Dry needling is frequently used for the treatment of neck pain but knowledge about its neurophysiological central effects is scarce.
ObjectivesTo compare the immediate effects of a single session of dry needling (DN) and sham needling (SN) on local and distant pressure pain thresholds and conditioned pain modulation in patients with chronic idiopathic neck pain.
MethodParticipants with chronic idiopathic neck pain were randomly allocated to a DN or SN group. The primary outcome measure was the pressure pain threshold (PPT) at one peripheral location: quadriceps muscle (Q). Secondary outcome measures were local PPTs at the treated (most painful) (tUT) and non-treated upper trapezius muscle (ntUT), absolute and relative conditioned pain modulation (CPM) effects and pain during hot water immersion. Patients were assessed at baseline and immediately post intervention. Linear mixed models were used to examine interaction effects as well as between- and within-group differences.
ResultsFifty-four participants were included for statistical analysis. Linear mixed model analyses showed no significant “group X time” interaction effects for any of the outcome measures. The relative CPM effect at the Q was significantly higher post-intervention, compared to baseline within the DN group (mean difference= 13.52%; 95% CI: 0.46, 26.59).
ConclusionThe present study shows no superior effect of DN, compared to SN, in the immediate effect on local and distant PPTs and CPM in patients with chronic idiopathic neck pain.
Over the last few years, the number of studies suggesting myofascial pain syndrome (MPS) as one of the possible underlying causes of chronic idiopathic neck pain (CINP) has increased.1–4 CINP can be associated with (referred) muscle pain caused by active or latent myofascial trigger points (MTrPs).5 The prolonged presence of MTrPs may lead to altered peripheral and central pain processing, also referred to as peripheral and central sensitization (CS).6–8 Peripheral primary sensory neurons and pain-processing neurons in the spinal cord and brain become more sensitive due to neuronal plasticity caused by continuous nociceptive afferent information coming from the MTrP to spinal cord neurons and supra-spinal structures of the central nervous system.6 Nevertheless, the presence and clinical importance of CS in CINP is still under discussion.9–13
Although there is no gold standard to diagnose CS, multiple screening and diagnostic tools have already been established.8 A screening questionnaire that identifies self-reported signs of CS is the Central Sensitization Inventory (CSI).14 Another option is the use of Quantitative Sensory Testing (QST).15 This testing includes, amongst others, the determination of local and distant pain sensititivy or hyperalgesia as assessed by pressure pain thresholds (PPTs) and endogenous pain inhibition efficiency as assessed by conditioned pain modulation (CPM) paradigms.16,17 Changes in central nociceptive processing may explain persistent and recurrent symptoms in CINP and failure of treatments to obtain long-lasting relief.11–13
A common intervention for treatment of MTrPs is dry needling (DN). Although several local and mechanical effects have already been established, more research is needed on the unclear underlying central neurophysiological effects of DN. Preliminary experimental evidence shows that the application of DN may be able to reduce the excitability of the central nervous system in patients with chronic pain.18,19 Niddam et al.18 found in an MRI study that pain mediation after DN happens through the periaqueductal gray substance in the brainstem, possibly indicating that DN may activate enkephalinergic inhibitory dorsal horn interneurons. Stieven et al.20 found that a single application of DN in CINP resulted in higher local and distant PPTs, compared to sham needling (SN).20 However, only a paucity of trials about the effect of DN on PPTs and CPM have been performed to date.19–21
Consequently, the aim of this randomized controlled trial was to compare the immediate effects of a single DN or SN session on distant and local PPTS and CPM in patients with CINP. It was hypothesized that DN would have immediate positive effects resulting in higher distant and local PPTs (reflecting a decrease in pain sensitivity) and higher CPM (reflecting more efficient pain inhibition).
MethodsProtocol and registrationThis study design was approved by the Ethics and Research Committee of Ghent University (project number EC2019/0980) and prospectively registered at Clinicaltrials.gov (registration number: NCT04725825). This trial was reported according to the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) statement.22,23
Study populationBetween February 2021 and July 2021, patients with CINP were recruited for this study. Patients were recruited by flyers at the waiting rooms for physical medicine and rehabilitation of the Ghent University Hospital and on social media. Before participating, patients were asked to complete an online questionnaire concerning their current neck complaints and general health. After completing the online questionnaire, all participants were selected based on inclusion and exclusion criteria as stated in Table 1. All eligible individuals provided informed consent and were informed about the study procedures before the trial started.
