Mobility is an important component of functioning. Motor and cognitive impairment in older people with Alzheimer's disease can exert a negative impact on life-space mobility.
ObjectiveTo compare life-space mobility in older adults with mild and moderate Alzheimer-type dementia and those without dementia and determine associations with health factors.
MethodsLife-space mobility was assessed using the Life Space Assessment (LSA) in 33 older adults with Alzheimer-type dementia (AD group) and 24 older adults without dementia (WD group). The World Health Organization Disability Assessment Schedule (WHODAS 2.0), Addenbrooke's Cognitive Examination (ACE-R), Geriatric Depression Scale (GDS), Modified Baecke Questionnaire for Older Adults (MBQOA), and Short Physical Performance Battery (SPPB) were completed. Statistical analysis was performed with unpaired t-test or Mann-Whitney tests for comparisons between groups and Spearman's correlation test.
ResultsThe AD group had a lower total LSA score compared to the WD group (44 vs 65, mean difference = −20.7 [95% CI: −28.6, −12.9]), 21% of the AD group were restricted to their homes when no assistance was available. In both groups, moderate correlations were found between LSA and both functioning and physical activity level. Symptoms of depression presented moderate correlation only in the WD group.
ConclusionsOlder adults with AD have lower life-space mobility and require assistance to achieve higher levels of mobility. Clinical implications: LSA can help assess life-space mobility. Encouraging and enabling assistance is fundamental to a greater life-space for older adults with dementia
Mobility is the capacity for movement and locomotion, which is an important component of functioning.1–3 The World Health Organization recognizes a broad description of mobility, which includes places inside and outside the home, as well as using assistive devices and transportation.4,5 Assessment tests to evaluate mobility in older adults can be classified in: (i) performance-based measurement, in which participants accomplish the test and a ratio score is generated, (ii) judgment-based measurement, in which observers score the test based on their examination, and (iii) self-report measurement, based on a questionnaire answered by the participants. One measure is not better or interchangeable with another, but can be complementary.6
Life-space assessment (LSA) is used to assess life-space mobility, which includes places older adults visit, the frequency with which they go to these places and whether they need help of another person or an assistance device.7 The LSA has been used in several studies. It is considered a predictor of the risk of mortality in older adults,8 and also of cognitive decline9 and the development of Alzheimer disease (AD).10
Many studies have examined the predictive potential of life-space mobility for physical and cognitive health.11 However little is known about the life-space mobility of older adults living with AD. Tung et al.12 assessed life-space mobility in older adults with AD for 3 days using a global positioning system (GPS), and found that the GPS-derived area, perimeter, and average distance from home were smaller than the control group. The results are important for understanding life-space mobility in this population, but the authors did not report important information for clinical practice, such as assessing mobility over a longer period of time (4 weeks), mobility within the home (moving between rooms), and especially the need and level of assistance (personal or equipment) for moving. This information could be provided by the LSA.
Thus, this study aimed to compare life-space mobility between older adults with AD and those without dementia (WD) and to determine whether LSA is associated with other health measures, such as functioning, cognitive function, physical performance, physical activity level, and symptoms of depression.
MethodsParticipants and eligibilityThe present cross-sectional observational study received approval from the Institutional Review Board of the Universidade Federal de São Carlos (UFSCar) (process number: 88,921,118.4.0000.5504; certificate number: 3.557.596). All participants in the WD group and the guardians of those in the AD group signed statements of informed consent. The participants were recruited through pamphlets, social media, and local television and radio programs.
Participants were considered eligible for the study if they were 65 years of age or older, lived in the community in the city of São Carlos - Brazil, were able to walk 10 m, did not have severe uncorrected visual or hearing impairments, and were available to participate in the evaluations. Participants were included in the AD group if they were diagnosed with mild to moderate AD by a neurologist using the criteria of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).13 The Clinical Dementia Rating (CDR)14,15 was considered for the classification of the stage of the disease. Only individuals with CDR 1 or 2 (mild and moderate) were included in the AD group. The inclusion criterion for the WD group was that participants had to score above the cutoff point adjusted for schooling in the Mini Mental State Examination (MMSE).16
The sample size of at least 52 participants (i.e., 26 participants per group) was estimated to detect an effect size of 0.8 or a minimum significant change of five points in the LSA total,17 using a t-test with a 5% significance level and 80%power. The G*Power 3.1.9.4 statistical program was used in the sample size calculation.
