In healthy people and people with nonspecific chronic spinal pain (nCSP) and/or insomnia, participation in physical activity on a regular basis has several physical and psychological health benefits. However, people with chronic conditions often tend to reduce physical activity participation which can lead to deconditioning over time. Currently, there are no known predictors for an (in)active lifestyle (before and after physical therapy treatment) in people with chronic spinal pain and comorbid insomnia.
ObjectiveTo examine predictors of pre-treatment moderate-to-vigorous physical activity (MVPA) and to examine determinants for a change in MVPA in response to 14-weeks of active physical therapy treatment in people with nonspecific chronic spinal pain (nCSP) and comorbid insomnia.
MethodsBaseline data and post-treatment data were analyzed for 66 participants. A linear multiple regression analysis was conducted to examine which factors predict MVPA at baseline. Linear mixed-effects modeling was used to identify determinants for change in MVPA in response to an active physical therapy treatment.
ResultsPhysical fatigue (b = -0.9; 95%CI: -1.59, -0.15), less limitations in functioning as a result of emotional problems (b = 0.1; 95%CI: 0.03, 0.10), mental fatigue (b = -1.0; 95%CI: -1.67, -0.43), lower general sleep quality (b= 0.7; 95%CI: 0.22, 1.17), and body mass index (b = -0.5; 95%CI: -0.93, -0.16) were significant predictors of baseline MVPA. The regression model explained 33.3% of the total variance in baseline MVPA. The change of MVPA in response to the treatment ranged from a decrease of 17.5 to an increase of 16.6 hours per week. No determinants for change in MVPA after treatment could be identified.
ConclusionPeople with nCSP and comorbid insomnia are more likely to engage in MVPA if they report, at baseline, lower sleep quality, fewer limitations in functioning resulting from emotional problems, lower body mass index, as well as less physical and mental fatigue.
Nonspecific chronic spinal pain (nCSP) is a common chronic pain condition associated with high healthcare use, high rates of disability, and consequently high direct and indirect costs for society.1-5 Furthermore, chronic pain is frequently associated with comorbidities such as other chronic diseases and mental disorders which generally harm the patient's functioning, treatment response, and economic burden.6,7 One of the most common comorbidities in people with nCSP is insomnia, with prevalence rates exceeding 50%.8,9
It is well known that physical activity has several physical and psychological health benefits.10-12 Specifically, in people with nCSP or insomnia, higher levels of physical activity are associated with less pain, better function, and several sleep promoting benefits.13-17 In general, ≥150 minutes of moderate-intensity physical activity throughout the week, ≥75 minutes of vigorous-intensity physical activity throughout the week, or an equivalent combination is recommended.18-20 Moreover, active treatment approaches promoting an increase in physical activity to aid recovery and reduce disability in people with nCSP are recommended over passive treatments.18-20 Despite the beneficial effects of physical activity, some people with chronic pain will avoid activities and change their activity patterns.21 Furthermore, comorbidities such as insomnia might negatively affect the possibility of maintaining and/or increasing physical activity level.22-24 Given the available evidence regarding the relation between chronic pain, sleep, and physical activity, it is clear that physical activity might play a key role in minimizing health concerns and improving quality of life in people with nCSP and comorbid insomnia.
Having knowledge about factors predicting whether someone is likely to be physically (in)active and knowing which subgroup of people are (less) likely to change their activity level after an active physical therapy treatment can be useful to identify these people early and anticipate an appropriate treatment response. Therefore, this study aims to examine predictors of moderate-to-vigorous physical activity (MVPA) at baseline and to examine determinants for a change in MVPA in response to 14-weeks of active physical therapy treatment in people with nCSP and comorbid insomnia.
MethodsDesign overviewThis study is a secondary analysis using real patient data (baseline and immediate post-treatment) from an ongoing multi-center, randomized controlled trial (expected finalization in June 2022) approved by the local ethics committees (University Hospital Ghent and University Hospital Brussels – ref no. B.U.N. 670201835625). The primary purpose of this ongoing trial is to examine the added value of cognitive-behavioral therapy for insomnia (CBT-I) to the current best physical therapy treatment for nCSP with comorbid insomnia. All participants gave informed consent. The full study protocol of the ongoing trial is registered at Clinicaltrials.gov (NCT03482856) and is published elsewhere.25
Setting, population, and sample sizeAdults with nCSP and comorbid insomnia were recruited via flyers in the participating universities, university hospitals (Ghent and Brussels), workplaces and public places, through advertisements, social media, primary care practices, and occupational health services. People had to send an email to an institutional email address specifically created for the original trial to show their interest. The inbox was only accessible by 3 researchers who worked on the original trial. Thereafter, the potential participants received a booklet with the study details and were requested to sign the informed consent and to fill out an online questionnaire which was used to perform a screening based on inclusion and exclusion criteria (Table 1). Potential participants were called by telephone for a verbal screening and to schedule the home-based polysomnography (Alice PDX system, Philips Respironics IncTM) assessment to screen for underlying sleep pathologies.26,27
Inclusion and exclusion criteria.