Inclusion and exclusion criteria.
BMI, body mass index; NPRS, numeric pain rating scale.
All procedures were performed at the Department of Rehabilitation Sciences, Ghent University. Participants were randomly allocated to one of the 2 study groups (DN or SN) by an independent researcher, using an internet-based randomization website (www.randomizer.org) with an allocation ratio of 1:1. Allocation concealment was guaranteed by using sealed opaque envelopes. All participants were informed that they would be randomly assigned to one of the two study groups and were blinded for treatment allocation. All outcome measures were assessed at baseline and immediately post-intervention by assessors blinded to treatment allocation.
Sample size determination and pilotA total sample size of at least 36 subjects had to be recruited based on an a priori sample size calculation (G*Power 3.1.9.2). This calculation was determined for the primary outcome measure “PPT Quadriceps” and was based on pilot data, which showed an effect size of 0.28 for the difference between a DN group (n = 9) and a SN group (n = 9) post intervention. The a priori sample size calculation was performed for the within-between interaction in a repeated-measures analysis of variance with two groups and two measurements, a minimum power of 0.90, an effect size of 0.28, and an α level of 0.05.
The PPT data from this pilot study were pooled with the data from the present study, which resulted in a total sample size of 54 participants for the PPT data and 36 participants for CPM data.
InterventionsBoth groups received one single needling intervention at the upper trapezius (UT) of the (most) painful side, there was no follow-up treatment. All interventions were performed by one of the three trained physical therapists with at least 4 years of experience in the treatment of MPS and manual therapy. All therapists performed both interventions. Prior to the intervention, therapists provided the same standardized information to all participants about MTrPs, the intervention and possible post-intervention effects. The interventions were performed with a solid filiform needle (0.30 × 0.40 mm C-Type acupuncture needle). Participants were placed in a prone position with their arms comfortably supported in 90° shoulder abduction.
Dry needlingThe DN was applied unilaterally at the (most) painful UT. First, the skin was cleaned with alcohol and a relevant MTrP was identified. Second, the skin was pierced subcutaneously at the MTrP location, followed by piercing into the muscle tissue in a posterior-anterior direction (from therapist's thumb to index), while the muscle belly was held in a pincer palpation. The “fast in, fast out” method was used, for this technique the needle was quickly moved up- and downwards into the muscle fibers of the taut band with the aim of provoking local twitch responses (LTRs) until extinction. In case no LTRs were elicited, the needle was moved up and downwards for 10 times in 3 slightly different directions and was then withdrawn from the muscle.
Sham needlingThe same procedure as for the DN group was implemented to replicate an authentic clinical experience and maintain credibility and participants’ blinding.24 The needle was inserted into the subcutaneous layer and went up and down 10 times on the MTrP location without penetrating the deep muscle fascia while the therapist pretended to change the direction of the needle 3 times. Because the needle did not penetrate the muscle fascia, no LTRs were provoked.21,25–27 Contextual clues associated with DN such as skin's cleaning, needle insertion, and manipulation (simulation in sham needling), and haemostatic compression after procedure were identical in both interventions.24
Outcome measuresThe outcome measurements were performed by three independent assessors who were blinded to treatment allocation. Baseline and post-intervention measurements for each participant were always performed by the same assessor. During the testing, the patient was placed in a seated position with a neutral spine and the feet flat on the ground. First, each patient was asked to score their NP at that moment on a numeric pain rating scale (NPRS). Second, PPTs were measured on both UT muscles (treated and non-treated side) and quadriceps muscle for the treated side. The sequence of PPT muscle testing was randomly selected via the online tool Randomizer (www.randomizer.org). Third, for the CPM protocol, the function of the descending pain inhibitory pathways was evaluated by examining the effect of a conditioning stimulus of the non-dominant hand (hot water immersion) on the PPTs. Additionally, pain intensity caused by the hot water immersion was assessed on a NPRS. After implementation of the intervention (DN or SN of the (most) painful UT), the same testing protocol was repeated.
