Clinical laxity tests are commonly used together to identify individuals with multidirectional instability (MDI). However, their biomechanical validity in distinguishing distinct biomechanical characteristics consistent with MDI has not been demonstrated.
ObjectiveTo determine if differences in glenohumeral (GH) joint laxity exist between individuals diagnosed with multidirectional instability (MDI) and asymptomatic matched controls without MDI.
MethodsEighteen participants (9 swimmers with MDI, 9 non-swimming asymptomatic matched controls without MDI) participated in this observational study. Participants were classified as having MDI with a composite laxity score from three laxity tests (anterior/posterior drawer and sulcus tests). Single plane dynamic fluoroscopy captured joint motion with 2D-3D joint registration to derive 3D joint kinematics. Average GH translations occurring during the laxity tests were compared between groups using an independent sample's t-test. The relationship of composite laxity scores to overall translations was examined with a simple linear regression. Differences of each laxity test translation between groups were analyzed with a two-way repeated measures ANOVA.
ResultsMean composite translations for swimmers were 1.7 mm greater (p = 0.04, 95% Confidence Interval (CI): 0.1, 3.3 mm) compared to controls. A moderate association occurred (r2 = 0.40, p = 0.005) between composite laxity scores and composite translation. Greater translations for the posterior drawer (-2.4 mm, p = 0.04, 95% CI: -0.1, -4.6) and sulcus tests (-2.7 mm, p = 0.03, 95% CI: -0.3, -5.0) existed in swimmers compared to controls.
ConclusionSignificant differences in composite translation existed between symptomatic swimmers with MDI and asymptomatic control participants without MDI during GH joint laxity tests. The results provide initial biomechanically based construct validity for the clinical criteria used to identify individuals with MDI.
The normal shoulder possesses considerable joint laxity because it sacrifices elements of joint stability such as joint congruency to enhance functional range of motion.1 The diagnostic term for multidirectional instability (MDI) has been described as excessive shoulder joint laxity in at least two directions in combination with symptomatology.2 Excessive laxity or glenohumeral joint translation has been theorized to lead to increased risk of mechanical injury of the surrounding joint structures such as the rotator cuff, long head of the biceps, or the labrum.3–5 Therefore, investigating how best to identify cases of MDI is essential to developing targeted treatment and prevention techniques in this population. Clinical laxity tests such as the anterior drawer, posterior drawer, and the sulcus test are commonly used together to identify cases of MDI.6 However, evidence for their usefulness in distinguishing distinct biomechanical characteristics of groups with and without MDI is lacking.
Investigations into the biomechanical validity of laxity tests are complicated by motion tracking errors due to skin motion artifact. Skin motion artifact precludes the use of surface sensors to identify differences in joint laxity between groups.7 Multiple techniques have been described to measure joint motion including two-dimensional (2D) static x-ray imaging,8 ultrasound, and magnetic resonance imaging (MRI). All of these have limits to their ability to provide accurate data during in vivo motion.9–13 Single and biplane fluoroscope techniques have the capability to overcome skin motion artifact while being minimally invasive. Combining biplane or single plane fluoroscopic movement capture with three-dimensional (3D) participant specific anatomic modeling allows for dynamic imaging and 3D measurements of motion with 2D-3D shape matching techniques.14–18
Therefore, the purpose of this biomechanical validation study was to use fluoroscopic motion tracking with 2D-3D shape matching to determine if biomechanical differences in overall glenohumeral joint laxity existed between competitive symptomatic swimmers diagnosed with MDI and matched controls of asymptomatic non-swimmers without MDI. The distinct groups were recruited for this study to first establish baseline validity of the tests to find differences in groups that should differ biomechanically. The experimental hypothesis was that swimmers diagnosed with MDI would demonstrate increased overall glenohumeral joint translations during the laxity exams as compared to matched controls. A secondary exploratory purpose of the study was to examine if swimmers and matched controls differed in translation magnitudes for individual shoulder laxity tests.