nCSP, nonspecific chronic spinal pain; PSG, polysomnography.
Because the recruitment of participants for the main trial was still ongoing at the conception of this analysis, a sample size calculation was conducted specifically for estimating the required sample size for answering the research question of this analysis. Sample size calculations were performed with G*Power 3 (Düsseldorf, Germany). The required number of participants was calculated for a linear multiple regression analysis based on a medium effect size (Cohen's f²) of 0.20 and 4 predictors in the final model.28 A total of 65 participants was required allowing for a type I error of .05 and aiming for 80% power.
Interventions, randomization, and blindingThe experimental and control interventions had a similar structure, the same number of sessions and the same session duration, only the content was different. All participants received 18 sessions of therapy during 14 weeks. The control and experimental groups received pain neuroscience education (PNE) and cognition targeted exercise therapy (CTET) while the experimental group also received additional CBT-I. The first three session for both groups focused on PNE.29-34 The first session was a 1-hour group session addressing the general aspects of PNE with the possibility for patients to ask questions. After this session, all participants received an information leaflet to inform their significant other and as refresher for themselves. Patients were also asked to complete a form to indicate feared activities. The second session was a home-based online module which consists of videos alternating with online questioning. The last PNE session was a 30-minute individual session, addressing the patient's specific questions, translating the content to the patient's daily life, and questioning and discussing the patient's perceptions and goals.
All other sessions were individual, real life, 30-minutes face-to-face sessions. The purpose of the CTET was to change inappropriate beliefs and perceptions in combination with PNE by gradually confronting the patient with movements and activities that were feared and/or avoided using a time-contingent approach. The exercises gradually progressed towards more complex and physically, cognitively, and psychosocially demanding situations. The same exercises were also implemented in an individual home exercise program. Across all sessions, patient's cognitions and perceptions about their problem and about exercises were addressed. In addition to CTET the experimental group also received individual CBT-I (including general sleep education, sleep restriction therapy, stimulus control, sleep hygiene instructions, and cognitive therapy) within the same sessions. More details of both intervention groups can be found elsewhere.25 Both interventions were delivered by trained physical therapist with a master's degree and took place at the University Hospitals of Ghent and Brussels. Physical therapists were trained by experts in the field of chronic pain rehabilitation (JN, AM). Only the experimental therapists received CBT-I-training by a psychologist/behavioral somnologist with expertise on this matter (OM).
Participants were randomized by an independent researcher (AM) uninvolved in the treatment or assessment. Randomization lists were made available separately for both treatment centers and stratified for sex and dominant pain problems.
The participants, assessor, and statistician were blinded to the maximal extent for the study hypothesis and randomization. Therapists were not blinded (i.e., the experimental therapists knew they had to include CBT-I). However, to avoid therapist bias and contamination of the therapy arms, therapists involved in the experimental intervention were not involved in the control intervention, and vice versa.
Outcome measuresOnline questionnaires (in Dutch) were used to assess secondary self-reported outcomes, socio-demographic, and medical data. All outcome measures were assessed at baseline and immediately after treatment. Accelerometry was used to measure physical activity levels.
Primary outcomes measureThe primary outcome measure was time spent in MVPA for one week, because the beneficial effects of physical activity are mainly linked to MVPA and most guidelines use it as a criterion.20,35 Three-axis accelerometer activity monitors (GT9X Link, Actigraph Corporation, LLC, USA) were used to assess objective physical activity-related outcomes. Participants were asked to wear the activity monitors day and night at their non-dominant wrist, starting one week before the treatment (baseline) until one week after the treatment (post-treatment). The captured data were analyzed using ActiLife6 (Actigraph, Corporation, LLC, USA). The Freedson Adult (1988) algorithm was used to calculate the time in MVPA.36 All actigraph data were visually checked for collection errors such as non-wear or abnormalities in the registration.