Primary outcome measuresDistant PPTs – quadriceps (distant pain sensitivity/ hyperalgesia) –Fig. 2APPTs were measured at a standardized location with a hand-held pressure algometer (Wagner FPX 25 Force Gage). The quadriceps muscle on the painful side was assessed at the middle of the distance between the anterior superior iliac spine and the base of the patella.28,29 The probe (1cm2) was placed perpendicular to the test surface. The pressure was expressed in Newton (N) and the average was taken of two measurements with a 30-second interval between each application. Pressure was increased by 1 N/s until the participant reported this feeling as unpleasant.9 Digital algometry performed at the Q muscle is shown to have a good intrarater reliability (intraclass correlation, 0.74–0.85).30
Secondary outcome measuresLocal PPTs – treated and non-treated upper trapezius (local pain sensitivity/ hyperalgesia) – Fig. 2BPPTs were measured at the treated (tUT) and non-treated upper trapezius (ntUT). The reported treated side was the most painful side indicated by the patient. The average of two measurements at the middle between the spinous process of C7 and the center of the acromion was calculated.9 Digital algometry is shown to have sufficient intrarater reliability in measuring the PPT on the trigger point of the UT muscle in patients with CINP.31 The interrater reliability of PPT measurements has shown to be excellent.32 In a study of Walton et al., PPT at the UT showed a significant ability to detect global change (AUC=0.76), using minimal clinically important difference (MCID) change scores within a clinically reasonable range (between approximately 5 and 22 N/cm2).33 Minimal detectable change (MDC) values at the UT site ranged between approximately 4.45 and 11.12 N/cm2; intrarater reliability was almost perfect (ICC = 0.94–0.97).34
Conditioned pain modulation (Efficacy of pain inhibition) –Fig. 2CThe conditioning stimulus in this study was a 1 min hot water immersion (45.5 °C) of the non-dominant hand (up to the most distal point of the ulnar styloid process) in a VersaCool Circulating Bath (Thermo Fisher Scientific).29,35 PPTs were used as test stimulus, which are shown to be a valid tool to measure CPM.36 For analysis of CPM efficacy, absolute CPM effects were calculated: the mean PPT measured before the hot water immersion was subtracted from the mean PPT after hot water immersion (PPT post - PPT pre). Hence, a lower CPM value reflects a less efficient endogenous pain inhibition, whereas a higher CPM value reflects a more efficient endogenous pain inhibition. Additionally, the relative CPM effect (CPM efficacy expressed in percent change) was calculated: ((PPT post – PPT pre)/PPT pre) * 100. This resulted in either a pronociceptive value (CPM value less than or equal to zero, indicating a less efficient endogenous pain inhibition: no CPM effect) or antinociceptive value (CPM value more than zero, indicating a more efficient endogenous pain inhibition: CPM effect). No information about MCID has been found.
NPRS during hot water immersion (heat hyperalgesia)After placing the hand in the VersaCool for one minute, the patient was asked to score the pain caused by the hot water on an 11-point NPRS. The MDC and MCID are 2.1 and 1.3 points, respectively, in patients with mechanical neck pain.37
Statistical analysisData analysis was performed based on an intention-to-treat principle with IBM SPSS Statistics version 27.0 (IBM, Armonk, NY, USA) for all outcome measures. Data normality was assessed by means of the Shapiro-Wilk test, histograms and Q-Q plots. Boxplots were used as quality control to find any outliers and extreme values. Patients’ characteristics, baseline and post-intervention values between groups, were evaluated with the independent T-test, the Mann-Whitney U test (for the non-normally distributed data), and the Chi Square test (for categorical variables sex and affected side). Means and standard deviations were calculated for all demographic data. Linear mixed model analyses were used to determine the differences of all outcomes between and within the intervention groups over time for the PPTs, as well as for the absolute and relative CPM effects at the tUT, ntUT, and Q, and for heat hyperalgesia. Participant number was used as random intercept and residuals were checked for normality. Fixed factors were ‘intervention’ (DN and SN group), ‘time’ (baseline and post-intervention), and ‘intervention x time’. Sex was included as covariate in the linear mixed model analyses. All PPT analyses were performed on the entire group (DN; N = 26 and SN; N = 28). CPM data were only available from a subgroup (DN; N = 17 and SN; N = 19). Statistical significance was accepted at the 0.05 α-level.