MethodsTwo participant groups were recruited as a subset of a larger observational study through a sample of convenience. A subset of participants (n = 18) was necessary for this study to limit the repeated radiation to the examiner performing the laxity tests. The recruited participants were competitive swimmers with shoulder pain (n = 9; 4 females, age: 36 ± 10 years, body mass index [BMI]: 22.9 ± 2.2 kg/m2), and asymptomatic individuals without shoulder pain (n = 9; 4 females, age: 38.8 ± 8 years, BMI: 23.2 ± 1.8 kg/m2). Eligible participants were between the ages of 18 and 55 with no history of trauma or previous shoulder surgeries. Swimmers were required to be experiencing shoulder pain during functional activities for six weeks or greater, swim at least 3.2 km per session and train a minimum of three hours per week with a coach. The asymptomatic control group was matched on age, sex, BMI, and hand dominance to the swimmers. Matched controls were excluded if they reported a history of shoulder pain or regular participation in swimming, throwing, or overhead hitting activities (e.g. volleyball or tennis), which might contribute to the development of glenohumeral joint laxity.
A board-certified sports and orthopaedic specialist physical therapist with 15 years of experience performed the clinical examinations. Participants were excluded if demonstrating symptoms of cervical origin.19 Symptoms were considered of cervical origin if the participant tested positive on any of the tests described by Wainner et al.19 Participants were also excluded if they had symptoms that changed as a result of head movement through full active cervical range of motion or possessing humerothoracic elevation less than 120°. For the swimmers with pain to be classified with MDI, the inclusion criteria required two additional conditions. First a composite laxity score (perception of translation from the examiner) from the anterior, posterior, and sulcus laxity testing of greater than 1.5 20 with each individual test rated zero to three. The composite laxity score was calculated as the mean of the three laxity test scores and chosen because Staker et al.20 demonstrated an average difference in composite translations of 3.5 mm when comparing individuals above and below a composite laxity score of 1.5. For example, a participant would have an overall score greater than 1.5 if they scored greater than one on each test or greater than two on more than one test. The second condition was that either a positive anterior apprehension sign21 or a score of equal or greater than two on the Beighton's Index was obtained.22
The anterior and posterior drawer tests were performed as described by Gerber and Ganz,23 with grades ranging from 0 to 3.24 The participant was supine while the examiner held the arm in their axilla and provided an anterior or posterior directed force at the upper arm in a direction parallel to the glenoid. The other hand stabilized the scapula near the joint line. Additionally, manual joint compression prior to the drawer maneuver was added to the classically described position. This was done to standardize the starting position through concavity-compression as described by Matsen et al.1 The drawer tests were graded on a 0–3 scale, as described by Hawkins and Mohtadi.24 A grade of zero was provided when no humeral head translation was perceived during the anterior or posterior mobilization. A grade of one was provided when anterior or posterior mobilization up to 50% of the humeral head diameter was perceived. A grade of two was provided when greater than 50% humeral head diameter translation occurred. A grade of three was provided if the humeral head moved beyond the “confines” of the glenoid fossa.24 The sulcus test was performed and graded as described by Altchek et al.25 Briefly, the participants’ humerus was at their side with their forearm resting on their thigh. The examiner stabilized at the acromion with one hand while applying traction at the humeral epicondyles. Grading ranged from 1 to 3. Participants received a grade of one when less than one centimeter of distance from the acromion to humeral head was perceived. When one to two centimeters of distance was perceived between the acromion and humeral head; a grade of two was assigned. More than two centimeters distance received a grade of three.25
Participants in the asymptomatic non swimming control group were excluded if any of the following signs of MDI were present; a composite laxity score of greater than 1.5 or a Beighton's index of greater than two. Additionally, the control group participants could not have a positive anterior apprehension test or greater than one of four positive impingement tests.26
All ratings of laxity tests and additional tests were performed prior to undergoing the laxity test maneuvers during dynamic fluoroscopy. Swimmers’ numerical pain rating scale and Disabilities of the Arm Shoulder and Hand (DASH)27 scores were collected. Participants’ and swimmer's characteristics are located in Tables 1 and 2, respectively. All participants provided informed consent to participate in this study. The University of Minnesota Institutional Review Board Human Subjects Committee approved the study protocols.