Activity monitors are commonly used in research to provide objective measures of physical activity and their validity has been broadly investigated in adults in laboratory settings and in free-living conditions.37-41
Secondary outcomes measuresPain-related outcomes were assessed using the Brief Pain Inventory (BPI) and the Central sensitization inventory (CSI). The BPI is a reliable and valid questionnaire that allows to rate the intensity of the experienced pain and the impact of pain on functioning.42-44 The CSI is used to assess self-reported health symptoms indicative of central sensitization and has good psychometric properties.45-47
Sleep-related outcomes were evaluated using the Insomnia Severity Index (ISI), the Epworth Sleepiness Scale (ESS), the Brugmann Fatigue Scale (FBS), and the Pittsburgh Sleep Quality Index (PSQI). The ISI is a valid and reliable instrument to quantify perceived insomnia severity.48,49 The ESS is a reliable method to assess sleep propensity by questioning sleepiness in different situations.50,51 The BFS is a recently developed instrument for assessing fatigue and has the necessary psychometric characteristics to allow for a valid, reliable, linear, and unidimensional measurement of mental and physical rest propensity.52 The PSQI is a commonly used questionnaire to assess subjective sleep quality and has a high test-retest reliability and good validity.53,54
The hospital anxiety and depression scale (HADS) is a reliable tool with relatively high sensitivity and specificity for identifying and quantifying anxiety and depression.55
The Short Form Health Survey-36 (SF-36) is widely used to measure perceived health or health-related quality of life and has well-established psychometric properties.56
Data analysisStatistical analyses were performed with SPSS 26.0 (IBM, Armonk, NY, USA). Descriptive statistics were retrieved for all demographic characteristics and variables of interest at baseline. The normality assumptions were checked using histograms, Q-Q plots, and Kolmogorov-Smirnov tests. Correlations among all independent variables and variance inflation factors (VIF) for all variables included in the regression analysis were calculated to check for multicollinearity indicated by an absolute correlation coefficient ≥0.8 or a VIF ≥5.57 Outliers were determined as a difference of 2.2 times the interquartile range.58 Because previous studies in the general populations show there are differences in physical activity based on sex, age, and educational level, these variables were considered as control variables.59-63
Predictors of MVPA at baselineA linear multiple stepwise regression analysis, with forward selection of variables, was conducted to examine which factors are associated with MVPA at baseline. All control variables were entered first. Next, all demographics, baseline characteristics, and secondary outcomes presented in Table 2 were taken into consideration as a predictor. A stepwise variable selection procedure was used in which variables are sequentially entered into the model based on the absolute correlation coefficient with the primary outcomes. The next variable which was considered for entry was the independent variable with the largest partial correlation. To be entered, the variable had to pass the tolerance criterion (tolerance level: 0.0001). If a variable would cause the tolerance of another variable in the model to drop below the tolerance criterion, it was not entered in the model. Adjusted R² was used to determine how much of the total variance was explained by the regression model. After the best-fitting model with the inclusion of the control variables was constructed, the non-significant variables in this model (including control variables) were stepwise removed using backwards elimination to get better estimates for the explained variance by the predictors.
Demographics and baseline characteristics of patients with nCSP and comorbid insomnia (n=66).
