A growing body of evidence has demonstrated the importance of implementing movement-evoked pain in conventional pain assessments, with a significant role for psychological factors being suggested. Whether or not to include these factors in the assessment of movement-evoked pain has not yet been determined.
ObjectivesThe aim of this systematic review is to explore the association between psychological factors and movement-evoked pain scores in people with musculoskeletal pain.
MethodsFor this systematic review with meta-analysis, four electronic databases (PubMed, Medline, WOS, and Scopus) were searched. Cross-sectional studies, longitudinal cohort studies, and randomized controlled trials investigating the association between movement-evoked pain and psychological factors in adults with musculoskeletal pain were considered. Meta-analysis was conducted for outcomes with homogeneous data from at least 2 studies. Fischer-Z transformations were used as the measure of effect. Quality of evidence was assessed using the National Institutes of Health's Quality assessment tool for observational cohort and cross-sectional studies and Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.
ResultsMeta-analyses and grading the quality of evidence revealed moderate evidence for a relation between movement-evoked pain and depressive symptoms (Fisher-z=0.27; 95%CI: 0.17, 0.36; 5 studies (n=440)), pain-related fear (Fisher-z=0.35; 95%CI: 0.26, 0.44; 6 studies (n=492)), and pain catastrophizing (Fisher-z=0.47; 95%CI: 0.36, 0.58; 4 studies (n=312)) in people with musculoskeletal pain.
ConclusionsMovement-evoked pain is weakly to moderately associated to depressive symptoms, pain-related fear, and pain catastrophizing in people with musculoskeletal pain.
Persistent musculoskeletal pain is the most common type of chronic pain.1,2 It interferes with people's quality of life and is the global leader in disability.3 Movement-evoked pain (MEP) is a frequently reported complaint amongst people with musculoskeletal pain4,5 and a construct that provides unique information compared to pain at rest.5,6 MEP accounts for a significant amount of variance in self-reported disability7,8 and findings from Dailey et al.9 suggest that transcutaneous electrical nerve stimulation reduces MEP – but not pain at rest – in individuals with fibromyalgia. In the literature, two types of outcome measures are used to evaluate MEP: a maximum or average pain score; representing the pain experienced by patients during a specific movement task, and an index score; representing a maximum or average pain score, yet corrected for baseline pain (i.e., to calculate the MEP index score, the baseline pain score – assessed at rest, before completing any movement task – is subtracted from the maximum (or average) pain score).10-14 These two concepts thus capture a different experience, which may be an issue in reviews that combine all these measures in one analysis.15,16 The mechanisms behind MEP in patients with musculoskeletal pain remain partly unknown. It is, however, speculated that aside from peripheral mechanisms, centrally-driven mechanisms are involved.
Evidence supporting these speculated central contributions is provided by quantitative sensory testing measures of central pain processing in patients with musculoskeletal pain14,17 and significantly contributing psychological factors.18,19 Some of these psychological factors have been previously discussed in the literature,20,21 for which inconsistent results regarding their relation to MEP have been found (potentially due to differences in population [i.e., healthy people, people undergoing surgery or people experiencing musculoskeletal pain],15 as well as a lack of clear protocols to objectify MEP15,16). A greater understanding of how psychological factors relate to MEP may lead to enhanced assessment methods of this construct. Whether to implement psychological constructs in clinical testing procedures, and eventually in rehabilitation programs, has not yet been determined.
Given that MEP appears to be a significant barrier in activity-based interventions,22,23 and the positive association between psychological factors (i.e., pain-related beliefs) and improved functional outcomes,24,25 filling this knowledge gap seems essential. According to our knowledge, no clear overview addressing the relation between MEP scores and psychological factors exists. Therefore, this systematic review aims to explore the association between psychological factors and MEP scores in patients with musculoskeletal pain.
MethodsProtocol & registrationThis systematic review is reported consistent with the PRISMA guidelines.26 The review protocol was a priori registered in the PROSPERO database (CRD42020181138).
Literature searchA systematic search was performed by screening PubMed, Web of Science, Scopus, and Medline until the 15th of April 2022. The search strategy was based on the PICO(S)-framework (Patient=individuals with musculoskeletal pain; Instrument of measurement=self-reported questionnaires; Comparison was not applicable; Outcomes=MEP scores and psychological factors; Study design=cross-sectional and cohort study designs, and randomized controlled trials). Search items are listed in the Supplementary Material. In addition, we performed a citation tracking using PubMed, and a reference search of the eligible studies.
Eligibility criteria – study selectionStudies needed to be cross-sectional studies, longitudinal cohort, or randomized controlled trials, and report the relationship between psychological factors and MEP scores in adults with musculoskeletal pain (a full electronic search strategy for PubMed is in the Supplementary Material). Studies were included if the title or abstract contained outcome measures reporting pain during any kind of movement task,15 and intrinsic factors (situational, behavioral, and emotional) that cause patients to experience pain in a certain manner.27,28 As MEP is expressed by the maximum or average pain score during a particular movement task, or by a MEP index score (i.e., maximum or average pain, corrected for baseline pain), the results were categorized accordingly. Numerical coding was used to indicate whether the article discussed the relation between psychological factors and MEP (coded as 1) or a MEP index (coded as 2), Table 1. After screening titles and abstracts, eligible articles were read in full. Two independent researchers (LL and LA) screened the title and abstract, and the full text using Rayyan software.29 Disagreements were resolved by a consensus-based discussion.
