We evaluated the effects of posture, sex, and age on breathing pattern and chest wall motion during quiet breathing in healthy participants.
MethodsEighty-three participants aged 42.72 (SD=21.74) years presenting normal pulmonary function were evaluated by optoelectronic plethysmography in the seated, inclined (with 45° of trunk inclination), and supine positions. This method allowed to assess the chest wall in a three dimensional way considering the chest wall as three compartments: pulmonary rib cage, abdominal rib cage and abdomen.
ResultsPosture influenced all variables of breathing pattern and chest wall motion, except respiratory rate and duty cycle. Chest wall tidal volume and minute ventilation were reduced (p<0.05) in both sexes from seated to inclined and from seated to supine positions, mainly in males. Moreover, moving from seated to supine position significantly increased the percentage contribution of the abdomen to the tidal volume in both sexes (p<0.0001). Regarding sex, women showed higher contribution of thoracic compartment compared to men (p=0.008). Aging provided reductions on rib cage contributions to tidal volume that were compensated by increases of abdomen contributions (p<0.0001). In addition, increases in end-inspiratory and end-expiratory volumes over the years were observed.
ConclusionThe degree of contribution of chest wall compartments is dependent on posture, sex, and age. Therefore, verticalization increases expansion of pulmonary rib cage as well as horizontalization increases abdominal displacement. Women presented higher thoracic contribution to tidal volume than men. Aging reduces rib cage contributions to tidal volume that were compensated by increases of abdomen contributions.
The assessment of breathing pattern and chest wall motion provide important information about respiratory function.1 It is known that these parameters are influenced by different factors, including posture, sex, and age.1–9 The influence of posture on chest wall motion has been intensively studied.1,2,6,9 Nevertheless, its influence on breathing pattern as well as the effect of sex7,9 has been less investigated.3,8,9 Moreover, the influence of sex on chest wall kinematics is still controversial.1,5,7,9 Regarding age, the literature suggests that aging does not change the breathing pattern and chest wall motion.1,4,7
Although several studies have evaluated the influence of posture, sex, and age on breathing pattern and/or chest wall motion of healthy individuals, they focused on the isolated effects of these variables.1–9 In addition, respiratory inductive plethysmography was the main method used for the assessment.1,4,7,10,11
As advances had been made to elucidate the mechanisms involved in the control of breathing pattern and chest wall motion, improvements in instrumentation used for its evaluation have also been developed.12 In this context, optoelectronic plethysmography (OEP) has emerged as a noninvasive,2 valid,2 and reliable method15 capable of accurately providing an indirect measure of chest wall volume changes and its three compartments (pulmonary rib cage, abdominal rib cage, and abdomen) in different positions and situations.2,12–14 OEP allows analyzing volume variations, in a tricompartmental way, with no need to pre-establish degrees of freedom for the chest wall. This enables a more detailed study of these parameters in the different compartments of the chest wall, as well as the evaluations of end-inspiratory and end-expiratory volumes.2,12
Taking into account the relevance of breathing pattern and chest wall motion assessment and considering the great differential of evaluating the influence of posture, sex, and age both on breathing pattern and chest wall motion using OEP on healthy individuals, it is important to study the influence of these factors on chest wall compartments. The aim of this study was to assess the influence of posture, sex, and age, as well as their interaction on breathing pattern and chest wall motion at rest in healthy individuals using OEP.
MethodsParticipantsThis was a multicenter cross-sectional study with 83 participants who met the following inclusion criteria: age between 20 and 99 years; body mass index (BMI) between 18.5 and 29.99kg/m216; normal lung function17; self-reported absence of neuromuscular diseases and/or congestive heart failure; and preserved cognitive function assessed by the Mini-Mental State Examination (MMSE).18 The exclusion criteria were the inability to understand and/or perform any of the procedures and OEP data acquisition problems. The study was approved by the Ethics Committee of The Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil (ETIC 0194.0.2036000-11). All participants signed a written consent form.