Participant demographics and characteristics.
Values are means ± standard deviation, unless a count variable. Mean difference (control minus swimmer); CI: 95% confidence interval of difference (control minus swimmer); If values are non parametric (composite laxity test score and Beighton's Index), median with interquartile range (IQR) provided. RoM, Range of Motion; MDI, multidirectional instability; BMI, body mass index; Exact, Fisher's Exact statistic.
Swimmer's characteristics (n = 9).
Unless percentage, all values are mean ± standard deviation. NPRS, numeric pain rating scale; DASH, Disabilities of the Arm Shoulder Hand. One participant failed to complete the DASH.
Dynamic images for each laxity test were captured with a Phillips BV Pulsera mobile c-arm fluoroscopy unit (Philips Medical Systems, Netherlands) with a 30 cm field of view, 1024 × 1024 image resolution, 99.5 cm source to image distance, at 25 Hz. Images were acquired with voltages (kV) and current (mA) adjusted automatically by the fluoroscopy unit to maximize image quality and minimize dose. Acquisition parameters, image distortion correction, and 3D volume calibration were performed as described by Lawrence et al.18
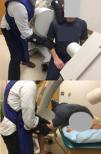
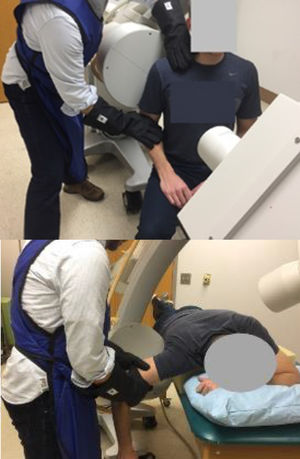
Muscle activity was monitored with a portable clinical surface electromyography (EMG) unit (PhsioPlux, Arruda Dos Vinhos, Portugal). Each laxity test was performed once unless an electromyographic muscle excitation signal from the deltoid of greater than 10% of a sub maximum effort was recorded, indicating participant guarding. For the sulcus test, participants were seated with the glenohumeral joint centered in the imaging field and trunk axially rotated approximately 40° away from the imaging plane (Fig. 1A).28 Imaging lasted approximately three seconds per test. Anterior and posterior drawer tests were performed in prone. The participant was positioned with their arm at 90° abduction and neutral external/internal rotation. The imaging source was placed superiorly approximately 15–30° off midline and toward the contralateral side being imaged (Fig. 1B). The image intensifier was placed as close to the axilla as possible without touching the subject's arm or interfering with the test maneuvers. Fluoroscopic imaging of the drawer tests began just prior to joint compression and ended just after reaching the end position. As required by the institutional radiation safety board, the examiner wore leaded gloves to perform the laxity tests during fluoroscopic imaging.
A. Sulcus test position. Examiner's left hand stabilizing the scapula proximally and right hand providing inferior distraction force at the elbow wearing black, leaded gloves. B. Anterior/posterior drawer test position. Prone position of participant with head rotated and flexed to the left to avoid image obstruction. Examiner's hands are as proximal on humerus as possible without image obstruction applying compression and then posterior or anterior (mobilization) forces on the humerus.
Aside from the prone positioning, the laxity tests were performed as previously described.23,24 An average of 50 images were captured for each laxity test. Two frames for shape matching were chosen. The first frame was defined as the image just prior to the onset of any movement of the scapula or humerus visible on the images. The second frame was the image observed visually to be at the end of the test maneuver when no further humeral movement in the test direction was occurring.