Procedures and instrumentsThe clinical assessment was performed on a single day in the Research Laboratory of Older Adults’ Health. Data collected included the following constructs: demographic and anthropometric characteristics, LSA, functioning, cognitive function, physical performance, physical activity level, and symptoms of depression. All instruments used in this study were translated and validated for the Brazilian population. The tests were administered in the same order and by a single physical therapist, who had five years’ experience in using the instruments. The examiner was not blinded to the allocation of the groups.
The AD group answered the questions related to cognitive function and symptoms of depression directly to the evaluator. Questionnaires involving memory recalls such as life-space mobility, functionality, and level of physical activity were answered by the caregivers of the older adults. The caregiver was considered the person who spent at least half a day with the older adult at least four times weekly.
Demographic and anthropometric characteristicsTo characterize the sample, the following data were collected: age (years), sex, weight (kg), height (cm), body mass index (BMI: kg/m²), schooling (years of study), number of medications taken, and self-reported falls in the previous six months.
Life-space mobilityLife-space mobility was assessed by the LSA. The LSA considers the following aspects of mobility: level, frequency in the last month, and degree of independence.1,2,7 The level is defined by the place where the person moves and includes five items (1 = home; 2 = outside home; 3 = neighborhood; 4 = town; and 5 =unlimited). Frequency is scored on each level: “less than once per week” = 1 point; “one to three times were week” = 2 points; “four to six times per week” = 3 points; “daily” = 4 points. The degree of dependence is also investigated and scored: “personal assistance” = 1 point; “only equipment” = 1.5 points; “no equipment or personal assistance” = 2 points. Equipment may be a gait-assistance device or furniture used for support, such as a chair. Personal assistance refers to a person (caregiver or family member) who assists the older person (e.g., getting up from bed, driving them to another neighborhood or city).
The composite LSA was calculated by the product of level, frequency, and degree of independence, the score range varies from 0 to 120. The maximum LSA is defined as the largest life-space reached. The independent LSA is the largest living space reached without the help of any equipment or person. The independent and maximum LSA only considers the level, and therefore the scoring range is between 1 and 5.
The translated and validated version of the LSA for the Brazilian population has adequate internal consistency (Cronbach alpha = 0.92) and reliability (Intraclass Correlation Coefficient[ICC]= 0.97; 95% CI: 0.95, 0.98; standard error of measurement: 4.12 points [3%]).2 The LSA is also validated to apply to a proxy.18
FunctioningThe 12-item World Health Organization Disability Assessment Schedule (WHODAS 2.0) was used to assess functioning. This WHODAS 2.0 measures the level of functioning in six domains: 1) cognition, 2) mobility, 3) self-care, 4) interpersonal relations, 5) life activities, and 6) participation in community activities and society.4 The total score is calculated by summing the score of each domain and ranges from 0 to 48, with higher scores denoting greater limitation and disability.19 The WHODAS 2.0 shows good internal consistency (Cronbach alpha= 0.86) and reproducibility (ICC= 0.77; 95% CI: 0.69, 0.83).20
Cognitive functionThe Addenbrooke's Cognitive Examination (ACE-R)21 was used to assess cognitive function. The ACE-R score is distributed across five domains: orientation and attention, memory, verbal fluency, language, and visuospatial skills. The total score ranges from 0 to 100 points, with higher scores denoting better cognitive performance.22 The reliability measured with Cronbach alpha coefficient has been reported to be 0.80.21
Physical performanceThe Short Physical Performance Battery (SPPB) consists of an assessment of balance, gait speed, and sit-to-stand. Each activity is scored from 0 to 4 points and the total score is calculated by adding the three activities.23 The SPPB has acceptable values of internal consistency (Cronbach alpha = 0.725) and inter-(ICC = 0.996) and intra-observer (ICC = 0.876) reliability.24
Physical activity levelThe Modified Baecke Questionnaire for Older Adults (MBQOA) was used to assess the physical activity level. This questionnaire measures household activities, sports, and leisure time activities performed in the previous year.25,26 The MBQOA showed excellent reproducibility (ICC= 0.76).27
Depressive symptomsThe 15-item Geriatric Depression Scale (GDS-15) was used to measure symptoms of depression. The GDS-15 has good reliability (kappa = 0.6).2829
Analysis of resultsStatistical analysis was performed using the Statistical Package for the Social Sciences (IBM SPSS statistics, version 20.0). The Shapiro-Wilk and Levene tests were used to assess normality and equality of variance, respectively. The unpaired t-test was used for variables with parametric distribution and the Mann-Whitney test was used for nonparametric variables. The data are reported as mean ± standard deviation, median [min-max], frequency (proportion), mean difference (95% confidence interval [CI]), median difference [95% CI]. The Chi-squared test was used for frequency measures. Correlations between LSA and health measures were analyzed using Spearman's correlation test, the results of which were interpreted as follows: r = 0.00 to 0.10 – insignificant correlation; r = 0.10 to 0.39 – weak correlation; r = 0.40 to 0.69 – moderate correlation; r = 0.70 to 0.89 – strong correlation; r = 0.90 to 1.00 – very strong correlation.30 The significance level was set at 5% (p < 0.05).