Measurement instrumentOptoelectronic plethysmography (OEP)The OEP (BTS Bioengineering, Milan, Italy) was used to evaluate the breathing pattern and chest wall motion, during quiet breathing, in three positions: seated, inclined (with 45° of trunk inclination), and supine. This method assesses the chest wall in a three dimensional way considering the chest wall as three compartments: pulmonary rib cage, abdominal rib cage and abdomen. Technical details including marker positions, calibration processes and clinical applicability of the OEP system were previously published.12 In this study, 89 markers were used for the seated position and 52 markers for the inclined and supine positions, based on anatomical reference points.19,20
ProceduresData were collected in one or two days, based on the availability of the participant. Initially, clinical and demographic data were registered. The participants then answered the MMSE administered by a trained investigator. Next, they immediately underwent the pulmonary function test (Koko®, PFT type; nSpireHealth Inc., CO, USA or Micro Medical Microloop MK8, UK) and subsequently answered the Human Activity Profile (HAP) to register their physical activity level. The HAP adjusted activity score (AAS) was used in this study to classify the participants as inactive (AAS<53), moderately active (AAS between 53 and 74) and active (AAS>74).21
Next, the examiner placed 89 markers on the participants’ chest wall, and the OEP was calibrated according to the established protocol.12,20 Quiet breathing – defined as the participants’ own breathing pattern – was assessed for 5min in each of the three positions. Breathing pattern and chest wall motion were registered for 5min in seated position, followed by 5min in inclined and 5min in supine positions. Calibration of OEP was performed before changing each position.
Variables analyzedThe following variables were analyzed: chest wall tidal volume (Vcw); respiratory rate (RR); minute ventilation (VE); duty cycle (Ti/Ttot); end-inspiratory chest wall volume (Veicw); end-expiratory chest wall volume (Veecw); pulmonary rib cage percentage contribution (VRCp%); abdominal rib cage percentage contribution (VRCa%); and abdomen percentage contribution (VAB%). More details regarding the description of the variables were previously published.12
Data reductionTo determine the breathing pattern and chest wall motion variables, the middle 100s from the 5min registered were used.
Sample size calculationSample size calculation was determined for mixed factorial analysis of variance (ANOVA) and linear regression model. For ANOVA, GPower® version 3.1 was used. Considering an effect size of 0.25, an alpha error probability of 0.05 and a power of 0.80, the estimated sample size was 44 participants. For regression analysis, considering the 5 independent variables in the model (age, sex, delta seated-inclined, delta inclined-supine, and delta seated-supine), the equation n≥30+10k22 was used, which resulted in a sample of at least 80 participants. Therefore, the higher number of individuals required for regression analysis was used for the recruitment of the individuals.
Statistical analysisData were presented as measures of central tendency and dispersion, and the normality was verified by the Shapiro–Wilk test. The comparisons between sexes for demographic, spirometric, and clinical variables were performed by Student t test for independent samples, and Mann–Whitney or chi-square test, according to the characteristic and/or variable distribution.
To verify the influence of position and sex on breathing pattern and chest wall motion, we performed mixed factorial ANOVA with repeated measures in a split-plot design 3×2 (positions and sex). The results regarding the comparisons of breathing pattern and chest wall motion variables in different positions and between sexes were presented as deltas. This allows a dynamic analysis of the results, as opposed to comparing the absolute values found in each position in a static way. Post hoc analysis of the differences among positions (delta seated-inclined, delta inclined-supine, delta seated-supine) were performed by Bonferroni test.
A stepwise multiple linear regression model was used for assessing the influence of age and was analyzed together with position and sex. For bivariate analysis, we used the Spearman correlation test. The final model was determined from the adjusted coefficient of determination (R2) and by the statistical significance. To determine the statistical quality of the model, the absence of multicollinearity was verified by the variance inflation factor as well as the presence of homogeneity and normal distribution of residuals, by graphic visual analysis. The level of significance was set at 5%. The Statistical Package for the Social Sciences v 15.0 (Chicago, IL, USA) was used for analyses.
ResultsSampleInitially, 161 individuals were approached to participate in the study in the three centers, of whom 7 refused to participate and 14 did not attend the data collection. From the 140 remaining participants, 57 were not included: 13 presented BMI over 29.99kg/m2 and 2 had BMI below 18.50kg/m2; 20 showed abnormal pulmonary function test results; two reported respiratory or neurological disease; and 20 were excluded due to problems with OEP data acquisition. Thus, data of 83 individuals were included in the study.
