (OR) together with the corresponding 95% confidence intervals (CI). A p ≤ 0.05 was considered statistically significant.
Linear regression models were used to examine associations between the distress and impact of PF dysfunction in women with and without breast cancer using a similar two-step modelling approach. All variables were standardised before computing linear regression models. Initial testing showed that assumptions for linear regression were met for both the PFDI-20 and PFIQ-7. Highly correlated predictors were identified when the variance inflation factor (VIF) was higher than 2.5 in all regression models, in which case only the variable with the higher R2 was retained in the multivariable model. All analyses were performed using Stata v16.0/IC (StataCorp, LLC).
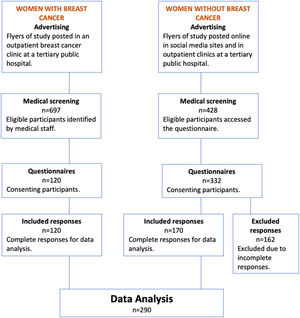
ResultsParticipantsFigure 1 illustrates the recruitment flowchart. 697 women with breast cancer were identified by medical staff to be eligible for this study and 120 consented to participate (17% consent rate). All women who consented to participate subsequently completed the questionnaires (100% completion rate). In the control group, the consent rate was calculated by dividing the number of women who consented to completing the questionnaire by the number of women who viewed the questionnaire online. Seventy-six percent consented to participate (332/428) and 51% completed the questionnaire (170/332). There were 162 responses that were either completely blank (n = 149) or partially completed (n = 13) from the control group. Only complete responses were included in this analysis.
Participant demographics and the magnitude of pelvic floor dysfunctionThe demographic and clinical characteristics of all participants, and occurrence of PF dysfunction are presented in Table 1. Women with breast cancer were significantly older (53 ± 12 years old) and had a higher BMI (31 ± 7 kg/m2). A larger proportion of women with breast cancer were post-menopausal (86/120, 72%) than women without breast cancer who were mostly pre-menopausal (109/170, 64%).
Participant demographics and clinical characteristics.
| Variables | Women with breast cancer (n = 120) | Women without breast cancer (n = 170) | Difference (95% CI) |
|---|---|---|---|
| Age, years mean ± SD | 53.3 ± 11.5 | 40.4 ± 11.9 | 12.9 (10.1, 15.6) |
| BMI, kg/m2 mean ± SD | 31.1 ± 6.6 | 26.9 ± 6.8 | 4.2 (2.5, 5.7) |
| Parity mean ± SD | 1.6 ± 1.3 | 1.3 ± 1.4 | 0.3 (0.1, 0.4) |
Participant reported menopausal status, n (%)*
| 15 (13)36 (30)65 (54) | ||
Clinician reported menopausal status, n (%)*
| 7 (6)23 (19)86 (72) | 109 (64)21 (12)36 (21) | 51% (23, 71) |
Medical history, n (%)
| 94 (78)26 (22) | 148 (87)22 (13) | 9% (−4, 16) |
| Smoking status, n (%)*Have never smokedSmoker | 50 (42)69 (58) | 130 (77)40 (23) | 35% (28, 37) |
Home situation, n (%)*
| 12 (10)108 (90) | 25 (15)145 (85) | 5% (3, 6) |
Relationship situation, n (%)
| 32 (27)88 (73) | 55 (32)115 (68) | 5% (−1, 9) |
Educational level, n (%)
| 36 (30)84 (70) | 16 (9)154 (91) | 21% (17, 35) |
Employment status, n (%)
| 58 (49)62 (51) | 144 (84)26 (16) | 35% (21, 59) |
Breast cancer stage, n (%)
| 15 (13)43 (36)35 (28)27 (23) | – | – |
Oestrogen receptor status, n (%)
| 71 (59)49 (41) | – | – |
Breast cancer treatments, n (%)β
| 72 (60)32 (27)87 (73)72 (60) | – | – |
| Time since diagnosis, years mean ± SD | 2.3 ± 1.8 | – | – |
Presence of pelvic floor dysfunction, n (%)
| 88 (74)21 (18)13 (11) | 97 (57)26 (15)23 (14) | 17% (7, 29)3% (−2, 6)−3% (−4, 1) |
PFDI-20 summary score, mean ± SD
| 47.3 ± 39.327.3 ± 22.611.9 ± 13.28.1 ± 11.8 | 46.9 ± 40.121.8 ± 21.612.8 ± 13.512.3 ± 13.8 | 0.4 (−9.6, 9.0)5.5 (0.3, 10.6)0.9 (−2.2, 4.1)4.2 (1.1, 7.2) |
PFIQ-7 summary score, mean ± SD
| 40.9 ± 46.619.8 ± 22.213.8 ± 19.87.4 ± 13.2 | 22.7 ± 33.97.8 ± 11.67.6 ± 11.87.3 ± 11.9 | 18.2 (8.9, 27.7)12.0 (8.6, 41.2)6.2 (−7.5, 43.5)0.1 (−1.2, 23.4) |
BMI, body mass index; ER, oestrogen receptor; PFDI, pelvic floor distress inventory; PFIQ, pelvic floor impact questionnaire. Significant results in bold.