ResultsCharacteristics of participantsA total of 171 older adults were assessed for eligibility from March to May 2019. Forty-one individuals were not eligible for the WD group due to mild neurocognitive disorder or neurological/orthopedic disorders that affected cognition or mobility. Seventy-three individuals were not eligible for the AD group because they were institutionalized, they were in the severe stage of Alzheimer's-type dementia (CDR = 3) or had sequelae caused by a stroke. Thus, the final sample comprised 57 participants: 33 in the AD group and 24 in the WD group. The AD group consisted of 64% of older adults classified in CDR 1 and 36% in CDR 2. There was no difference in sex, marital status, and income between the groups.
Table 1 shows the characteristics of participants. We found significant differences between groups for the number of medications in use, for the total scores on the WHODAS, MBQOA, SPPB, and ACE-R, and for percentage of fallers in the previous 6 months.
Demographic characteristics of participants.
| Variables | WD (n = 24) | AD (n = 33) | Median difference [95% CI] or Mean difference (95% CI) |
|---|---|---|---|
| Age (years) | 75.5 ± 6.1 | 77.6 ± 4.9 | 2.1 (−0.8, 5.0) |
| Female | 15 (62,5%) | 22 (66,7%) | – |
| Weight (kg) | 70.9 ± 11.6 | 66.0 ± 12.2 | −4.96 (−11.4, 1.5) |
| Height (cm) | 158.3 ± 8.8 | 157.9 ± 9.7 | −0.4 (−0.5, 4.6) |
| BMI (kg/m2) | 27.5 [20.8 - 42.0] | 26.18 [19.2- 42.5] | 1.5 [−0.2, 4.2] |
| Schooling (years of study) | 4 [2 - 17] | 4 [0 - 16] | 0 [−1, 1] |
| Marital status | |||
| Single | 5 (21%) | 0 (0%) | – |
| Married | 14 (58%) | 23 (70%) | – |
| Divorced | 1 (4%) | 2 (6%) | – |
| Widowed | 4 (17%) | 8 (24%) | – |
| Income | |||
| 1 to 2 x BMMW | 9 (38%) | 13 (39.4%) | – |
| 3 to 5 x BMMW | 8 (33%) | 14 (42.4%) | – |
| ≥ 6 x BMMW | 0 (0%) | 3 (9.1%) | – |
| Did not know/did not report | 7 (29%) | 3 (9.1%) | – |
| Fallers in previous 6 months | 2 (8.3%) | 28 (84.8%) | – |
| Number of medications | 2 [0 - 7] | 5 [1 - 14] | −3 [−4, −2]* |
| GDS-15 (0-15) | 2 [0 −7] | 2 [0 - 9] | −1 [−2, 0] |
| WHODAS (0-48) | 13 [12 - 21] | 24 [13 - 46] | −11 [−7, 14]* |
| MBQOA | 9.9 [2.1- 30.6] | 3.7 [0.2 - 14.0] | 4.8 [2.2, 7.6]* |
| ACE-R (0-100) | 84 [73 - 96] | 52 [22 - 80] | 34 [27, 40]* |
| SPPB(0-12) | 8 [3 - 11] | 7 [3 - 9] | 1 [0, 3]* |
| CDR, n (%) | |||
| Without dementia | 24 (100%) | 0 (0%) | – |
| Mild dementia | – | 21 (64%) | – |
| Moderate dementia | – | 12 (36%) | – |
Data expressed as mean ± standard deviation, median [min - max], frequency (proportion), mean difference (95% confidence interval), median difference [95% confidence interval]. WD, without dementia; BMI, body mass index; BMMW, Brazilian monthly minimum wage; GDS, Geriatric Depression Scale; WHODAS, World Health Organization Disability Assessment Schedule; MBQOA, Modified Baecke Questionnaire for Older Adults; ACE-R, Addenbrooke's Cognitive Examination - revised; SPPB, Short Physical Performance Battery. High school equals 12 years of study.