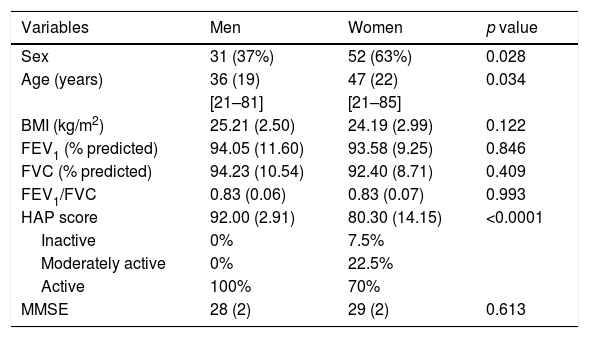
Table 1 presents demographic, anthropometric, spirometric, and clinical data of the individuals by sex. The sample comprised mostly women and compared with men, women were older. There was a significant difference in the HAP score between men and women, but it did not influence the classification as both were considered active.
Demographic, anthrophometric, spirometric, and clinical data of participants by sex.
| Variables | Men | Women | p value |
|---|---|---|---|
| Sex | 31 (37%) | 52 (63%) | 0.028 |
| Age (years) | 36 (19) | 47 (22) | 0.034 |
| [21–81] | [21–85] | ||
| BMI (kg/m2) | 25.21 (2.50) | 24.19 (2.99) | 0.122 |
| FEV1 (% predicted) | 94.05 (11.60) | 93.58 (9.25) | 0.846 |
| FVC (% predicted) | 94.23 (10.54) | 92.40 (8.71) | 0.409 |
| FEV1/FVC | 0.83 (0.06) | 0.83 (0.07) | 0.993 |
| HAP score | 92.00 (2.91) | 80.30 (14.15) | <0.0001 |
| Inactive | 0% | 7.5% | |
| Moderately active | 0% | 22.5% | |
| Active | 100% | 70% | |
| MMSE | 28 (2) | 29 (2) | 0.613 |
Values are expressed as mean (standard deviation), except sex (%). Minimal and maximal values for age are shown in brackets. BMI, body mass index; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1 to FVC; HAP, Human Activity Profile; MMSE, Mini-Mental State Examination.
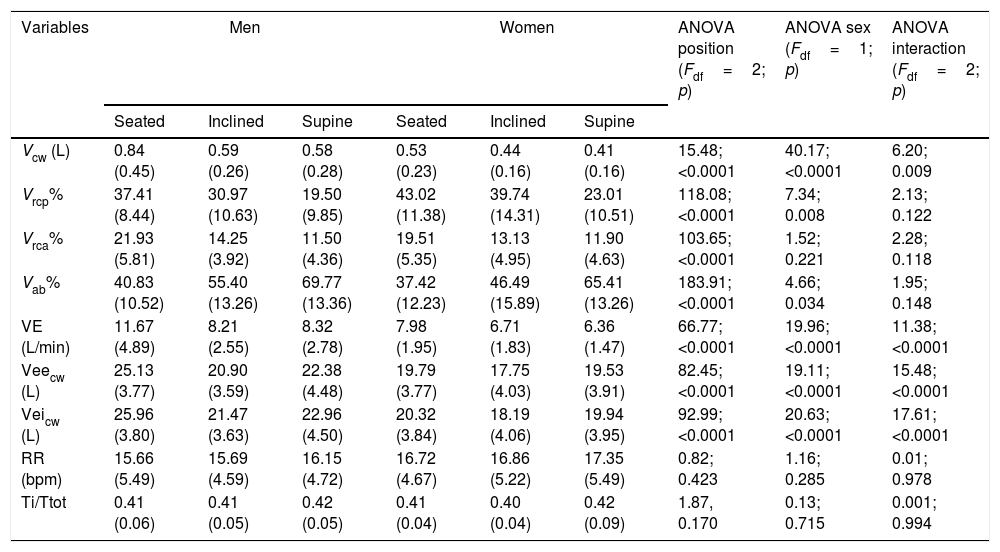
Table 2 presents breathing pattern and chest wall motion data in men and women in the three positions. We observed significant differences between the sexes for the variables VRCp% and VAB% and among positions (seated, inclined, and supine) for the variables VRCp%, VRCa%, and VAB%. In addition, there was an interaction effect (sex×position) for the variables Vcw, VE, Veicw, and Veecw. Neither sex nor position significantly influenced RR or Ti/Ttot.