Participant specific 3D models of the humerus and scapula were necessary for single plane 2D-3D shape matching as has been previously described.18 Briefly, 3D models were developed by obtaining MRIs of the tested shoulder using a three Tesla Siemens Magnetom SKYRA (Siemens Healthineers, Erlangen, Germany) with a shoulder coil. The imaging protocol utilized a 3D gradient echo sequence T1-VIBE with a slice thickness of 0.6 mm. The MRIs were checked to ensure that the entire scapula and at least the upper 1/3 of the humerus were captured. The digital image files (DICOMs) were imported into Mimics software (Materialise, Leuven, Belgium) for manual bone segmentation, and creation of the participant-specific 3D anatomical models.18 The 3D models of participants’ humerus and scapula were manually manipulated using an open source model registration software (JointTrack, available at https://sourceforge.net/projects/jointtrack/) until the 2D bone edges visually aligned between the 3D model and the fluoroscopy image for each of the laxity tests. The primary investigator, blinded to group assignment, performed the 2D-3D shape matching. Anatomical coordinate systems were embedded in the 3D models to allow derivation of 3D kinematics in clinically interpretable ways.29 Accuracy of single plane shape matching within our lab with cadaveric specimens was established in a previous study by Lawrence et al.18 utilizing radiostereometric analysis. With the arm at the side, in a position similar to sulcus testing, superior/inferior translation had a root-mean-square (RMS) error of 1.2 mm (bias 0.3 mm, precision 1.1mm). Prone positioning accuracy was tested similarly by the primary investigator on a single cadaveric specimen. Joint position RMS errors for prone positioning were 2.1 mm anterior/posterior (bias -1.1 mm, precision 2.0).
Glenohumeral joint translations were described as displacement calculations (end minus start position) from the center of the glenoid along the scapular X (anterior/posterior) axis for anterior and posterior drawer tests. Sulcus test glenohumeral joint translations were described along the scapular Y (superior/inferior) axis. If a small translation was calculated in the opposite direction of the intended motion (eg, a positive translation for a posterior drawer or sulcus test), the magnitude of translation was set to zero. A RMS calculation was performed to determine the composite translation.20 The RMS was calculated as the square root of the mean of the squared magnitudes of translation for each of the three laxity tests in each individual.
Checks for normality of data distribution were performed by assessing skewness and kurtosis, by performing Shapiro-Wilk tests, and visually assessing histograms of the dependent variables. Group demographic data were analyzed using independent samples t-tests. Non-normally distributed data were analyzed with Wilcoxon's tests for non-parametric data. Frequency counts between group demographics were analyzed with Chi square analyses. If any cell counts less than five were encountered, a Fisher's Exact test was performed. For the primary analysis, composite translation results were compared using an independent samples t-test and the relationship of composite scores to overall translations was examined with a simple linear regression. For the secondary, exploratory analysis examining difference of individual laxity test translations between groups, a two-way repeated measures analysis of variance (ANOVA) with factors of group (controls and swimmers) and test (anterior drawer, posterior drawer, sulcus tests) was used. Simple effects of group were also examined. A Bonferroni correction was applied for multiple comparisons in the exploratory analysis. All statistical analyses were performed with IBM SPSS Statistics for Macintosh, Version 26 (IBM Corp, Armonk, NY, USA).
ResultsDemographic data were normally distributed with exception to Beighton's index and the composite laxity score which were then analyzed using non-parametric statistics. Swimmers with MDI had a significantly higher median (interquartile range) composite laxity score of 1.7 (1.7–2.0) compared to a score of 1.0 (1.0–1.2) of the control group and a significantly higher Beighton Index median score of 4.0 (2.5–5.0) compared the controls with a score of 0 (0–1.0) (Table 1). Raw data of the laxity tests grades, translations, and composite values are located in the supplementary table.
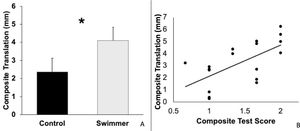
Composite laxity translations were normally distributed. Mean composite translations for swimmers (4.1 ± 1.7 mm standard deviation [SD]) were significantly greater (p = 0.04) compared to controls (2.4 ± 1.5 mm, mean difference between groups of 1.7 mm, 95% Confidence Interval (CI) 0.1, 3.3 mm; Fig. 2A). The simple linear regression analysis between the composite laxity score and composite translation revealed a moderate, significant association (r = 0.63, r2 = 0.40, p = 0.005; Fig. 2B).