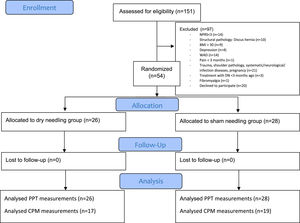
ResultsParticipantsFifty-four patients with CINP were randomly allocated to the DN group (n = 26) or the SN group (n = 28) (Fig. 1). Demographic features of both groups are presented in Table 2. Patients’ characteristics between groups (except for sex) and outcome measures were comparable at baseline (Tables 1 and 2). The mean NDI and CSI-scores in both groups were considered to represent mild disability levels and presence of mild features of CS. The mean CSI-score did not reach the clinically relevant cutoff value of 40/100, although some participants reached higher CSI-scores on an individual level.14
Patients’ characteristics of the dry needling and sham needling group.
Values are expressed as means ± standard deviation for continuous variables and absolute frequency (%) for categorical variables. Abbreviations: n, number of participants; BMI, body mass index; NDI, Neck Disability Index; CSI, Central Sensitization Inventory; NPRS, Numeric Pain Rating Scale; UT, upper trapezius muscle.
Descriptive statistics and within-group change scores (post-pre intervention) for pressure pain thresholds and conditioned pain modulation.
Data are expressed as mean ± standard deviation and mean difference (95% confidence interval).
Within-group difference (Baseline – post-intervention).
*= statistically significant.
Abbreviations: CI, confidence interval; CPM, conditioned pain modulation; DN, dry needling; NPRS, numeric pain rating scale; ntUT, non-treated upper trapezius muscle; PPT, pressure pain threshold; Q, Quadriceps muscle; SN, sham needling; tU, treated (most painful) upper trapezius muscle.
The linear mixed-models revealed no significant “group x time” interaction effect for PPTs at the tUT, ntUT, or Q. No post hoc pairwise comparisons for between-group or within group-comparisons showed any significant results. No difference in PPTs between DN and SN groups was found for tUT (mean difference [MD]=−1.38; 95% CI: −8.13, 5.37); ntUT (MD= −2.15; 95% CI:−9.52, 5.23); and Q (MD= −0.13; 95% CI:−9.78, 9.52). Between-group differences in mean changes from baseline to post-intervention were smaller than the reported MDC and MCID.
Absolute CPM effectThe linear mixed-models revealed no significant “group x time” interaction effects for tUT, ntUT, or Q. No post hoc pairwise comparisons for between-group or within group-comparison show any significant results. No difference in absolute CPM effect between DN and SN groups was found for tUT (MD= −0.96; 95% CI: −3.22, 1.30); ntUT (MD= −0.38; 95% CI:−4.08, 3.33); and Q (MD= 3.44; 95% CI: −0.51, 7.40).
Relative CPM effectNo significant “group x time” interaction effect for tUT, ntUT, or Q was found. There were no significant results for the between-group or within group-comparison at the tUT and ntUT. The within group difference in the DN group for the Q indicated that the CPM efficiency was significantly higher post-intervention compared to baseline (MD= 13.52%; 95% CI: 0.46, 26.59). In the SN group, no significant differences were found. No between-group mean differences were found for the tUT, ntUT, and Q.
NPRS during hot water immersionNo significant “group x time” interaction effect for NPRS water temperature were found, reflecting the absence of heat hyperalgesia. The post hoc pairwise comparisons for between-group or within group-comparison also showed no significant results. Between-group differences in mean changes from baseline to post-intervention were smaller than the reported MDC and MCID.
Adverse eventsDuring the trial, no adverse events were registered.