Overview of studies, categorized by psychological factors in patients with musculoskeletal pain.
| Author and year | Study design | Participants (N; Pain condition; women (%)) | MEP (Movement task; Calculation; Assessment tool) | Psychological factor (Construct (Assessment tool)) | Results (p-value) |
|---|---|---|---|---|---|
| Anxiety | |||||
| 1 Hadlandsmyth 201748 | CS | N=346; knee OA; 54% | Active flexion/extension of the affected knee; Average score between flexion and extension pain ratings was used; NRS (0-20) | Sate anxiety (STAI) | r = N.R. |
| 1 Murphy 199744 | CS | N=20; CLBP; 70% | Walking test, stand-up test, stair climbing; Experienced pain during each exercise; Verbal VAS (0-10) | State anxiety (STAI) | β (walking)= N.R. (p > 0.05) |
| β (stand-up test) = -0.764, T = -2.877 (p = 0.010) | |||||
| β (stair climbing) = N.R. (p > 0.05) | |||||
| 1 Tonelli 201113 | C | N=208, Knee OA, 66.3% | Flexion, extension and walking; N.R.; NRS (0-20) | State anxiety (STAI) | β (walking) = 0.075 (p = 0.081) |
| β (flexion and extension): N.R. | |||||
| Chronic pain acceptance | |||||
| 2 Rabey 201659 | CS | N= 294; CLBP; 57.1% | Repeated spinal bending (20 forward and 20 backward spinal bends); MEP index (pain intensity score after last 5 repetitions subtracted from baseline pain score (first 5 repetitions)); NRS (0-10) | Chronic pain acceptance (CPAQ 8) | MEP index: Cluster 1 had a significantly greater proportion of people with no increase in pain following repeated movements and a lesser proportion of people with increased pain following repeated movement c |
| Depressive symptoms | |||||
| 1 Adams 200849‡ | CS | N = 83; musculoskeletal pain; 51% | Canister lifting task; Average pain (18 canister lifts); VRS (0-10) | Depressive symptoms (BDI-II) | Women: r = 0.39 (p < 0.01) |
| Men: r = 0.03 (p > 0.05) | |||||
| 1 Bartley 2019a50 | CS | N = 60; CLBP; 56% | Back Performance Scale; Average mean score of current LBP immediately after movement tasks; N.R. (0-100) | Depressive symptoms (PROMIS) | No differences (p = 0.08) were detected across cluster groups a |
| 1 Cruz-Almeida 201751 | CS | N=270; knee OA, 63% | Standing balance, 4-m walking, rise from a chair; Average pain; VAS (0-100) | Depressive symptoms (CES-D) | Cluster 3 reported significantly greater depressive symptoms than individuals in Cluster 1 b |
| 1 Hadlandsmyth 201748 | CS | N=346; knee OA; 54% | Active flexion/extension of the affected knee; Average score between flexion and extension pain ratings was used; NRS (0-20) | Depressive symptoms (GDS) | r = N.R. |
| 1,2 Lambin 201112‡∇ | CS | N=100; fibromyalgia (n=50) and CLBP (n=50); 100% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Depressive symptoms (BDI-II) | r = 0.284 (p < 0.01) |
| MEP index: r = 0.088 (p > 0.05) | |||||
| 1 O'Sullivan 201452 | CS | N=53; mechanical CLBP (N=17), non-mechanical, CLBP (N=19), and pain free controls (N=19); 64% | Mechanical pain; where pain is related to processes of peripheral sanitization and some degree of activity dependent central sensitization; VAS (0-10) | Depression, anxiety and stress (DASS 21) | Significant differences for DASS score between (p < 0.001) |
| - non-mechanical CLBP (median (IQR)): 30 (34)- mechanical CLBP (median (IQR)): 20 (18)- controls (median (IQR)): 10 (14) | |||||
| 1 Penn 202053‡ | CS | N=105; CLBP; 59% | Standing balance, 4-m walking, rise from a chair; pain experienced during the activity; NRS (0-100) | Depressive symptoms (CES-D) | r = 0.206 (p < 0.05) |
| 2 Rabey 201659 | CS | N= 294; CLBP; 57.1% | Repeated spinal bending (20 forward and 20 backward spinal bends); MEP index (pain intensity score after last 5 repetitions subtracted from baseline pain score (first 5 repetitions)); NRS (0-10) | Depression, anxiety and stress (DASS 21) | MEP index: Cluster 1 had a significantly greater proportion of people with no increase in pain following repeated movements (p < 0.001) and a lesser proportion of people with increased pain following repeated movement (p < 0.001) c |
| 1,2 Sullivan 200911‡∇ | CS | N=90; CLBP; 49% | Canister lifting task; Mean of activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Depressive symptoms (BDI-II) | r = 0.25 (p < 0.05) |
| MEP index: r = 0.10 (p > 0.05) | |||||
| 1,2 Sullivan 201010‡∇ | CS | N=62; whiplash injuries; 48% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Depressive symptoms (BDI-II) | r = 0.37 (p < 0.01) |
| MEP index: r = -0.20 (p > 0.05) | |||||
| 1 Tonelli 201113 | C | N=208, Knee OA, 66.3% | Flexion, extension and walking; N.R.; NRS (0-20) | Depressive symptoms (GDS-SF) | β (walking) = N.R. (p > 0.05) |
| β (flexion and extension): N.R. (p > 0.05) | |||||
| 2 Wideman 201414∇ | CS | N= 107; Knee OA; 70.1% | 6-minute walk test, average pain score MEP index (subtracting first pain ratings from peak pain ratings) over 2 trails; VRS (0-100) | Depressive symptoms (POMS) | MEP index: r = -0.072 (p > 0.05) |
| Distress | |||||
| 1 Booker 201954 | CS | N= 162; knee OA; 61% | Standing balance, walking, chair stand, maximal isometric strength test; Mean intensity pain; NRS (0-100) | Perceived distress (PSS) | F (standing balance) = 1.37 (p = 0.24) |
| F (walking) = 3.59 (p = 0.06) | |||||
| F (chair stand) = 1.69 (p = 0.20) | |||||
| F (index knee strength test) = 0.02 (p = 0.88) | |||||
| F (non-index knee strength test) = 3.52 (p = 0.06) | |||||
| 1 Damsgard 201046 | CS | N=232; various musculoskeletal complaints; 53% | Average pain experienced during latest week during activity; NRS (0-10) | Psychological distress (HSCL 25) | β = 1.28 (p = 0.001) |