Analysis of breathing pattern and chest wall motion variables by sex in three corporal positions.
| Variables | Men | Women | ANOVA position (Fdf=2; p) | ANOVA sex (Fdf=1; p) | ANOVA interaction (Fdf=2; p) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Seated | Inclined | Supine | Seated | Inclined | Supine | ||||
| Vcw (L) | 0.84 (0.45) | 0.59 (0.26) | 0.58 (0.28) | 0.53 (0.23) | 0.44 (0.16) | 0.41 (0.16) | 15.48; <0.0001 | 40.17; <0.0001 | 6.20; 0.009 |
| Vrcp% | 37.41 (8.44) | 30.97 (10.63) | 19.50 (9.85) | 43.02 (11.38) | 39.74 (14.31) | 23.01 (10.51) | 118.08; <0.0001 | 7.34; 0.008 | 2.13; 0.122 |
| Vrca% | 21.93 (5.81) | 14.25 (3.92) | 11.50 (4.36) | 19.51 (5.35) | 13.13 (4.95) | 11.90 (4.63) | 103.65; <0.0001 | 1.52; 0.221 | 2.28; 0.118 |
| Vab% | 40.83 (10.52) | 55.40 (13.26) | 69.77 (13.36) | 37.42 (12.23) | 46.49 (15.89) | 65.41 (13.26) | 183.91; <0.0001 | 4.66; 0.034 | 1.95; 0.148 |
| VE (L/min) | 11.67 (4.89) | 8.21 (2.55) | 8.32 (2.78) | 7.98 (1.95) | 6.71 (1.83) | 6.36 (1.47) | 66.77; <0.0001 | 19.96; <0.0001 | 11.38; <0.0001 |
| Veecw (L) | 25.13 (3.77) | 20.90 (3.59) | 22.38 (4.48) | 19.79 (3.77) | 17.75 (4.03) | 19.53 (3.91) | 82.45; <0.0001 | 19.11; <0.0001 | 15.48; <0.0001 |
| Veicw (L) | 25.96 (3.80) | 21.47 (3.63) | 22.96 (4.50) | 20.32 (3.84) | 18.19 (4.06) | 19.94 (3.95) | 92.99; <0.0001 | 20.63; <0.0001 | 17.61; <0.0001 |
| RR (bpm) | 15.66 (5.49) | 15.69 (4.59) | 16.15 (4.72) | 16.72 (4.67) | 16.86 (5.22) | 17.35 (5.49) | 0.82; 0.423 | 1.16; 0.285 | 0.01; 0.978 |
| Ti/Ttot | 0.41 (0.06) | 0.41 (0.05) | 0.42 (0.05) | 0.41 (0.04) | 0.40 (0.04) | 0.42 (0.09) | 1.87, 0.170 | 0.13; 0.715 | 0.001; 0.994 |
Values are expressed as mean (standard deviation). Abbreviations: Vcw, chest wall volume; VRCp%, percentage contribution of the pulmonary rib cage; VRCa%, percentage contribution of the abdominal rib cage; VAB%, percentage contribution of the abdomen; VE, minute ventilation, Veecw, end-expiratory chest wall volume; Veicw, end-inspiratory chest wall volume; RR, respiratory rate; Ti/Ttot, duty cycle.