| Demographic characteristics | n | Mean ± SD or n (%) | Range |
|---|---|---|---|
| Demographics | |||
| Sex,a female | 66 | 44 (66.7) | |
| Duration of pain, mo | 63 | 98.9 ± 93.8 | 3 – 444 |
| Age, y | 66 | 41.7 ± 11.3 | 21 – 62 |
| BMI, kg/m2 | 66 | 23.7 ± 3.4 | 16 – 30 |
| Level of educationa | 66 | ||
| - Higher secondary | 11 (16.7) | ||
| - Higher professional education | 2 (3.0) | ||
| - Professional bachelor | 27 (40.9) | ||
| - Academic bachelor | 5 (7.5) | ||
| - Master | 20 (30.3) | ||
| - Doctorate | 1 (1.5) | ||
| Baseline characteristics | |||
| BPI – Pain severity questions | 66 | 4.5 ± 1.5 | 1.8 – 7.0 |
| BPI – Pain interference questions | 66 | 3.1 ± 1.7 | 0.1 – 7.4 |
| CSI | 66 | 43.0 ± 11.0 | 19 – 70 |
| ISI | 66 | 14.5 ± 4.4 | 4 – 25 |
| PSQI | 66 | 9.4 ± 2.9 | 4 – 16 |
| BFS – Mental fatigue | 66 | 2.8 ± 2.3 | 0 – 10 |
| BFS – Physical fatigue | 66 | 3.2 ± 2.0 | 0 – 9 |
| ESS | 66 | 8.6 ± 4.3 | 0 – 19 |
| HADS – Anxiety | 66 | 8.3 ± 3.1 | 1 – 16 |
| HADS – Depression | 66 | 5.0 ± 2.8 | 0 – 14 |
| SF-36 Physical functioning | 66 | 69.2 ± 19.3 | 35 – 100 |
| SF-36 Role physical functioning | 66 | 52.3 ± 41.1 | 0 – 100 |
| SF-36 Role emotional functioning | 66 | 20.7 ± 36.0 | 0 – 100 |
| SF-36 Energy / fatigue | 66 | 51.4 ± 17.6 | 5 – 85 |
| SF-36 Emotional well-being | 66 | 65.6 ± 14.8 | 24 – 96 |
| SF-36 Social functioning | 66 | 74.2 ± 17.7 | 37.5 – 100 |
| SF-36 Pain | 66 | 55.6 ± 17.8 | 20 – 90 |
| SF-36 General health | 66 | 55.8 ± 16.7 | 15 – 95 |
| MVPA in hours/week | 59 | 18.7 ± 6.0 | 8.0 – 32.1 |
| Treatment allocationa control | 66 | 36 (54.5) | |
| Change primary outcome after treatment (14 weeks later) | |||
| MVPA difference in hours/week (pre-post difference) | 57 | -0.1 ± 5.5 | -17.5 – 16.6 |
BFS, Brugmann Fatigue Scale; BPI, Brief Pain Inventory; CSI, Central Sensitization Inventory; ESS, Epworth Sleepiness Scale; F, female; HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; M, male; mo, months; MVPA, moderate vigorous physical activity; PSQI, The Pittsburgh Sleep Quality Index; SF-36, 36-Item Short Form Survey; SD, Standard Deviation; y, years.
Linear mixed-effects modeling including a random intercept for each participant was used to investigate influencing factors (fixed effects) for MVPA and whether the factors might lead to a change of MVPA after treatment. This method accommodates missing data by creating estimates using all data available for each participant. All variables presented in Table 2 were considered as a potential influencing