| 1 Hadlandsmyth 201748 | CS | N=346; knee OA; 54% | Active flexion/extension of the affected knee; Average score between flexion and extension pain ratings was used; NRS (0-20) | Pain related distress (NRS) | r = 0.86 (p < 0.01) |
| Pain-related fear | |||||
| 1 Crombez 1999 (2)55‡ | CS | N=38; CLBP; 66% | Trunk-extension-flexion task; Maximum back pain experienced; Verbal graphical rating scale (0-100) | Fear Avoidance Beliefs (FABQ) | FABQ-physical: r = 0.18 (p > 0.05) |
| FABQ-work: r = 0.42 (p < 0.01) | |||||
| Pain-related fear (TSK) | r = 0.16 (p > 0.05) | ||||
| 1 Damsgard 201046 | CS | N=232; various musculoskeletal complaints; 53% | Average pain experienced during latest week during activity; NRS (0-10) | Pain-related fear (TSK) | β = 0.70 (p < 0.001) |
| 1,2 Lambin 201112‡∇ | CS | N=100; fibromyalgia (n=50) and CLBP (n=50); 100% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain-related fear (TSK) | r = 0.360 (p < 0.01) |
| MEP index:r = 0.208 (p < 0.05) | |||||
| 2 La Touche 201960 | CS | N=60; nonspecific CLBP; 58% | Canister lifting task; MEP index (subtracting first pain ratings from peak pain ratings); VAS (0-10). | Fear avoidance beliefs (FABQ) | Patients were classified as having "high" or "low" self-efficacy based on CPSS-scores. |
| Pain-related fear (TSK) | MEP index: | ||||
| High self-efficacy group: r = 0.335 (p > 0.05) | |||||
| Low self-efficacy group: r = 0.206 (p > 0.05) | |||||
| MEP index: | |||||
| High self-efficacy group: r = 0.711 (p < 0.01) | |||||
| Low self-efficacy group: r = 0.705 (p < 0.01) | |||||
| 1,2 Mankovsky-Arnold 20147‡∇ | CS | N=142; whiplash; 48% | Canister lifting task; pain evoked by one lift and MEP index (pain intensity score after first 3 lifts subtracted pain intensity score after last 3 lifts); NRS (0-10) | Pain-related fear (TSK) | r = 0.369 (p < 0.01) |
| MEP index: | |||||
| r = 0.088 (p > 0.05) | |||||
| 1 Palit 201947‡ | C | N=60; LBP; 56.7% | Back performance scale; average of pain ratings; N.R. (0-100) | Fear-avoidance beliefs (FABQ) | r = 0.26 (p < 0.05) |
| β = 0.46, t = 0.91 (p < 0.01) | |||||
| 2 Rabey 201659 | CS | N= 294; CLBP; 57.1% | Repeated spinal bending (20 forward and 20 backward spinal bends); MEP index (pain intensity score after last 5 repetitions subtracted from baseline pain score (first 5 repetitions)); NRS (0-10) | Fear avoidance beliefs (FABQ) | MEP index: Cluster 1 had a significantly greater proportion of people with no increase in pain following repeated movements (p < 0.001) and a lesser proportion of people with bidirectional increases in pain following repeated movement (p < 0.001) c |
| 1,2 Sullivan 200911‡∇ | CS | N=90; CLBP; 49% | Canister lifting task; Mean of activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain-related fear (TSK) | r = 0.36 (p < 0.01) |
| MEP index: | |||||
| r = 0.26 (p < 0.05) | |||||
| 1,2 Sullivan 201010‡∇ | CS | N=62; whiplash injuries; 48% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain-related fear (TSK) | r = 0.33 (p < 0.01) |
| MEP index: | |||||
| r = 0.16 (p > 0.05) | |||||
| 2 Woznowski-Vu 201945∇ | CS | N=116; Musculoskeletal pain; 69,8% | Self-paced walk, standardized lift, tailored lift; MEP index (subtracting first pain ratings from peak pain ratings); NRS (0-100) | Pain-related fear (TSK) | MEP index: |
| r (walking) = 0.140 (p > 0.05) | |||||
| r (standardized lift) = 0.052 (p > 0.05) | |||||
| r (tailored task) = 0.110 (p > 0.05) | |||||
| Pain catastrophizing | |||||
| 1 Cruz-Almeida 201751 | CS | N=270; knee OA, 63% | Standing balance, 4-m walking, rise from a chair; Average pain; VAS (0-100) | Coping strategies and pain catastrophizing (CSQ-R) | Cluster 3 reported significantly greater use of coping strategies, more catastrophizing individuals in Cluster 1 b |
| 1 Hadlandsmyth 201748 | CS | N=346; knee OA; 54% | Active flexion/extension of the affected knee; Average score between flexion and extension pain ratings was used; NRS (0-20) | Pain catastrophizing (PCS) | r = N.R. |
| 2 La Touche 201860 | CS | N=60; nonspecific CLBP; 58% | Canister lifting task; MEP index (subtracting first pain ratings from peak pain ratings); VAS (0-10). | Pain catastrophizing (PCS) | Patients were classified as having “high” or “low” self-efficacy based on CPSS-scores. |
| MEP index: | |||||
| High self-efficacy group: r = 0.606 (p < 0.01) | |||||
| Low self-efficacy group: r = 0.765 (p < 0.01) | |||||
| 1,2 Lambin 201112‡∇ | CS | N=100; fibromyalgia (n=50) and CLBP (n=50); 100% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain catastrophizing (PCS) | r = 0.380 (p < 0.01) |
| MEP index: | |||||
| r = 0.151 (p > 0.05) | |||||
| 1 Palit 201947‡ | C | N=60; LBP; 56.7% | Back performance scale; average of pain ratings; N.R. (0-100) | Pain catastrophizing (PCS) | r = 0.46 (p < 0.01) |
| β = 0.58, t = 2.13 (p < 0.001) | |||||
| 2 Rabey 201659 | CS | N= 294; CLBP; 57.1% | Repeated spinal bending (20 forward and 20 backward spinal bends); MEP index (pain intensity score after last 5 repetitions subtracted from baseline pain score (first 5 repetitions)); NRS (0-10) | Pain catastrophizing (PCS) | MEP index: Cluster 1 had a significantly greater proportion of people with no increase in pain following repeated movements (p < 0.001) and a lesser proportion of people with increased pain following repeated movement (p < 0.001) c |
| 1,2 Sullivan 200911‡∇ | CS | N=90; CLBP; 49% | Canister lifting task; Mean of activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain catastrophizing (PCS) | r = 0.46 (p < 0.01) |