Table 2 shows LSA in the WD and AD groups. Significant differences were found for both independent and maximum LSA. The median maximum LSA was higher in the WD group compared to the AD group [median difference = 1, 95%CI: 0, 1]. Regarding the independent LSA, the WD group showed also a higher median score compared to the AD group [median difference = 2, 95%CI: 2, 2]. The mean score on the composite LSA was 65.2 ± 13.04 in the WD group and 44.5 ± 15.71 in the AD group, with a significant difference between groups (mean difference = −20.7, 95%CI: −28.6, −12.9). Regarding the life-space level, no significant differences between groups were found for levels 1, 2, and 3. In contrast, the WD group had higher median scores for level 4 (town) [median difference = 12, 95%CI: 8, 16], and level 5 (unlimited) compared to the AD group [median difference = 5, 95%CI: 5, 10].
Median scores on each level of LSA in WD group and AD group.
| Life Space | WD group | AD group | Median difference [95% CI] or Mean difference (95% CI) |
|---|---|---|---|
| Composite LSA (0–120) | 65.3 ± 13.0 | 44.5 ± 15.7 | −20.7 (−28.6, −12.9)* |
| Maximum LSA (0–5) | 5 [4 – 5] | 4 [4 – 5] | 1 [0, 1]* |
| Independent LSA (0–5) | 5 [4 – 5] | 3 [0 −4] | 2 [2, 2]* |
| Level 1 (0–8): home | 8 [8 – 8] | 8 [6 – 8] | 0 [0, 0] |
| Level 2 (0–16): outside home | 16 [8 – 16] | 16 [6 −16] | 0 [0, 0] |
| Level 3 (0–24): neighborhood | 12 [0 −24] | 9 [0 – 24] | 6 [0, 12] |
| Level 4 (0–32): town | 16 [8 −32] | 8 [0 −32] | 12 [8, 16]* |
| Level 5 (0–40): unlimited | 10 [0 – 10] | 0 [0 – 5] | 5 [5, 10]* |
Data expressed mean ± standard deviation, median [min -max], mean difference (95% confidence interval), median difference[95% confidence interval]. LSA Life-Space assessment.
Note: each mobility level has a score range, which varies from zero when the individual does not have mobility at this level to a maximum score that corresponds to the product of the mobility level attained, the frequency of attainment and the degree of independence. For example at level 1, if the individual leaves the room where he/she sleeps (1 point) with a daily frequency (4 points) and without assistance (2 points), the maximum score is reached, which is 8 points (1 × 4 × 2).
Correlations between LSA and other health measures.
95%CI, 95% confidence interval; WD, Without Dementia; ACE-R, Addenbrooke's Cognitive Examination – revised version; GDS, Geriatric Depression Scale; WHODAS, World Health Organization Disability Assessment Schedule; SPPB, Short Physical Performance Battery; MBQOA, Modified Baecke Questionnaire for Older Adults.
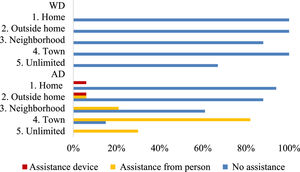
Fig. 1 shows the levels and degree of assistance participants reported.