DiscussionNo significant differences between DN and SN were found for PPTs at the local and remote locations and change in PPT values did not exceed the SE, MDC, or MCID, as identified by Walton et al.33,34 Walton et al. stated that local PPTs appears to be a useful tool for measuring change over time, but remote (measured at the tibialis anterior muscle in their study) PPT is not useful for this purpose.34 Our result contradicts the findings of Stieven et al., who found that a single session of DN or manual release, but not SN, resulted in an increase in PPTs at the UT bilaterally and at the ipsilateral and contralateral proximal head of the radius in patients with CINP.20 Pecos-Martin et al. also reported a significant increase in PPT over the lower trapezius after one DN session performed in an active MTrP, immediately and up to at least one month after the treatment session, compared to DN on another location of the same muscle (but not a MTrP).38 Mejuto-Vázquez et al. found superior effects of DN, compared to no treatment on local (C5-C6 zygapophyseal joint) and remote (second metacarpal and tibialis anterior muscle) PPTs in patients with acute neck pain 10 min and one week after intervention.39 However, because no control group was included, placebo effects cannot be ruled out. Although our results show a general increase in PPTs for all DN locations, in contrast to the SN group where only a local increase was seen at the treated location, the results were statistically non-significant. A possible explanation is that DN is often accompanied by local post needling soreness, lasting up to 48 h.40,41 This soreness is the result of a direct local hyperalgesia response at the treated area and thus can mask effects immediately after intervention.19,42
This study found no differences in absolute CPM effects within or between groups. To our knowledge, there are no other studies evaluating CPM effect after DN in patients with neck pain, which makes comparing results difficult. One study evaluated the CPM effect of DN in patients with knee osteoarthritis and found no larger effect of DN on central pain processing, compared to SN, immediately and 3 days postintervention.21 Nevertheless, it may be hypothesized that eliciting LTRs during DN, which is mostly experienced as ‘painful’, may be considered an extra painful conditioning stimulus, which may have influenced the CPM protocol. In this case, the LTR might blur the CPM response, as this acts as a third pain stimulus besides the test and conditioning stimulus.43 This contrasts with SN, which may not have influenced the testing protocol in a similar way since no LTRs were elicited and less pain was present.
When considering the relative CPM effects; there was a significant increase in the percentage of change at the Q location in the DN group, indicating a possible amelioration of the antinociceptive pain modulation. This may be caused by activation of descending inhibitory pain mechanisms.5,18 This is in line with the generalized, however not significant, increase in PPTs after DN.
The sample in this study included patients with CINP with mild disability and mild features of CS. Generelizability of the results to other patient groups (eg. whiplash, patients with cervical radiculopathy or generalized musculoskeletal complaints) is not applicable because previous research has shown that QST-features differ in these patient groups.9,44–46
Considering the complexity of blinding in physical intervention research, two Delphi studies have been performed to evaluate the most important elements of shams for DN research.47 Experts placed high importance on the entire intervention experience for active and sham protocols. Sham credibility may be maintained using cognitive strategies, potentially relinquishing the need for indistinguishable shams that have proved problematic to design.24
StrengthsThis study is to our knowledge one of the first studies investigating pain modulatory effects of DN. The combination of evaluating PPT measurements and both absolute and relative CPM effects on local and remote locations is an added value to the insights on central neurophysiological effects of DN. The intervention was performed by three experienced DN therapists, who actively searched for trigger points instead of needling a predetermined point. All outcome assessors were blinded for the intervention. Therapists were trained to give identical verbal and non-verbal communication to both groups to maximize blinding of the participants.
LimitationsFirst, only one measurement was performed, making it impossible to evaluate and discuss the long-term effects and the possible influence of muscle soreness on the results immediately post-intervention. Second, patients who experienced DN in the past were not excluded. Although participants were not aware of the group allocation and were blinded to their treatment, expectations and previous experience with DN may have influenced the results. Nevertheless, in a recent study, evaluating the effects of previous experience with DN therapy on blinding effectiveness and pain outcomes in people with neck pain, participants with previous experience were 22% more accurate at identifying their group allocation than those without experience, but the difference was not significant. Previous experience did not influence most clinical outcomes, except for pain intensity after real DN, although the difference was not clinically relevant.48 Lastly, SN may have provoked neurophysiological effects as well, resulting in the comparison of two interventional groups instead of comparing an intervention to a control group.49,50 The insertion of a needle in the skin is interpreted as a noxious stimulus that can result in the activation of central inhibitory mechanisms and cause the excitation of Aδ and Aβ nerve fibers, which automatically provokes an analgesic effect.51,52 Nevertheless, there are no high-quality alternatives that may counter this possible effect.
Implications for clinical practice & future researchFuture trials are needed to examine the effects of DN on central pain processing, after recovering from the associated post needling soreness. A follow-up period of more than 48 h post-intervention should therefore be indicated. Because there is no widely accepted sham protocol for DN research, researchers should incorporate cognitive influences that extend beyond mimicking of tactile sensations to create a believable simulation of active dry needling. Assessment of blinding, using a blinding index might provide more robustness to the results.24
ConclusionBased on the results of this study, we cannot conclude that DN has better effects on pain sensitivity and central pain modulation immediately post-intervention, compared to SN. Future trials are needed to examine the effects after post-needling soreness is resolved.
The authors like to thank and acknowledge the contribution of all master students who collaborated in recruiting and testing the participants.