| MEP index: r = 0.19 (p > 0.05) | |||||
| 1,2 Sullivan 201010‡∇ | CS | N=62; whiplash injuries; 48% | Canister lifting task; Mean activity-related pain and MEP index (subtracting first pain ratings from peak pain ratings); VRS (0-10) | Pain catastrophizing (PCS) | r = 0.48 (p < 0.01) |
| MEP index: r = 0.28 (p < 0.05) | |||||
| 1 Tonelli 201113 | C | N=208, Knee OA, 66.3% | Flexion, extension and walking; N.R.; NRS (0-20) | Pain catastrophizing (PCS) | β (walking): N.R. (p > 0.05) |
| β (flexion and extension): N.R. (p > 0.05) | |||||
| 2 Wideman 201414∇ | CS | N= 107; Knee OA; 70.1% | 6-minute walk test, average pain score MEP index (subtracting first pain ratings from peak pain ratings) over 2 trails; VRS (0-100) | Pain catastrophizing (PCS) | MEP index: |
| r = 0.215 (p < 0.05) | |||||
| β = 0.222, T= 2.508 (p < 0.05) | |||||
| 2 Woznowski-Vu 201945∇ | CS | N=116; Musculoskeletal pain; 69,8% | Self-paced walk, standardized lift, tailored lift; MEP index (subtracting first pain ratings from peak pain ratings); NRS (0-100) | Pain catastrophizing (PCS) | MEP index: |
| r (walking) = 0.068 (p > 0.05) | |||||
| r (standardized lift) = 0.060 (p > 0.05) | |||||
| r (tailored lift) = -0.039 (p > 0.05) | |||||
| Pain hypervigilance | |||||
| 1 Cruz-Almeida 201751 | CS | N=270; knee OA, 63% | Standing balance, 4-m walking, rise from a chair; Average pain; VAS (0-100) | Pain vigilance (PVAQ) | Cluster 3 reported significantly more pain hypervigilance than individuals in Cluster 1 b |
| Perceived injustice | |||||
| 1 Penn 202053 | CS | N=105; CLBP; 59% | Standing balance, 4-m walking, rise from a chair; pain experienced during the activity; NRS (0-100) | Perceived injustice (IEQ) | r = 0.496 (p < 0.001) |
| Positive and negative affect | |||||
| 1 Bartley 2019a50 | CS | N = 60; CLBP; 56% | Back Performance Scale; Average mean score of current LBP immediately after movement tasks; N.R. (0-100) | Positive and negative affect (PANAS) | No differences (p = 0.08) were detected across cluster groups a |
| 1 Bartley 2019b56 | CS | N= 201; knee OA; 61% | Standing balance, 4-m walking, rise from a chair; Average mean score of LBP immediately after movement tasks; N.R. (0-100) | Positive and negative affect (PANAS) | r = -0.09 (p > 0.05) |
| 1 Crombez 1999 (2)55‡ | CS | N=38; CLBP; 66% | Trunk-extension-flexion task; Maximum back pain experienced; Verbal graphical rating scale (0-100) | Negative affect (NEM) | r = 0.23 (p > 0.05) |
| 1 Cruz-Almeida 201751 | CS | N=270; knee OA, 63% | Standing balance, 4-m walking, rise from a chair; Average pain; VAS (0-100) | Positive and negative affect (PANAS) | Cluster 3 reported significantly more negative affect than individuals in Cluster 1. All three clusters reported similar levels of positive affect (p > 0.05) b |
| 1 Wideman 201657‡ | CS | N=108, Knee OA; 70.4% | 6MWT, TUG test; post-task discomfort; VRS (0-100) | Positive and negative affect (VRS) | Positive affect: r (6MWT) = 0.36 (p < 0.05) |
| Positive affect: r (TUG) = -0.25 (p < 0.05) | |||||
| Negative affect: r (6MWT) = 0.56 (p < 0.05) | |||||
| Negative affect: r (TUG) = 0.26 (p < 0.05) | |||||
| Positive well-being | |||||
| 1 Bartley 2019a50 | CS | N = 60; CLBP; 56% | Back Performance Scale; Average mean score of current LBP immediately after movement tasks; N.R. (0-100) | Positive well-being (PROMIS positive affect and well-being) | No differences (p = 0.08) were detected across cluster groups a |
| 1 Bartley 2019b56 | CS | N= 201; knee OA; 61% | Standing balance, 4-m walking, rise from a chair; Average mean score of LBP immediately after movement tasks; N.R. (0-100) | Positive well-being (PAW-SF) | r = -0.16 (p < 0.05) |
| Resilience | |||||
| 1 Bartley 2019a50 | CS | N = 60; CLBP; 56% | Back Performance Scale; Average mean score of current LBP immediately after movement tasks; N.R. (0-100) | Trait resilience (BRS) | No differences (p = 0.08) were detected across cluster groups a |
| Optimism (LOT-R) | |||||
| 1 Bartley 2019b56 | CS | N= 201; knee OA; 61% | Standing balance, 4-m walking, rise from a chair; Average mean score of LBP immediately after movement tasks; N.R. (0-100) | Trait resilience (BRS) | r = -0.17 (p < 0.05) |
| Optimism (LOT-R) | r = -0.22 (p < 0.01) | ||||
| 1 Palit 201947 | C | N=60; LBP; 56.7% | Back performance scale; average of pain ratings; N.R. (0-100) | Pain resilience (PRS) | r = -0.11 (p > 0.05) |
| β = -0.03, t = -0.11 (p = 0.91) | |||||
| Self-efficacy | |||||
| 1 Adegoke 201758 | CS | N = 51; unilateral knee OA; 57% | Stair test (STT), 20m walking test (20MWT), Timed Up and Go Test (TUG)); Present pain; Box NRS (BNPS, 0-10) | Pain self-efficacy (PSE) and function (FSE) subscale | PSE: r = -0.56 (p < 0.01) |
| FSE: r = -0.52 (p < 0.01) | |||||
| 1 Damsgard 201046 | CS | N=232; various musculoskeletal complaints; 53% | Average pain experienced during latest week during activity; NRS (0-10) | Self-efficacy (ASES) | β = -0.05 (p < 0.001) |
| 2 Rabey 201659 | CS | N= 294; CLBP; 57.1% | Repeated spinal bending (20 forward and 20 backward spinal bends); MEP index (pain intensity score after last 5 repetitions subtracted from baseline pain score (first 5 repetitions)); NRS (0-10) | Pain self-efficacy (PSE) | MEP index Cluster 1 had a significantly greater proportion of people with no increase in pain following repeated movements (p < 0.001) and a lesser proportion of people with bidirectional increases in pain following repeated movement (p < 0.001) c |
: included in a meta-analysis MEP; ∇: included in meta-analysis MEP index.