factor. To investigate whether a specific variable had an influence on the change of MVPA, interaction effects with time (baseline or post-treatment measurement) were added to the model. The base model only included the control variables. To adjust for the possible difference between the two treatment groups, treatment allocation was also added as control variable. Every time a factor or interaction was added to the model, the goodness of fit of the new model was compared to the previous model using the Akaike's Information Criterion (AIC).64,65 When the AIC of the new model was at least 10 units lower compared to previous model, the new model was considered a better fit for the data. Subsequently, the model was narrowed down to ensure better power by stepwise removal of variables which contributed little. The model with the removed variable was compared to the previous model using the AIC. The best-fitting model was considered the final model.
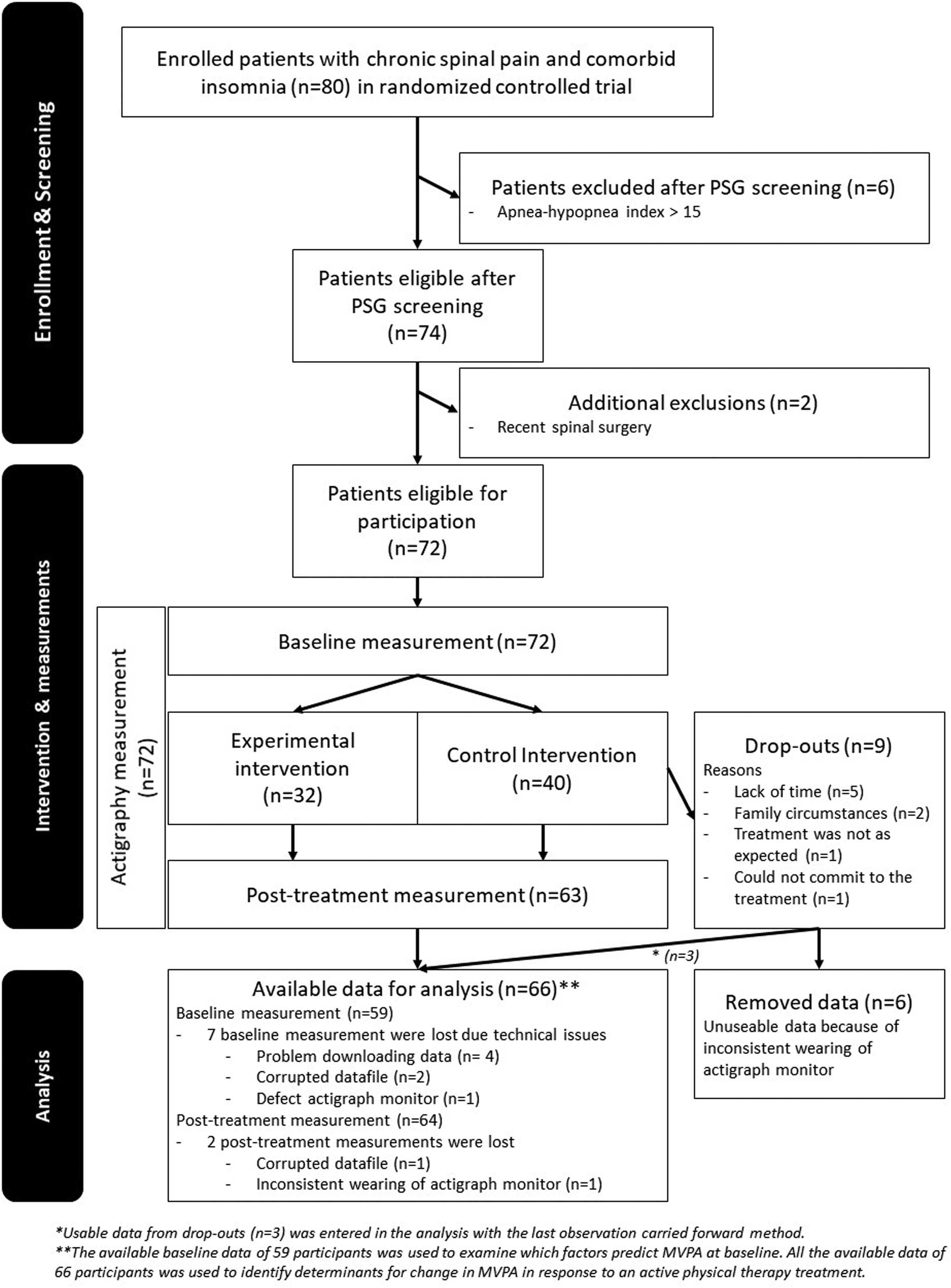
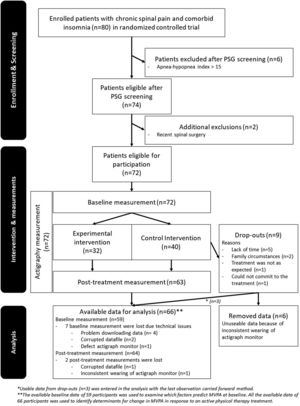
ResultsThe data were analyzed for 66 participants. A detailed overview with reasons for exclusion, drop-out, and missing data is presented in Fig. 1.
The mean ± standard deviation age of the participants was 41.7 ± 11.3 years (range: 21-62) and about 67% were female. No adverse treatment effects were reported. A mean of 18.7 hours MVPA per week was observed at baseline. The average change in MVPA of the total sample in response to the treatment was rather small and negligible (-0.1 ± 5.5 hours per week). However, the change of MVPA varied per individual (range: -17.5 to +16.6 hours per week). Vigorous and very vigorous activity were not performed at any time, except for two participants at post-treatment (respectively 20 and 111 minutes). No multicollinearity between independent variables was detected (range r: <.01-.64; range VIF: 1.0-1.4). All baselines values are presented in Table 2.
Predictors of baseline MVPAThe multivariate regression model explained 33.3% of the total variance in MVPA at baseline (Table 3). Physical and mental fatigue were both negatively associated with MVPA (b = -0.9; 95%CI: -1.59, -0.15, and b = -1.0; 95%CI: -1.67, -0.43). Body mass index (BMI) was also negatively related to the amount of MVPA (b = -0.5; 95%CI: -0.93, -0.16). Lower subjective sleep quality was related to greater MVPA (b = 0.7; 95%CI: 0.22, 1.17). Fewer limitations in functioning as a result of emotional problems was associated with greater MVPA (b = 0.1; 95%CI: 0.03, 0.10).