Seventy-four percent of women with breast cancer (88/120) experienced any type of UI, compared to 57% in the control group (97/170) which was a statistically significant difference between groups (percentage difference = 17%; 95% CI: 7, 29) (Table 1). Further analysis of the occurrence of UI according to menopausal status in women with breast cancer demonstrated that pre-menopausal women (7/7, 100%) experienced higher rates of UI compared to peri or post-menopausal women (77/109, 70%). Conversely, women in the control group who were either peri or post-menopausal experienced UI at higher rates (47/57, 82%) than women who were pre-menopausal (46/109, 42%). The factors associated with the presence of PF dysfunction in both groups are presented in Table 2. After controlling for known risk factors of PF dysfunction and factors with a moderate association on univariable analysis, there were no statistically significant associations between breast cancer and the presence of incontinence or pelvic organ prolapse.
Factors associated with the presence of pelvic floor dysfunction in women with (n = 120) and without (n = 170) breast cancer*.
95%CI, 95% confidence interval; BMI, body mass index; OR, odds ratio. Significant results (p < 0.05) in bold.
The distress and impact of PF dysfunction are presented in Table 1. The PFDI-20 urinary domain score showed the highest distress value in both groups, suggesting that urinary symptoms were the most distressing in both groups of women. Women with breast cancer experienced a significantly higher distress of urinary symptoms than women without breast cancer (mean difference = 5.5; 95% CI: 0.3, 10.6).
There was a significant difference in impact summary scores between groups; women with breast cancer experienced higher impact of PF dysfunction than women without breast cancer (mean difference = 18.2; 95% CI: 8.9, 27.7). The urinary domain score of the PFIQ-7 was the one with the highest impact value in both groups. Women with breast cancer experienced a significantly higher impact of urinary symptoms than women without breast cancer (mean difference = 12; 95% CI: 8.6, 41.2).
Association between breast cancer and the distress and impact of pelvic floor dysfunctionIn Table 3, univariable analyses showed that higher age, higher BMI, not having a university degree and not currently working were moderately associated with distress of PF dysfunction, based on the overall PFDI-20 score. Of the variables that were entered into the multivariable model, age (β 0.21; 95%CI: 0.06, 0.36) and not having a university degree (β −0.45; 95%CI: −0.77, −0.13) were the strongest predictors of distress of PF dysfunction. The presence of breast cancer was not associated with the distress of PF dysfunction in the multivariable model. This model explained 6.3% of variance in overall distress scores.
Factors associated with the distress and impact of pelvic floor dysfunction in women with (n = 120) and without (n = 170) breast cancer*.
β: standardised beta coefficient refers to the degree of change in distress or impact scores for every standard deviation of change in the predictor variable.
R2: adjusted R2 value shows the contribution of each predictor (independent) variable to the variance in symptom distress or impact scores expressed as a percentage. 95%CI, 95% confidence interval; BMI, body mass index. Significant results (p < 0.05) in bold.