ASES, Arthritis Self-Efficacy Scale; BDI-II, Beck Depression Inventory-II; BRS, Brief Resilience Scale; C, cohort study; CES-D, Center for Epidemiological Studies – Depression; CLBP, chronic low back pain; CPAQ-8, Chronic Pain Acceptance Questionnaire 8; CPSS, Chronic Pain Self-Efficacy Scale; CS, cross-sectional study; CSQ-R, Coping Strategies Questionnaire-Revised; DASS-21, Depression Anxiety Stress Scale; FABQ, Fear Avoidance Beliefs Questionnaire; FSE, Function Self-Efficacy Scale; GDS, Geriatric Depression Scale; GDS-SF, Geriatric Depression Scale – Short Form; HSCL-25, Hopkins Symptoms Checklist – 25; IEQ, Injustice Experience Questionnaire; IQR, inter-quartile range; LBP, low back pain; LOT-R, Life Orientation Test-Revised; m, meter; MEP, movement-evoked pain; N, number; NEM, Negative Emotionality Scale; N.R., not reported; NRS, numeric rating scale; OA, osteoarthritis; PANAS, Positive And Negative Affect Schedule; PAW-SF, Positive Affect and Well-being – Short Form; PCS, Pain Catastrophizing Scale; POMS, Profile of Mood States; PROMIS, Patients-Reported Outcomes Measurement Information System; PRS, Pain Resilience Scale; PSE, Pain Self-Efficacy Scale; PSS, Perceived Stress Questionnaire; PVAQ, Pain Vigilance Awareness Questionnaire; STAI, State-Trait Anxiety Questionnaire; TSK, Tampa Scale for Kinesiophobia; VAS, visual analogue scale; VRS, Verbal rating scale.
Four clusters were identified: (1) High Resilience group: high levels of psychological, health and social support functioning; (2) High Health/Low psychosocial group: optimal health related functioning, low levels of psychosocial function, (3) High psychosocial/Low health group: poor health functioning, high psychological functioning, moderate to high social support, (4) Low resilience group: low levels of functioning across psychological, social and health-related factors;
Three clusters were identified: (1) High physical function and minimal MEP, (2) Moderate physical function and mild MEP, (3) Low physical function and severe MEP.
Three clusters were identified: (1) Low cognitive and affective questionnaire scores, with exception of fear-avoidance beliefs, (2) elevated thought suppression, catastrophizing and fear-avoidance beliefs, but low pain self-efficacy, depression, anxiety and stress, (3) highest scores across cognitive and affective questionnaires.
The methodological quality was rated as good, fair, or poor, using the National Institutes of Health's (NIH) Quality Assessment tool for observational cohort and cross-sectional studies,30 and as low risk of bias, some concerns, or high risk of bias using the Revised Cochrane risk-of-bias tool for randomized trials.31 The appraisal was independently performed by 2 blinded reviewers (LL and LA). Disagreements were resolved by a consensus-based discussion.
Summary measures and methods of analysesAll data regarding the associations between psychological factors and MEP were retrieved from the eligible papers. If the title or abstract included MEP and psychological factors as outcome measures, but no corresponding data were provided in the full-text article, authors were contacted. The mean correlation coefficient was calculated using Fischer-Z transformations as the measure of effect in the meta-analysis (MA). We interpreted .10 as a weak correlation, .30 as moderate, and .50 as a strong correlation.32 Heterogeneity was assessed by the prediction interval (PI)33 and by the I2 statistic.34 An I2 value >50% was classified as important heterogeneity.25 In this case, a subgroup analysis was conducted, investigating possible underlying differences that may explain heterogeneity. For the subgroup analyses, we followed the suggestions to examine only a small number of characteristics – based on meta-analyses and clinical studies.26 If authors used multiple movement tasks to assess MEP, a sensitivity analysis was conducted.26 We assessed confidence in the effect estimates using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach.35 There are four levels of quality, the highest initial rating being for randomized controlled trial evidence, and low-quality ratings for observational studies. We acknowledge, however, that not all observational studies are of low quality.36 Therefore, the initial rating of “moderate” quality37 could be downgraded to low, or very low quality evidence based on the following criteria: risk of bias, inconsistency (i.e., the presence of significant heterogeneity and inconsistent findings), indirectness (i.e., generalisability of the findings, the research does not address the intervention, population or outcomes of interest), imprecision (i.e., the total number of participants is less than the number of participants generated by a conventional sample size calculation for a single adequately powered study (i.e., less than 400 for continuous outcomes38,39)), and publication bias (i.e., an under-estimation or over-estimation of the underlying effect due to selective publication of studies, e.g., inclusion of small studies, industry sponsored studies or asymmetrical funnel plots40). If performing a MA was not possible due to clinical or statistical heterogeneity (i.e., differences in assessment tool or questionnaire, or correlation coefficients could not be calculated based on given beta coefficients),41,42 a best evidence synthesis (BES)43 was performed.
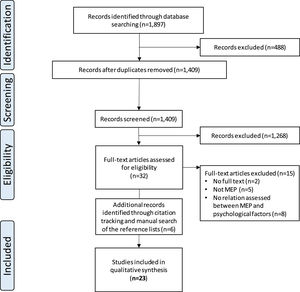
ResultsStudy selectionThe initial search of PubMed, Medline, WOS, and Scopus resulted in a total of 1897 hits, of which 1409 papers remained after deduplication. After screening papers on title and abstract, full text of 32 studies was examined in more detail. Finally, 23 suitable articles were included (Fig. 1).