Prediction model of time in moderate-to-vigorous physical activity in patients with chronic spinal pain and comorbid insomnia.
| Dependent variable | Independent variable | n | b (95% CI) | Β | Adjusted R² | P | VIF | Unadjusted b (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Final model including control variables | ||||||||
| MVPA | Age# | 59 | -0.1 (-0.18, 0.08) | -0.1 | .414 | 1.3 | -0.1 (-0.23, 0.06) | |
| Sex#a | 59 | 1.7 (-1.18, 4.53) | 0.1 | .245 | 1.2 | 3.3 (0.11, 6.44) | ||
| Level of education# | 59 | 1.2 (-0.67, 2.98) | 0.14 | .210 | 1.0 | 2.0 (-0.14, 4.17) | ||
| BFS – Physical fatigue b | 59 | -0.9 (-1.65, -0.20) | -0.3 | .014 | 1.2 | -0.6 (-1.40, 0.21) | ||
| SF-36 Role emotional functioning c | 59 | 0.1 (0.02, 0.10) | 0.4 | .004 | 1.2 | 0.0 (0.00, 0.09) | ||
| BFS – Mental fatigue b | 59 | -1.0 (-1.63, -0.33) | -0.4 | .004 | 1.4 | -0.6 (-1.26, 0.05) | ||
| PSQI d | 59 | 0.7 (0.17, 1.13) | 0.3 | .009 | 1.3 | 0.3 (-0.20, 0.83) | ||
| BMI | 59 | -0.4 (-0.83, -0.00) | -0.2 | .050 | 1.3 | -0.4 (-0.84, 0.05) | ||
| (Constant) | 25.7 (14.62, 36.85) | <.001 | ||||||
| Predictive model for MVPA = 25.735 - 0.053 × (Age) + 1.675 × (Sex) + 1.154 × (LoE) - 0.925 × (Physical fatigue) + 0.057 × (SF-36 emo. Funct.) - 0.980 × (Mental fatigue) + 0.650 × (PSQI) - 0.417 × (BMI) | 59 | 0.35 | <.001 | |||||
| Final model after stepwise removal of the weakest, non-significant variables | ||||||||
| BFS – Physical fatigue b | 59 | -0.9 (-1.59, 0.15) | -0.3 | .019 | 1.2 | -0.6 (-1.40, 0.21) | ||
| SF-36 Role emotional functioning c | 59 | 0.1 (0.03, 0.10) | 0.4 | <.001 | 1.2 | 0.0 (0.00, 0.09) | ||
| BFS – Mental fatigue b | 59 | -1.0 (-1.67, -0.43) | -0.4 | .001 | 1.3 | -0.6 (-1.26, 0.05) | ||
| PSQI d | 59 | 0.7 (0.22, 1.17) | 0.4 | .005 | 1.3 | 0.3 (-0.20, 0.83) | ||
| BMI | 59 | -0.5 (-0.93, -0.16) | -0.3 | .006 | 1.0 | -0.4 (-0.84, 0.05) | ||
| (Constant) | 29.4 (20.04, 38.80) | <.001 | ||||||
| Predictive model for MVPA = 29.423 - 0.867 × (Physical fatigue) + 0.065 × (SF-36 emo. Funct.) - 1.045 × (Mental fatigue) + 0.697 × (PSQI) -0.546 x (BMI) | 59 | 0.33 | <.001 | |||||
Linear regression modeling including dependent variable, variables to control for, independent variables, number of participants, unstandardized coefficient B values for each variable and 95% confidence interval, standardized coefficient B values, individual p values, model p value, model adjusted R², variance inflation factor, the equation of the final model, and unadjusted coefficient B values for each variable with corresponding 95% confidence interval and p-values.
b, unstandardized coefficient; β, standardized coefficient Beta; BMI, body mass index; BFS, Brugmann Fatigue Scale; CI, confidence interval; MVPA, Moderate-to-vigorous physical activity; SF-36, 36-Item Short Form Survey; VIF, variance inflation factor.
The final model included the variable “pain duration” and the interaction “education × time” (Table 4). A main effect for sex was found (estimate [SE]: -4.5 [2.0], p=.03). No other significant main and/or interaction effects improved the prediction model. Three post-treatment data points and two pre-post differences in MVPA were identified as outliers. No collection errors from the actigraph in the participants with data outliers were identified. Consequently, all outliers were considered a realistic part of the data set and maintained in all analyses.