Correlations between mobility and other health measuresA moderate positive correlation between the composite LSA and physical activity level (MBQOA), and a moderate negative correlation between the composite LSA and functioning (WHODAS) were found in both groups. A moderate negative correlation was also found between the composite LSA and symptoms of depression (GDS) only in the WD group.
DiscussionThe hypothesis related to decreased life-space in the AD group was confirmed. Older adults with AD had significantly lower maximum and independent LSA than older adults without dementia. In addition, the AD group had a lower score in the composite LSA than the WD group and required assistance to achieve higher levels of mobility. The most affected levels of life-space were those related to mobility outside the neighborhood and outside the city (levels 4 and 5). Additionally, moderate correlations were found between the composite LSA with functionality and physical activity level in both groups, and symptoms of depression only in the WD group.
The composite LSA score of life-space for community-dwelling older adults varied between 64 to 93.31,32 According to Baker et al.,7 values equal to or greater than 60 indicate an unrestricted life space. Therefore, the WD group had a normal life-space that could also be observed by the values of maximum LSA, who reached areas within and outside the town (levels 4 and 5).33 In the present study, 67% had mobility outside the town, all of them independently. Kuspinar et al.34 reported similar findings, as 77.8% of the older adults visited places out of town, 95% of whom were able to do so without any assistance.
The values of the composite LSA total and also the maximum LSA score in the AD group was lower than the WD group, indicating greater restriction in the living space of older adults with AD. Similar results are reported by Tung et al.12 who evaluated life-space mobility using a GPS tracker and demonstrated a smaller area, perimeter, and mean distance from older adults’ homes with mild-to-moderate AD compared to cognitively intact older adults. A likely reason for this finding is that individuals with AD may not be considered safe to go out on their own and require someone with them, so that the maximum LSA is dependent on the availability of a caregiver, or the caregiver's encouraging such displacements. Ullrich et al.35 reported maximum LSA mean value of 3.7 ± 1.2, which was slightly lower than our results, but the sample consisted of older adults with cognitive impairment who had recently been discharged from hospital. Thus, the physical impairment resulting from hospitalization may have overlapped with cognitive deficits and contributed to reduced values of maximum LSA.
Moreover, the WD group did not need any type of assistance to reach maximum LSA, meaning that their maximum LSA and independent LSA were equivalent. The same did not occur in the AD group, indicating the importance of providing assistance to older adults with AD so that they may achieve greater life spaces. In the present study, the independent LSA of the AD group was restricted to the neighborhood. Ullrich et al.35 reported that 50% of the older adults with cognitive impairment were able to increase their life spaces by at least two levels when receiving assistance from another person. When not receiving assistance, 21% were restricted to their homes (levels 1 and 2), which was associated with a reduction in opportunities for social participation and community activities.36
In the present study, 82% of the individuals in the AD group required the assistance of another person to achieve mobility in the town and less than 50% achieved mobility outside the town. Caregivers play a critical role in ensuring mobility and independence by providing assistance whenever it is required. Nonetheless, caregivers expressed a fear that the person they cared for might fall and a need for constant vigilance.37 These factors can limit travel to more distant areas such as out of town. In a recent study, caregiver life-space was associated with that of older adults with dementia, indicating that the life-space of caregivers is influenced by the mobility of the older adults with AD and the life-space of older people with AD is affected by the mobility behavior of their caregivers.38 Thus, encouraging caregivers to support mobility inside and outside the home seems to be an intervention that should be explored.
Another important topic is the reduction in barriers and the diversity of amenities in the neighborhood. These factors may have a direct effect on mobility and may slow down mobility decline.39,40 Bergefurt et al.41 suggested that public policies should focus on creating transitable, accessible neighborhoods with green spaces and adequate public transportation to enable the socialization of the residents, promoting the practice of physical activity, such as walking and cycling.41
We evaluated associations between the composite LSA and other health measures. Studies indicated that the SPPB is a determinant of LSA in older populations.42,43,1 However, this variable was not correlated with LSA in either of the groups we evaluated. The divergence may be attributed to the fact that the participants in the AD group had a diagnosis of AD rather than mild cognitive impairment and had not been institutionalized or hospitalized recently. A study44 reported that restricted life-space was more related to the fear of falls, slow cognitive processing speed, and limitations regarding instrumental activities of daily living (IADL) than sex or physical performance. It is important to note that the SPPB and LSA measure different constructs. Although physical functioning is a determinant for mobility, it does not necessarily determine social participation and life-space. For example, if the city does not provide adequate public transportation and security, this can be a barrier to achieving higher levels of life-space, even in the presence of good physical performance of the lower limbs.