Assumptions of normality were met for the exploratory analyses. A significant interaction between group and test was observed (p = 0.02). Pairwise comparisons (Table 3) revealed no significant difference existed between swimmers (2.1 ± 0.8 mm) and controls (1.6 ± 1.6 mm) in anterior translations for the anterior drawer test (p = 0.50). Significant differences were observed for the posterior drawer test (p = 0.04). Swimmers demonstrated an average of 3.3 ± 3.0 mm posterior translations compared to controls with 0.9 ± 1.1 mm (mean difference: -2.4 mm, 95% CI: -0.1, -4.6 mm). Significant differences in translation magnitude between groups were found for the sulcus test (p = 0.03) with the swimmers demonstrating 5.6 ± 2.1 mm translations and controls 2.9 ± 2.5 mm (mean difference: -2.7 mm, 95% CI: -0.3, -5.0 mm).
Pairwise comparisons of mean translations between controls and swimmers.
| Laxity Test (mm) | Controls | Swimmers | Mean difference | 95% CI of difference | p-value |
|---|---|---|---|---|---|
| Anterior | 2.1 | 1.6 | 0.4 | -0.9, 1.7 | 0.49 |
| Posterior | 0.9 | 3.3 | -2.4 | -0.1, -4.6 | 0.04 |
| Sulcus | 2.9 | 5.6 | -2.7 | -0.3, -5.0 | 0.03 |
Mean difference (control minus swimmer); CI, 95% confidence interval of difference (control minus swimmer).
Swimmers diagnosed with MDI demonstrated increased overall glenohumeral joint translations during the laxity exams as compared to matched controls. Our results suggest that laxity tests, taken together, may be a useful method for identifying cases of excessive multidirectional joint laxity consistent with the diagnosis of MDI. This study demonstrated significant differences between groups in the amount of composite translation that occurred during the laxity tests.
Our study excluded individuals with a history of dislocation. Translations for participants with such a history have been described with magnitudes greater than 10 mm.30 In comparison, our data showed a mean significant difference in translations between the controls and swimmers for a single laxity test was -2.4 mm (95% CI -0.1, -4.6 mm) for the posterior drawer and -2.7 mm (95% CI -0.3, -5.0) for the sulcus test. This suggests that it may be possible to detect differences in multidirectional shoulder joint laxity between groups without a history of dislocation or obvious signs of unidirectional instability. Identifying subtle joint laxity differences between a control group and individuals identified with MDI without a history of dislocation has not been previously demonstrated in the literature. Biomechanically differentiating subtle signs of multidirectional instability1 is an important step in the development of targeted treatment and prevention techniques for individuals with MDI.
This study used inclusion criteria similar to the current recommendations for clinical diagnosis of MDI.2,31 Unlike the present study, inclusion and exclusion criteria for most prior studies of MDI have been based on singular aspects of MDI such as a history of swimming, assumed pathologic findings, or one positive special test.8,9 The heterogeneity of joint laxity within these study groups may have prevented finding glenohumeral translation differences between the groups. Consequently, the inclusion criteria in this study were developed to match the clinical construct of MDI. This may account for why our data demonstrated differences between groups. Therefore, this study provides important biomechanical information supporting the utility of these diagnostic criteria for diagnosing individuals with MDI.
In addition, in this study, there was a moderately strong relationship between the composite laxity score and composite translations. This supports previous findings by Staker et al,20 that demonstrated a relationship between composite laxity scores in one group of patients with shoulder pain using intracortical pin tracking of translations. The current study builds upon that work by further demonstrating differences between two groups using commonly accepted diagnostic criteria and showing that these criteria also manifest with detectable biomechanical differences between groups. Taken together the two studies suggest that combining laxity tests may be critical to identify the level of overall shoulder joint laxity in patients. Further work is required with multiple raters across a wider range of patient populations to more fully explore this potential.