Study characteristicsTwenty-three studies were found eligible, of which six had an observational cohort study design and 21 a cross-sectional study design. A total sample of 2968 people with musculoskeletal pain was included. Characteristics of the included articles are presented in Table 1.
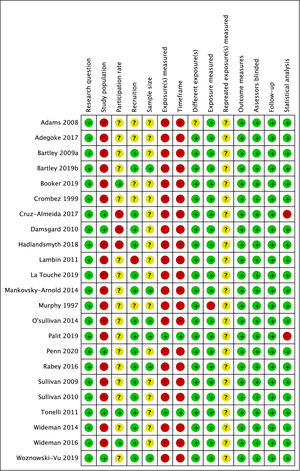
Risk of bias assessmentThe percentage agreement between both reviewers was 87.4%. The methodological quality of the included studies is summarized in Fig. 2. Weaknesses were lack of information on recruitment strategy and recruitment period. Also, adequate representation of the target population was mostly unclear and should be considered a potential bias in this review. Seventeen studies performed cross-sectional analyses. Therefore, questions 6 and 7 (i.e., exposure(s) measured and timeframe) of the NIH Quality Assessment tool30 were answered as “no.” In 4 studies performing (additional) regression analyses,14,44-46 exposure was assessed prior to the outcome, yet during the same timeframe. In 2 studies, the authors spread the measurements by approximately 1 week.13,47 Publication bias was not detected and is illustrated with funnel plots in the Supplementary Material.
Associations between psychological factors and MEPDefinitions of the included psychological factors can be found in Supplementary Material.
AnxietyThe relation between state anxiety and MEP was assessed in three studies.13,44,48 In two studies,13,44 it was not possible to calculate the correlation coefficient based on the given data42 and no MA could be conducted. A BES43 indicated limited evidence for a negative relation between state anxiety and MEP in patients with chronic low back pain (LBP), and limited evidence that state anxiety and MEP do not relate in patients with knee osteoarthritis (KOA).
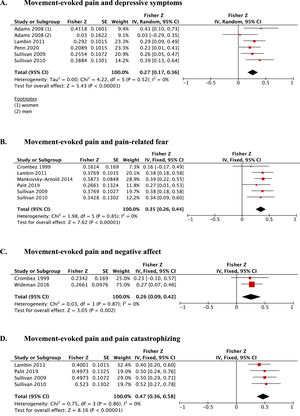
Depressive symptomsTen papers10-13,48-53 investigated the association between depressive symptoms and MEP. Data from five studies (n=440) indicated a significant and small estimated mean correlation coefficient (Fisher-z(Fz)=0.27; 95%CI: 0.17, 0.36; PI:0.13, 0.40; I2=0%, Fig. 3A) in patients with musculoskeletal pain. The quality of evidence for this association estimate was moderate (Table 2). In five studies,13,48,50-52 no correlation coefficient was reported or could be calculated,42 and could not be included in the MA. Two studies51,52 reported data consistent with the results of the MA.
GRADE evidence profile: associations between psychological factors and movement-evoked pain (index) scores in patients with musculoskeletal pain.
| Certainty Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other considerations | No of patients | Mean Correlation (95% C.I.) | Certainty | Comments |
| Movement-evoked pain and depressive symptoms | ||||||||||
| 5 | observational studies | serious1 | not serious | not serious | not serious | none | 440 | EMC 0.27 (0.17 to 0.36) p < 0.001* | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a weak association between MEP and depressive symptoms. |
| Movement-evoked pain and pain-related fear | ||||||||||
| 6 | observational studies | serious1 | not serious | not serious | not serious | none | 492 | EMC 0.35 (0.26 to 0.44) p < 0.001* | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a weak association between MEP and pain-related fear. |
| Movement-evoked pain and negative affect | ||||||||||
| 2 | observational studies | serious1 | not serious | not serious | serious3 | none | 146 | EMC 0.26 (0.09 to 0.42) p = 0.002* | ⨁⨁○○ LOW | There is limited evidence for a weak association between MEP and negative affect. |
| Movement-evoked pain and pain catastrophizing | ||||||||||
| 4 | observational studies | serious1 | not serious | not serious | not serious | none | 312 | EMC 0.47 (0.36 to 0.58) p < 0.001* | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a moderate association between MEP and pain catastrophizing. |
| Movement-evoked pain index and depressive symptoms | ||||||||||
| 4 | observational studies | serious1 | not serious | not serious | not serious | none | 359 | EMC -0.01 (-0.14 to 0.12) p = 0.88 | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a weak association between MEP and depressive symptoms. |
| Movement-evoked pain index and pain-related fear | ||||||||||
| 5 | observational studies | serious1 | not serious | not serious | not serious | none | 510 | EMC 0.14 (0.06 to 0.23) p = 0.001* | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a weak association between MEP and pain-related fear. |
| Movement-evoked pain index and pain catastrophizing | ||||||||||
| 5 | observational studies | serious1 | not serious | not serious | not serious | none | 475 | EMC 0.17 (0.08 to 0.26) p < 0.001* | ⨁⨁⨁○ MODERATE2 | There is moderate evidence for a weak association between MEP and pain catastrophizing |
CI, confidence interval; EMC, estimated mean correlation (Fisher z); MEP, movement-evoked pain; *, statistically significant.
As the overall risk of bias of the included studies can be considered high risk of bias, level of evidence was downgraded for within study risk of bias.