Best-fitting linear mixed models of time in moderate-to-vigorous physical activity in patients with chronic spinal pain and comorbid insomnia, constructed with the aim to explore determinants for a change in MVPA in response to physical therapy treatment.
| MVPA models | Main effects | Model with interactions | ||
|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | |
| Final model including control variables | ||||
| Sex (Male) | -4.6 (2.1) | .03* | -4.5 (2.1) | .03* |
| Age | -0.0 (0.1) | .71 | -0.0 (0.1) | .69 |
| Education | .62 | .20 | ||
| Lower | -1.7 (2.7) | -7.4 (4.2) | ||
| Bachelor | 0.9 (2.1) | -4.2 (3.3) | ||
| Master or higher | (reference) | (reference) | ||
| Allocation | -1.1 (1.9) | .55 | -1.1 (1.9) | .56 |
| Time | 0.1 (0.8) | .87 | -2.2 (1.3) | .85/.10 |
| Pain duration | 0.9 (1.0) | .33 | 1.0 (1.0) | .30 |
| Education × time | .10 | |||
| Lower | 3.7 (2.2) | |||
| Bachelor | 3.3 (1.7) | |||
| Master or higher | (reference) | |||
| Final model after removal of the worst fitting variables | ||||
| Sex (Male) | -4.5 (2.0) | .03* | -4.5 (2.0) | .03* |
| Education | .54 | .17 | ||
| Lower | -2.0 (2.6) | -7.6 (4.2) | ||
| Bachelor | 0.8 (2.1) | -4.3 (3.3) | ||
| Master or higher | (reference) | (reference) | ||
| Time | 0.1 (0.8) | .86 | -2.2 (1.3) | .85/.10 |
| Pain duration | 0.8 (0.9) | .37 | 0.8 (0.9) | .35 |
| Education × time | .10 | |||
| Lower | 3.7 (2.2) | |||
| Bachelor | 3.3 (1.7) | |||
| Master or higher | (reference) | |||
Estimates are unstandardized. MVPA, moderate to vigorous physical activity; SE, standard error.
This study aimed to explore predictors of baseline MVPA and determinants for a change in MVPA in response to a multimodal active physical therapy intervention in people with nCSP and comorbid insomnia. The results of the regression model suggest that 33.3% of the variance of MVPA at baseline can be explained by physical fatigue, mental fatigue, less limitations in functioning as a result of any emotional problems, lower perceived general sleep quality, and BMI.
A mean of 18.7 hours MVPA per week was observed. This seems high and might be explained by the use of wrist-worn actigraphy which might have led to elevated accelerometry scores. Despite having good accuracy, wrist placements seem to be less accurate than hip placements and can yield higher outputs.37,40 However, Montoye et al.66 found that left wrist-worn accelerometers (90% non-dominant) had higher sensitivity and specificity then the hip- and right wrist-worn accelerometers.66 Data from National Health and Nutrition Examination Survey (NHANES) studies using waist-worn accelerometers of US adults found that only 77.8% attained sufficient physical activity based on unbouted MVPA (using lifestyle activity cut points) to meet the recommended 150 minutes of MVPA per week.63 However, the higher obesity rate and the lower actigraph wear time in the NHANES studies are likely to affect the results.63,67-69 More recent NHANES studies also shifted to wrist-worn accelerometry because of better compliance.63,70 Still, time in MVPA is exceeding the recommendations by a large amount which possibly could be explained by lite activity registered as MVPA and the lack of specific validation studies.
Consistent with the existing evidence, lower levels of MVPA were found with higher levels of physical and/or mental fatigue.71,72 In people with chronic low back pain, fatigue seems to have several negative effects including more pain, depressive symptoms, and increased disability.73 Available evidence indicates that fatigue leads to less motivation for physically active behavior, can act as a barrier for physical activity, and has a negatively accelerating dose-response relationship with physical activity (i.e., increasing physical activity reduces the risk of experiencing fatigue).24,71-76 This suggests that fatigue and the relationship with physical activity might lead to a vicious cycle.
Both acute and regular physical activity have beneficial effects on several aspects of sleep quality and better sleep also leads to more involvement in physical activity.77-79 However, our results show that lower perceived sleep quality is associated with greater baseline MVPA. This might potentially be explained by overactivity (i.e., high-intensity activity and fluctuations in activity) which is associated with poor sleep,80 or by participants spending less time in bed leading to more active hours.