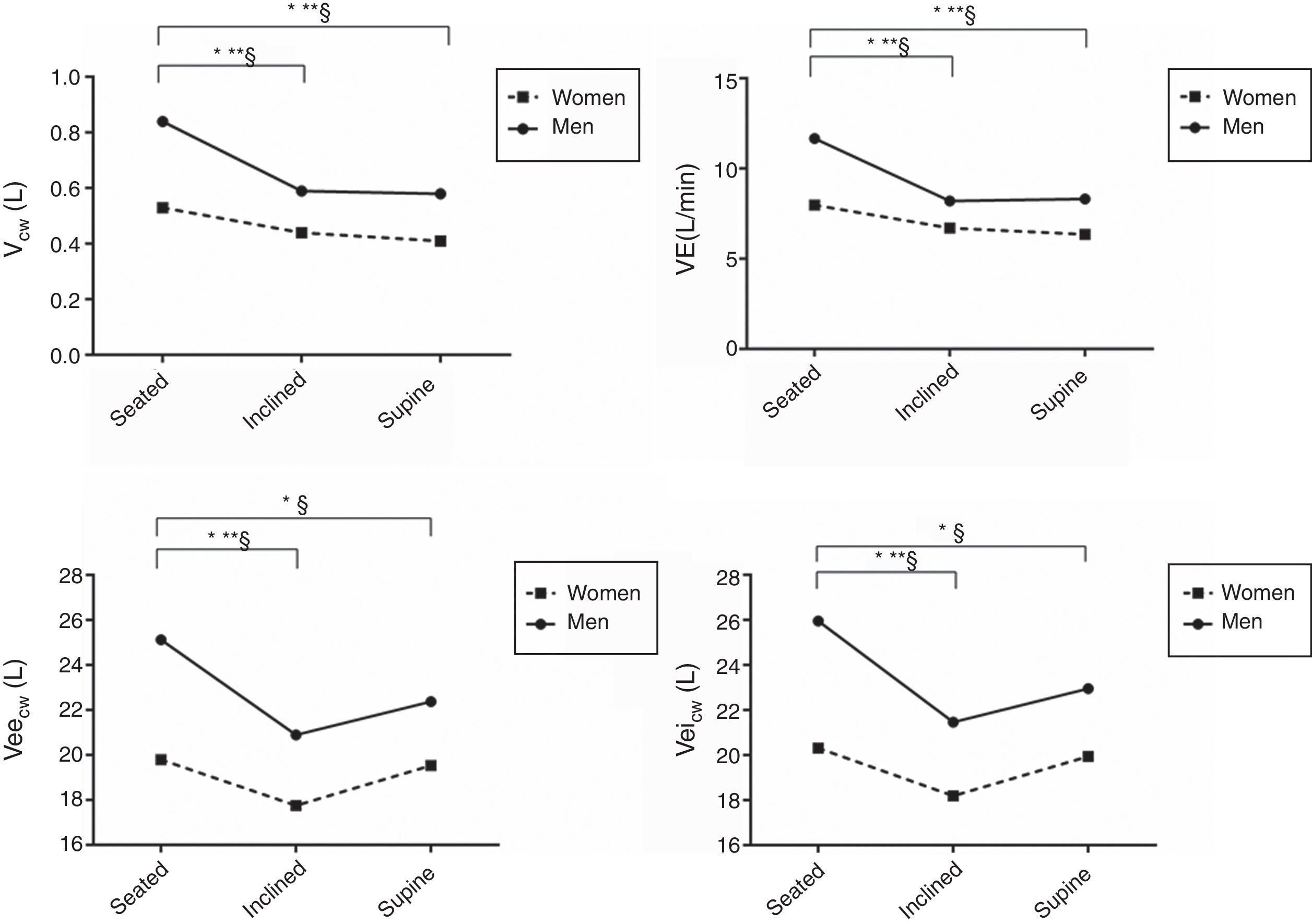
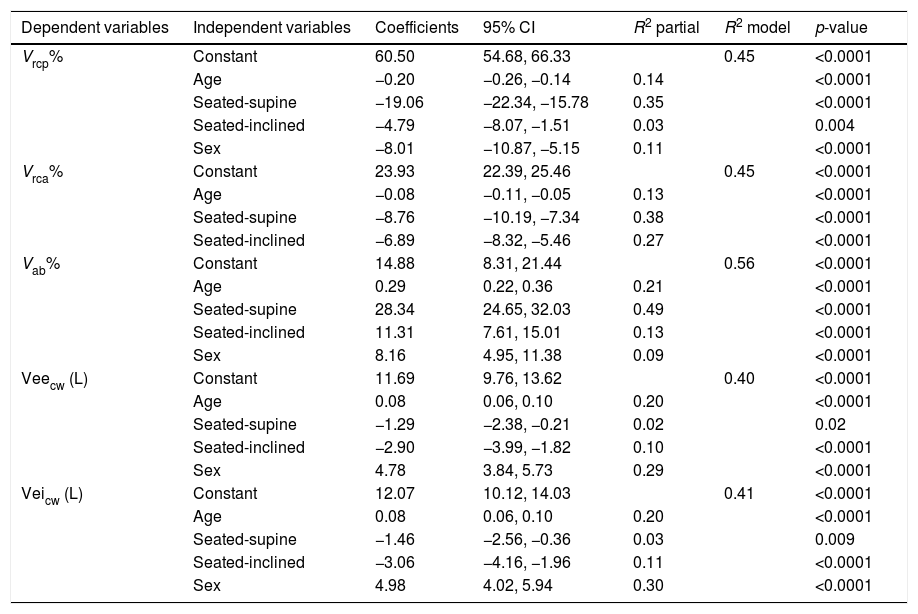
Fig. 1 presents the results for the influence of posture and sex on breathing pattern. There were interaction effects for all variables, except for RR and Ti/Ttot. The Vcw and VE were significantly reduced in both sexes for positions seated to inclined and for seated to supine, with a greater reduction mainly in males. The variables Veicw and Veecw were significantly decreased from seated to inclined positions in both sexes, mainly in males and from seated to the supine position only in males. The variables RR and Ti/Ttot were not influenced by sex or position.
Influence of posture and sex on breathing pattern.
Data are presented as mean values. Abbreviations: Vcw, chest wall tidal volume; VE, minute ventilation; Veicw, end-inspiratory chest wall volume; Veecw, end-expiratory chest wall volume. *, significant difference for men between posture; **, significant difference for women; §, significant difference between sexes.
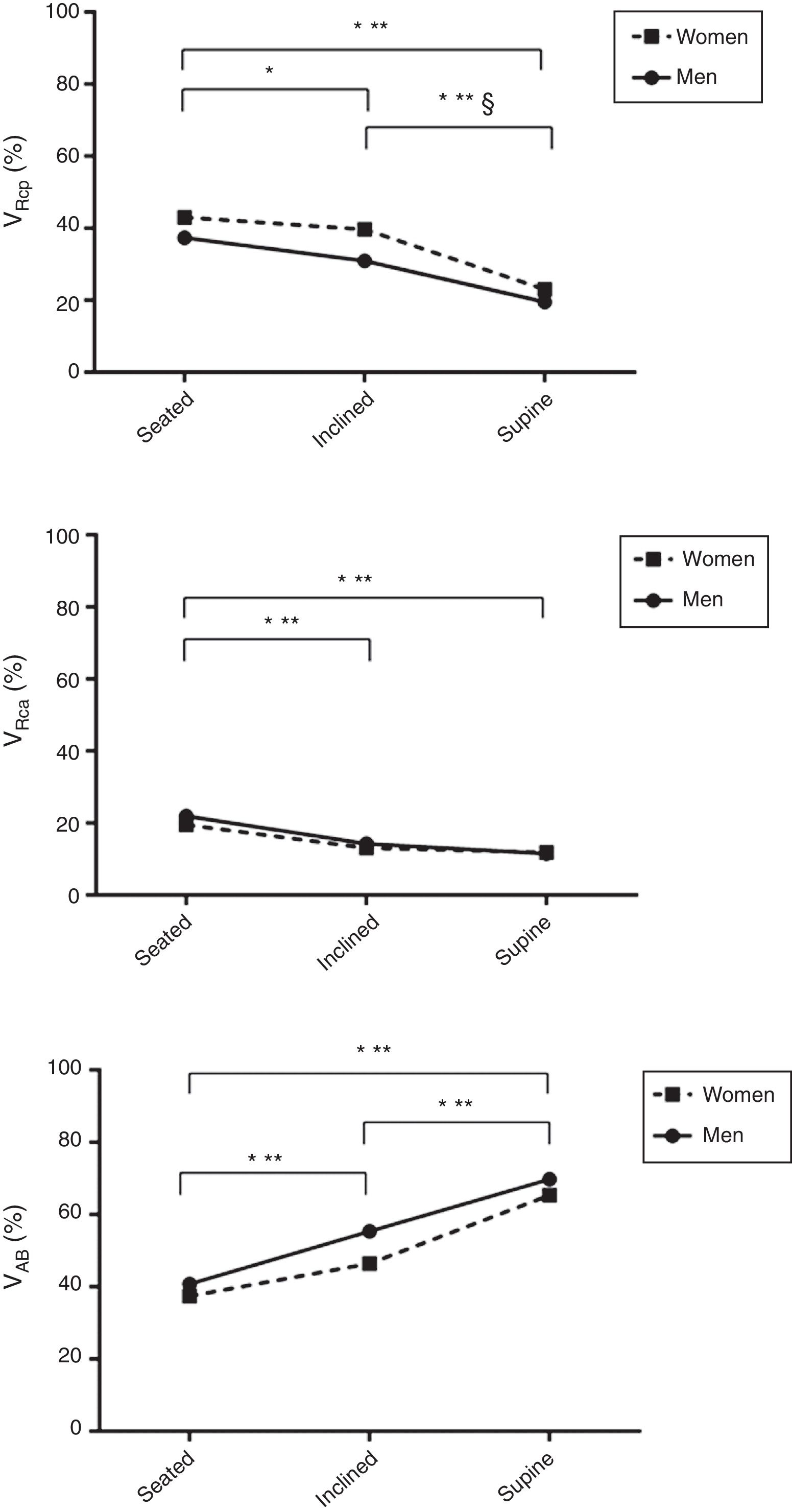
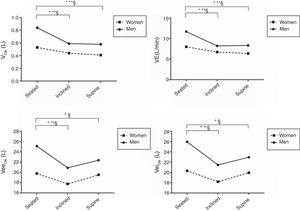