Significantly increased translations of the swimming group were found for the posterior drawer and sulcus tests compared to the controls. This finding is consistent with the clinical construct of the MDI diagnosis requiring excessive laxity in at least two directions. The lack of excessive laxity in the anterior direction was not anticipated. A retrospective power analysis revealed that with an a priori meaningful difference derived from a previous study20 set at 2.0 mm translation and overall observed measurement variance of 1.7 mm, the study achieved 80% power to detect differences. The lack of differences during anterior drawer testing may be explained by the prone positioning required for fluoroscopic testing. Manual, counter stabilization of the scapula to the anterior humeral movement was not possible which caused some difficulty in determining an end point of translation. Given that excessive anterior laxity is commonly described in MDI,32 future studies should consider different positioning to allow for scapular stabilization to determine if there is increased anterior laxity in this population.
This study used unique methods to classify participants with distinct clinical features of MDI to determine if this would yield evidence of biomechanical differences as well. By combining participants’ clinical features to diagnose MDI and quantifying laxity test translations through dynamic fluoroscopy, this study assessed the utility of a diagnostic process through biomechanical means that closely resembles clinical testing mechanics.
Limitations of this study should be considered. There was a single, unblinded examiner responsible for identifying the groups and performing the laxity tests. Including a blinded rater to perform the laxity test during fluoroscopy may have limited the potential for examiner bias. However, a lack of significant group differences for the anterior drawer test argues against examiner bias as a predominant factor. One reason for a lack of differences between groups during the anterior drawer test could have been a bias to avoid the laxity endpoint in swimmers through an unconscious desire to limit iatrogenic symptoms and therefore resulted in diminished differences between groups.
The use of radioprotective gloves with each participant also could have affected the sense of “end feel” by the examiner. However, gloves were used for both groups (swimmers with MDI and controls) and therefore the effect of the gloves on translations is distributed across both groups. Therefore, any effect would have likely resulted in no differences in translations as opposed to the differences in overall laxity found in the current study. Lastly, generalizability of the results would be improved by including multiple examiners to ensure, at minimum, imparted translations were repeatable between examiners. Although previous work by Staker et al.20 suggests laxity tests translations are repeatable between at least two examiners.20
A pragmatic testing approach was used to reproduce clinical conditions and to facilitate imaging positioning requirements. In doing so, the scapula was not mechanically stabilized with a belt or other mechanism during laxity testing. Fluoroscopic imaging and 2D-3D motion tracking techniques do not require this stabilization as humeral motion relative to the scapula can be isolated regardless of how the scapula moves on the thorax. (See supplementary online videos of an example of three dimensional motion of the three laxity tests). Additionally, the magnitudes of translations detected were similar to studies that did stabilize the scapula.10,33,34 We therefore believe the impact of foregoing a mechanically stabilized scapula in this study is minor and does not outweigh the benefits of investigating the tests in a manner similar to clinical test conditions.
The single plane imaging technique utilized for the 2D-3D shape matching in this study is a potential source of error. Even so, shape matching errors have been found to be relatively small (RMS 1.2 mm, bias 0.3 mm, precision 1.1mm)18 in the imaging plane. A priori validation testing performed by the authors demonstrated a -1.1 mm error bias for the anterior/position bone positions compared to a 0.3 mm bias for superior/inferior bone positions. The lack of group differences in translation during the anterior drawer test, however, may partly be caused by the error associated with shape matching in that direction. Future studies using biplane fluoroscopy would limit these perspective errors and potentially demonstrate more substantial differences in composite translations between groups.
ConclusionsSignificant differences in composite translation existed between symptomatic swimmers with MDI and asymptomatic control participants without MDI during glenohumeral joint laxity tests. The results provide initial biomechanically based construct validity for the clinical criteria used to identify individuals with MDI. The work represents an important first step in developing more precise clinical criteria that could lead to consistency in diagnosis and treatment of individuals with MDI.
This work was supported by the American Physical Therapy Association Academy of Orthopaedic Physical Therapy, New Investigator Grant. The authors would like to thank Rebekah L. Lawrence, PT, PhD for her assistance in developing the Matlab code for data analysis, Rodrigo P. G. Baretto, PT, PhD for his assistance in data collection and Oliver A. Silverson ATC, MS for manuscript preparation assistance.