Symptoms of depression, anxiety, and emotional distress are common in people with chronic pain, can influence pain, and contribute to long-term outcomes.81-85 We found that “Role limitations due to emotional problems” was a significant predictor, indicating that greater MVPA at baseline was associated with experiencing fewer limitations in functioning as a result of emotional problems.
Last, higher BMI was associated with less MVPA at baseline. It should be noted that people with a high BMI (>30 kg/m2) were excluded. Nevertheless, this finding is consistent with previous research showing lower activity levels in people with higher BMI, a higher risk for obesity as a result of physical deactivation and deconditioning, weight problems acting as a barrier to participate in physical activity, a negative impact of both pain and being overweight on each other, and less effective treatment outcomes in patients with low back pain who are obese.19,69,86-90 Based on the available evidence, it seems warranted to address weight control together with other lifestyle factors as a part of the pain rehabilitation.19,91
The unadjusted regression and final mixed model showed a significant main effect for sex indicating lower MVPA in men. There was no sex-dependent change of MVPA after treatment. Despite not being significant, “education × time” was a part of the model. No other interaction effect with time was identified.
LimitationsTo our knowledge, this is the first study to investigate predictors of (baseline) MVPA and determinants for a change in MVPA after a treatment in people with nCSP and comorbid insomnia. The data were derived from a randomized trial using valid and reliable research questionnaires. However, this study has some limitations. First, only short-term changes after the treatment were investigated. Second, the findings might not be generalizable to other chronic pain populations. However, nCSP is one of the most common chronic pain condition and similarities were found in other populations. Third, the included variables are limited to the measured outcomes in the original trial. Possibly several influencing factors were not included (e.g., pain catastrophizing, social factors, and environmental factors). Fourth, there might be a potential lack of power given that the sample size calculation for the linear regression model was based on four predictors with a medium effect size. Possibly some weaker predictors could not be identified. Nevertheless, we were able to identify five predictors. No determinants for a change in MVPA after treatment could be identified. No a priori power analysis was conducted for the corresponding linear mixed model analysis because of the explorative nature of the analysis (i.e., included variables and interactions in the final model where unknown at study initiation). Fifth, there is lack of information about the validity of wrist-worn accelerometers to assess physical activity (in free-living conditions) in people with nCSP with comorbid insomnia. One study in older adults investigated the validity of wrist-worn actigraphy and found that different physical activity intensity levels can be identified accurately.38 However, they propose to use different cut-off points for identifying intensity.38 Similarly, validation studies identifying optimal cut-off values in specific populations with chronic conditions are warranted. Last, while the English equivalents of the questionnaires used in this study are validated, the psychometric properties of some of the Dutch versions are not yet investigated. Still, the psychometric properties of the Dutch version of the SF-36 and CSI are well-established.46,92 As most questionnaires are developed for and validated in English-speaking populations, the questionnaires should be cross-culturally adapted and validated before being used in clinical studies.
Nevertheless, this study can serve as a basis for future studies investigating whether targeting one of the associated variables with MVPA as part of a treatment has an impact on the physical behavior and short- and long-term disability. Furthermore, current findings can be useful to identify people early who are more likely to be inactive and who might experience or develop negative consequences related to inactivity or overactivity. Early identification makes it possible to address undesirable physical behavior early.
ConclusionMVPA levels vary in people with nCSP and comorbid insomnia. People reporting lower sleep quality, lower BMI, less limitations in functioning resulting from emotional problems, as well as less physical and mental fatigue are more likely to engage in MVPA. No determinants for change in MVPA in response to an active physical therapy treatment were identified.
The authors would like to thank the therapists who contributed in conducting the trial: Elise Cnockaert, Louis Desmet, and Andreas Delaere. Furthermore, we thank all participants who took part in the study.
This study was funded by the Applied Biomedical Research Program (TBM) of the Agency for Innovation by Science and Technology (IWT) and the Research Foundation Flanders (FWO), Belgium (Grant project nr T001117N). Kelly Ickmans and Anneleen Malfliet are both funded by the Research Foundation Flanders (FWO), Belgium. Eveline Van Looveren and Thomas Bilterys are both funded by the Applied Biomedical Research Program (TBM) of the Agency for Innovation by Science and Technology (IWT) and the Research Foundation Flanders (FWO), Belgium. The funder played no role in the design, conduct, or reporting of this study.