Three studies46,48,54 investigated the association between distress and MEP. Since only Hadlandsmyth et al.48 reported correlation coefficients and the data reported by the two other studies46,54 did not allow to calculate correlation coefficients,42 no MA was conducted. A BES43 indicated conflicting evidence for a relation between distress and MEP in patients with KOA,48,54 and limited evidence for a positive relation in patients with musculoskeletal pain.46
Pain-related fearSeven studies7,10-12,46,47,55 assessed the relation between pain-related fear and MEP. Data from six studies (n=492) indicated a significant and small estimated mean correlation coefficient (Fisher-z(Fz)=0.35; 95%CI: 0.26, 0.44; p<0.001; PI:0.22 to 0.47; I2=0%, Fig. 3B). The quality of evidence was moderate (Table 2). Damsgard et al.46 could not be included in the MA as the data did not allow to calculate a correlation coefficient,42 and Crombez et al.55 reported results based on Fear Avoidance Beliefs Questionnaire (FABQ) subscales. A BES43 indicated limited evidence for a positive relation between MEP and the FABQ-work subscale score55 in patients with LBP.
Pain catastrophizingSix trials10-13,47,51 investigated the relation between pain catastrophizing and MEP in patients with musculoskeletal pain. Data from four papers10-12,47 (n=312) reported a moderate estimated mean correlation coefficient (Fisher-z(Fz)=0.47; 95%CI: 0.36, 0.58; PI:0.17, 0.69; I2=0%, Fig. 3D). The quality of evidence for this correlation estimate was moderate (Table 2). Two studies13,51 could not be included in the MA because no correlation coefficient was reported or could be calculated.42 One study51 reported data consistent with the results of the MA.
Pain hypervigilanceCruz-Almeida et al.51 assessed the relation between pain vigilance and MEP in patients with KOA. A BES43 indicated limited evidence for an association between pain vigilance and MEP in patients with KOA.
Perceived injusticePenn et al.53 investigated the relation between perceived injustice and MEP in patients with chronic LBP. A BES43 indicated limited evidence for a positive association between perceived injustice and MEP in patients with chronic LBP.
Positive and negative affectTwo studies50,56 investigated the relation between positive and negative affect and MEP. However, only one study56 reported a correlation coefficient and the data reported by Bartley et al. (a)50 did not allow to calculate the correlation coefficient. Therefore, no MA could be conducted. A BES43 indicated limited evidence that positive and negative affect and MEP are not associated in patients with KOA and chronic LBP. Two papers51,57 assessed the association between positive affect and MEP in patients with KOA. However, only Wideman et al.57 reported a correlation coefficient, and the data reported by Cruz-Almeida et al.51 did not allow to calculate the correlation coefficient. Therefore, no MA could be conducted. A BES43 indicated conflicting evidence for an association between positive affect and MEP in patients with KOA. Three studies51,55,57 investigated the association between negative affect and MEP. Data from two papers55,57 (n=146) reported a small estimated mean correlation coefficient (Fisher-z(Fz)=0.26; 95%CI: 0.09, 0.49; I2=0%, Fig. 3C). The quality of evidence for this association estimate was low (Table 2). One study51 could not be included in this MA because the data did not allow to calculate the correlation coefficient, yet was consistent with the results of the MA.
Positive well-beingThe relation between positive well-being and MEP was assessed in two studies.50,56 However, only one study56 reported a correlation coefficient and the data reported by Bartley et al. (a)50 did not allow to calculate the correlation coefficient, so no MA could be conducted. A BES43 indicated limited evidence for a small and negative association in patients with KOA, and limited evidence that positive well-being and MEP are not related in patients with chronic LBP.
Resilience and optimismThree studies assessed the relation between resilience47,50,56 and MEP in patients with KOA and LBP.47,50 Because the data reported by Bartley et al. (a)50 did not allow to calculate a correlation coefficient, and Palit et al. included pain resilience whereas Bartley et al. (b)56 investigated trait resilience, no MA was conducted. A BES43 indicated moderate evidence that resilience and MEP are not related in patients with LBP,47,50 and limited evidence for a small and negative relation in patients with KOA.56 Two studies reported the association between optimism and MEP in patients with KOA56 and chronic LBP.50 Because the data reported by Bartley et al. (a)50 did not allow to calculate a correlation coefficient, no MA was conducted. A BES43 indicated limited evidence for a negative relation between MEP and optimism in patients with KOA, and for no relation in patients with chronic LBP.
Self-efficacyTwo papers46,58 reported the association between self-efficacy and MEP in patients with KOA58 and musculoskeletal pain.46 However, Adegoke et al.58 reported correlation coefficients and the data reported by Damsgard et al.46 did not allow to calculate the correlation coefficient. A BES43 indicated limited evidence for a negative relation in patients with KOA and musculoskeletal pain.
Associations between psychological factors and MEP index scoresChronic pain acceptanceRabey et al.59 investigated the association between chronic pain acceptance and MEP index scores in patients with chronic LBP. A BES43 indicated limited evidence for a positive relation in patients with chronic LBP.
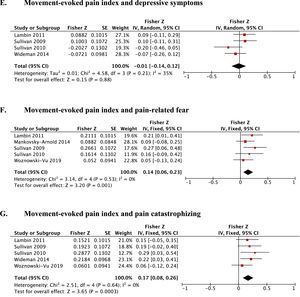
Depressive symptomsFour studies10-12,14 reported the relation between depressive symptoms and MEP index scores in patients with musculoskeletal pain. Data (n=359) indicated a small, but non-significant estimated mean correlation coefficient (Fisher-z(Fz)=-0.01; 95%CI: -0.14, 0.12; PI:-0.47, 0.45; I2=35%, Fig. 3E). The quality of evidence for this association estimate was moderate (Table 2).
Pain-related fearSeven studies7,10-12,45,59,60 assessed the relation between pain-related fear and MEP index scores in patients with chronic LBP. Data from five studies7,10-12,45 (n=510) reported a small estimated mean correlation coefficient (Fisher-z(Fz)=0.14; 95%CI: 0.06, 0.23; PI:-0.01, 0.28; I2=0%, Fig. 3F). The quality of evidence for this correlation estimate was moderate (Table 2). Because La Touche et al.60 classified the participants as “low” or “high self-efficacy groups,” this paper was not included in the MA yet reported data consistent with the results of the MA. Also, data reported by Rabey et al.59 did not allow to calculate the correlation coefficient, and was therefore not included in the MA.