A significant moderate correlation was found between LSA and functioning in both groups. According to Barnes et al.,45 LSA is a measure that reflects both the functional and psychological aspects of mobility, enabling an assessment of broader dimensions of social integration and participation in the community. Thus, the measure not only captures the actual spatial extent of the movement, but an interest in moving around and being involved in the wider social environment.
Physical activity level was another variable that exhibited a moderate correlation with LSA in both groups. Previous studies involving community-dwelling older adults with cognitive impairment reported similar findings.35,46 It seems that a greater physical activity level is accompanied by a greater spatial extension of mobility. Both physical activity level and life-space are aspects of motor behavior. Physical activity level concerns activity per se, regardless of the location, and life-space concerns location and spatial extension irrespective of whether locomotion is performed actively or passively.35 However, a limitation is that the caregiver provided the information about physical activity in the AD group. This could cause a bias in results, but there are no validated questionnaires to assess the level of physical activity in older adults with dementia. Farina et al.47 highlighted that 55.6% of the studies that sought to assess physical activity in this population also used interviews with caregivers, but we need more research to validate the use of proxy-report measures of physical activity in people with dementia.
Global cognitive status evaluation was not associated with LSA in either group. Previous studies presented conflicting results.48,35,34 Ullrich et al.35 found a low correlation between the MMSE and LSA in older adults with cognitive impairment. Kuspinar et al.34 found that cognitive function in community-dwelling older adults was not a strong predictor of LSA, stating that global cognition does not appear to affect life-space, but perhaps sub-domain analyses, such as attention and processing speed would demonstrate different results. Uemura et al.44 found that cognitive processing speed in older adults with mild cognitive impairment is important to explain the variability in life-space. Another issue is that in the presence of AD, whether mild or moderate, the cognitive decline affects the performance of IADL. For safety reasons, family members tend to restrict activities such as shopping, handling money, and driving.49 Consequently, older adults with AD will not go alone to the bakery or pharmacy close to their home, nor will they go further away. Therefore, the size of the life-space could be more related to the issue of dependence on performing activities of daily living than to a score on a scale that assesses global cognitive function.
A lower LSA has been associated with a greater likelihood of symptoms of depression; this association is mediated by locomotion difficulties, chronic conditions, and a lower sense of autonomy regarding participation outside the home.50 In our study, an association between LSA and depressive symptoms was only found in the WD group. Ambiguous results are found in older adults without cognitive impairment.7,33,51,1,50,43 In older adults with cognitive impairment, Ullrich et al.42 also found no correlation between LSA and symptoms of depression. Tung et al.12 reported that spatial movement behavior is correlated more strongly with apathy than depression.
Some limitations should be noted. The present findings cannot be extrapolated to individuals with severe AD but provide important information about AD in mild and moderate stages. The sample was non-probabilistic (convenience), but the participants were recruited through pamphlets, social media, and local television and radio programs. Thus, the individuals who were interested and available to participate in the evaluations were assessed.
LSA can help in assessing the life-space mobility as it is easy to use. Furthermore, rehabilitation programs should consider expanding the life-space of older adults with AD in the future as this is related to social participation and can bring benefits to functionality. Further studies could evaluate the effect of traditional rehabilitation programs that includes the enlargement of the life-space, encouraging correct use of assistance devices, support for caregivers, motivation of participants and performance of exercises in the neighborhood.
ConclusionsThe results show that life-space mobility was substantially lower in older adults with mild to moderate AD compared to a control group. Personal assistance played a key role in enabling the older adults with AD to achieve higher life-space levels. Encouraging and enabling assistance is fundamental to a greater life-space for older adults with dementia.
This work was supported by the following Brazilian research agencies: Coordenaçãode Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) –Finance Code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Instagram account: lapesi_ufscar