The presence of breast cancer, higher age, higher BMI, not having a university degree, not currently working and being a current smoker were moderately associated with the impact of PF dysfunction on univariable analysis (Table 3). Multivariable analysis showed that these variables accounted for 13.3% of the variance in overall impact scores with not currently working (β −0.50; 95%CI: −0.77, −0.23), being a current smoker (β 0.33; 95%CI: 0.10, 0.59) and the presence of breast cancer (β 0.09; 95%CI: 0.02, 0.18) being statistically significant predictors of impact scores. Having breast cancer was positively associated with impact scores demonstrating that women with breast cancer reported a higher impact of PF dysfunction than women in the control group.
As we observed higher distress and impact scores in the urinary domain of the PFDI-20 and PFIQ-7 in women with breast cancer than women without breast cancer, the association between breast cancer and the distress and impact of urinary symptoms were also computed (see Supplemental material online 1). The presence of breast cancer was not associated with the distress of urinary symptoms. Only age (β 0.21; 95%CI: 0.04, 0.29) and not having a tertiary education (β −0.47; 95%CI: −0.80, −0.15) were statistically significant predictors in the multivariable model that explained 7.2% of the variance in urinary distress scores. In contrast, having breast cancer (β 0.33; 95%CI: 0.08, 0.51) and currently working (β 0.21; 95%CI: 0.05, 0.37) were significantly associated with the impact of urinary symptoms on multivariable analysis. This model explained 5.8% of variance in urinary impact scores.
Despite our efforts to minimise selection bias by recruiting a larger number of women without breast cancer, we observed statistically significant differences in the known risk factors of PF dysfunction (age, BMI, and parity) between groups. Post hoc matched analyses were conducted to minimise the potential confounding effects of these variables. A total of 77 pairs were identified when women with and without breast cancer were sequentially matched according to five-year age groups, BMI (categorised into underweight, normal, overweight, and obese) and parity (nulliparous or parous) using SPSS v25 (IBM Corp, Armonk, NY). Differences in demographic characteristics, prevalence, distress, and impact of PF dysfunction between the two paired groups were assessed using paired t-tests for continuous data and McNemar's test for categorical variables.
Accuracy of matching between the two groups was confirmed as there were no significant differences found in age, BMI, and parity. A similar proportion of women were post-menopausal in each group: women with breast cancer (n = 49/77, 63%); women without breast cancer (n = 43/77, 56%) (Table 4).
Post hoc analyses of participant demographics and prevalence, distress, and impact of pelvic floor dysfunction.
BMI, body mass index; PFDI, pelvic floor distress inventory; PFIQ, pelvic floor impact questionnaire; SD, standard deviation.
Sixty-eight percent (n = 52/77) of women with breast cancer experienced UI compared to 39% (n = 30/77) in women without breast cancer which was significantly different between groups (percentage difference = 29%; 95%CI: 9, 37%). There were no differences in the occurrence of faecal incontinence and pelvic organ prolapse between groups. Women with breast cancer reported a significantly higher distress of PF dysfunction (in all domains) than women without breast cancer (mean difference = 23.5; 95%CI: 11.8, 35.2). They also reported a significantly greater impact of PF dysfunction than women without breast cancer (mean difference = 21.5; 95%CI: 10.4, 32.6).
DiscussionIn this study, the occurrence of UI was significantly higher in woman with breast cancer than women without breast cancer. There were however no statistically significant associations between breast cancer and the presence of UI, faecal incontinence, or pelvic organ prolapse. Women with breast cancer experienced higher impact of urinary symptoms, than women without breast cancer. While the presence of breast cancer was not associated with the distress of PF dysfunction, it was significantly associated with the impact of PF dysfunction. These findings suggest that UI may have a significant and previously unrecognised impact on the lives of women with breast cancer.