Pain catastrophizingFive studies10-12,14,45 assessed the association between pain catastrophizing and MEP index scores in patients with musculoskeletal pain. Data (n=475) indicated a small estimated mean correlation coefficient (Fisher-z(Fz)=0.17; 95%CI: 0.08, 0.26; PI:0.02, 0.31; I2=0%, Fig. 3G). The quality of evidence for this correlation estimate was moderate (Table 2). Because La Touche et al.60 classified the participants as “low” or “high self-efficacy groups”, this paper was not included in the MA, yet reported consistent results.
Self-efficacyOne study59 investigated the relation between self-efficacy and MEP index scores in patients with chronic LBP. A BES43 indicated limited evidence for a negative relation in patients with chronic LBP.
Sensitivity analysesBoth Wideman et al.57 and Woznowski-Vu et al.45 used different movement tasks to assess MEP. Therefore, sensitivity analyses were conducted. To investigate the relation between MEP and negative affect in patients with KOA, Wideman et al.57 used the timed up and go (TUG) and a 6-minute walk test to assess MEP. Sensitivity analyses showed different results by including different movement tasks. Including the TUG resulted in a small estimated mean correlation coefficient (Fisher-z=0.26; 95%CI: 0.09, 0.49; PI: 0.10, 0.41; I2=0%). When the 6-minute walk test was included, this resulted in a large estimated mean correlation coefficient (Fisher-z=0.53; 95%CI: 0.37, 0.70, PI: 0.40, 0.64; I2=76%). However, heterogeneity was high. Furthermore, to investigate how the MEP-index relates to pain-related fear and pain catastrophizing, Woznowski-Vu et al.45 utilised three movement tasks (i.e., self-paced walk, standardized lift, tailored lift). However, sensitivity analyses did not result in different results.
DiscussionThis systematic review and MA aimed to provide an overview of the association between MEP and psychological factors in patients with musculoskeletal pain. According to the GRADE-approach35 (Table 2), there is moderate evidence for a weak relationship between MEP and depressive symptoms and pain-related fear in patients with musculoskeletal pain. There is also moderate evidence for a moderate relationship between MEP and pain catastrophizing in patients with musculoskeletal pain. Additionally, this review provided moderate evidence for a relationship between MEP index scores and pain-related fear and pain catastrophizing in patients with musculoskeletal pain. The results from the BES can be found in the Supplementary Material for both MEP and MEP index scores respectively.
The results of the current study indicate that MEP is associated with depressive symptoms, pain-related fear, and pain catastrophizing in patients with musculoskeletal pain. As the perception of pain is influenced by biological, psychological, and movement system factors, incorporating all contributing aspects during treatment seems warranted. Unfortunately, this review reports correlations, which prevents drawing specific hypotheses on causality and consequently does not provide an answer to the contributing aspect of psychological factors in MEP. Few studies included in this review reported longitudinal data and results using linear regression techniques14,46,47: pain-related fear significantly predicted MEP in patients with chronic musculoskeletal disorders46 and pain catastrophizing significantly predicted MEP in patients with chronic LBP47 and KOA.14 In the management of (chronic) pain, a mechanism-based approach is suggested,61 indicating that psychosocial approaches (e.g., pain education) tackling pain mechanisms and maladaptive psychological factors are recommended in subgroups where central mechanisms play a (significant) role. This subgroup with involvement of central mechanisms is often referred to as patients with a predominance of nociplastic pain.62 The growing evidence that educating patients positively affects central pain processing (e.g., increased pain thresholds63,64 and conditioned pain modulation64,65) creates an exciting window for MEP-rehabilitation. We hope that this review will encourage researchers to gain insight into the role of pain education when addressing MEP in patients with musculoskeletal pain and, perhaps even more important, in patients with a predominance of nociplastic pain.
The relation between psychological factors and MEP is not always assessed identically. Some authors use an average/maximum activity-related pain score, while others include a MEP index score (i.e., maximum or average pain, corrected for baseline pain). This index is associated with elevated scores of clinical indices of hypersensitivity.11,66 Because hypersensitivity is associated with psychological factors,67-69 it is not surprising that the present study found a weak but significant association between MEP index scores and pain-related fear and pain catastrophizing in patients with musculoskeletal pain. Furthermore, analyzing studies including pain populations with a predominance of nociplastic pain7,11 (such as fibromyalgia and chronic whiplash syndrome61) resulted in stronger correlation coefficients for depressive symptoms (Fisher-z(SE)=-0.20 (0.13)), pain catastrophizing (Fisher-z(SE)=0.29 (0.13)), and pain-related fear (Fisher-z(SE)=0.16 (0.13)) compared to the estimated mean correlation coefficients (Table 2). The presence of these associations supports the notion that MEP can be influenced by both peripheral and central mechanisms, and that the contribution of these central mechanisms seems to increase in populations with a predominance of nociplastic pain.
Limitations and strengthsThe heterogeneity in terms of reported outcomes and statistical analysis methods prevents drawing firm conclusions. Also, due to the observational study designs, no conclusions could be drawn on the causality of the observed associations between MEP and psychological factors. It is not possible to differentiate whether psychological factors affect MEP or MEP affects psychological factors in patients with musculoskeletal pain. Future studies using multiple data points are needed to further clarify potential causality between both constructs.70 Furthermore, the pain conditions represented in the BES are limited to KOA and (chronic) LBP. Despite these limitations, this review has several important strengths as well. A systematic and transparent methodology was implemented and a priori registered, incorporating the evaluation of internal (risk of bias) and external validity (given the broad range of musculoskeletal conditions included in this systematic review). In addition, we applied the GRADE framework to determine the overall quality of evidence. For studies that could not be included in a MA, qualitative analyses were performed according to the BES43 principle.
ConclusionsMEP measures are weakly to moderately associated with depressive symptoms, pain-related fear, and pain catastrophizing in patients with musculoskeletal pain. Future research should investigate whether addressing these maladaptive psychological factors can help improve MEP.
We wish to thank Dr Nicolas Delvaux and Dr Trudy Bekkering from the Belgian Centre for Evidence-Based Medicine for their help with the design and finalization the summary of findings tables. Funding for this study was obtained from the grant Wetenschappelijk Fonds Willy Gepts (WFWG) of the UZ Brussels.