The occurrence rates of UI in both groups were considerably higher than expected. One systematic review reported that 19–47% of women with breast cancer experienced any type of UI7 which is much lower than the occurrence rate we found in this study. This may be the result of selection bias as women who experienced UI may have been more likely to agree to participate in our study, but this bias is likely to have also been present in previous studies.19,21 One possible reason for the overestimation of the occurrence of UI in the control group may be the use of systemic hormone replacement therapy (HRT), because systemic HRT use is known to be associated with the development and worsening of UI in peri or post-menopausal women without breast cancer.24 As we did not collect data on HRT use, we are unable to determine the association between HRT and the occurrence of UI in the control group. However, we continued to see a significant difference in the occurrence of UI after closely matching groups in the post hoc analysis, indicating that women with breast cancer may in fact experience UI at higher rates than women without breast cancer.
We collected both participant-reported, and clinician-reported menopausal status in women with breast cancer but noted discrepancies between these responses. Due to this discrepancy, menopausal status was not included in our analyses. Instead, menopausal status was indirectly adjusted through age which is often used as a proxy to classify menopausal status in epidemiologic studies.25 Future studies may need to determine whether menopausal status contributes to the occurrence of UI in women with breast cancer by conducting studies with matched cohorts and comparing women with various degrees of hypoestrogenism (e.g. women on tamoxifen or aromatase inhibitors).
The overall distress of PF dysfunction did not differ between groups. This result should be interpreted with caution as our sample size estimation was not calculated based on the distress of PF dysfunction, and our results may be underpowered. It is also important to note that both groups reported relatively low distress on the overall scale of the PFDI-20 which ranges from 0 to 300. Nevertheless, post hoc analyses showed significant differences in PF distress between groups in all domains when women were closely matched. This highlights that further studies with a larger sample size and a longitudinal study design are needed to understand the true distress of PF dysfunction in women with breast cancer and whether this distress changes as women recover from breast cancer.
A significant difference between groups was observed in the impact of PF dysfunction, including the urinary domain. It is however important to note that both groups reported relatively low impact on the overall scale of the PFIQ-7 which ranges from 0 to 300. Despite this, multivariable regression analyses showed that having breast cancer was associated with the impact of PF dysfunction overall and urinary symptoms specifically. It is plausible that women with breast cancer reduce or avoid situations in their daily activities that would provoke UI, to cope and minimise the distress of UI,26 which may explain both the low distress and high impact scores observed in this study. One qualitative study investigating the impact of genitourinary symptoms in women with breast cancer reported that these symptoms profoundly impacted on their ability to engage in activities of daily living.27 Further qualitative research is required to understand how women with breast cancer are impacted by PF dysfunction and the coping strategies they have employed the minimise the distress and impact of their symptoms. Women in this population may benefit from routine screening and treatment for UI by physical therapists.
LimitationsSome study limitations need to be noted. Firstly, the cross-sectional design limits our ability to draw causal inferences regarding the association between breast cancer and PF symptoms. Our method for selecting variables (based on differences between groups) into the final multivariable regression model may have resulted in a lower goodness of fit compared to other methods such as step-wise or hierarchical regression. However, all significant variables were likely captured as we included moderately associated variables (p ≤ 0.1) into the final model. Previous history of PF symptoms prior to breast cancer diagnosis was not collected as part of this study. Therefore, there is a possibility that women with breast cancer experienced PF dysfunction prior to cancer treatment. Further studies with a longitudinal design are warranted to determine the incidence of PF symptoms throughout the breast cancer treatment trajectory. A large proportion of participants in the control group also did not respond to any question in the questionnaires. As these responses were missing at random and not included in our analyses, we were unable to determine whether this may have produced different outcomes in this group, leading to invalid conclusions. However, this study had high participation rates and a large sample size compared to other similar studies.18 It was also powered to assess the difference in occurrence of UI in women with and without breast cancer.
ConclusionThis study compared the magnitude of PF dysfunction in women with and without breast cancer. Participants with breast cancer reported higher occurrence of UI than those without breast cancer. There were, however, no statistically significant associations between breast cancer and the presence of UI, faecal incontinence, and pelvic organ prolapse. Women with breast cancer experienced a significantly higher distress and impact of urinary symptoms than women without breast cancer. There were no significant differences in the impact of colorectal or prolapse domains. Given the cross-sectional nature of this study, further studies with a larger sample size and a longitudinal design are warranted to understand the true burden of UI in women with breast cancer and whether these changes as women recover from breast cancer.