Fig. 2 presents the influence of posture and sex on chest wall motion variables. From seated to supine and from inclined to supine positions VRCp% was significantly reduced in both sexes, whereas from seated to the inclined position, we observed a reduction of VRCp% only in men. From the inclined to the supine position, women presented a greater reduction in VRCp% than men. Regarding VRCa%, a significant reduction was observed from seated to inclined as well as from seated to supine positions, in both sexes without other changes. The VAB% significantly increased with trunk horizontalization (from seated to inclined and to supine) in both sexes.
Influence of posture and sex on chest wall motion.
Data are presented as mean values. Abbreviations: VRCp%, pulmonary rib cage percent contribution; VRCa%, abdominal rib cage percent contribution; VAB%, abdomen percent contribution. *, significant difference for men; **, significant difference for women; §, significant difference between sexes.
Besides the groups were significantly different for HAP score, there was no correlation between the physical activity level and most of the analyzed variables, except for VRCa% in the inclined position (r=0.294; p=0.028), VAB% in the supine position (r=−0.281; p=0.032), RR in the supine position (r=−0.285; p=0.032) and VE in the seated position (r=0.271; p=0.042), where significant correlations, however, weak, were observed.
Influence of ageTable 3 presents the influence of age adjusted for sex and position, on breathing pattern and chest wall motion. Age significantly influenced VRCp%, VRCa%, VAB%, Veicw, and Veecw. Between 21 and 85 years, for each year of increase in age, we observed an average reduction of 0.20% in VRCp% and 0.08% in VRCa% which was associated with an increase of 0.29% in VAB%. With regard to the values for Veicw and Veecw, we observed an increase of 0.08% for each year of increase in age.
Models obtained by regression analysis for the influence of age on breathing pattern and chest wall motion variables.
| Dependent variables | Independent variables | Coefficients | 95% CI | R2 partial | R2 model | p-value |
|---|---|---|---|---|---|---|
| Vrcp% | Constant | 60.50 | 54.68, 66.33 | 0.45 | <0.0001 | |
| Age | −0.20 | −0.26, −0.14 | 0.14 | <0.0001 | ||
| Seated-supine | −19.06 | −22.34, −15.78 | 0.35 | <0.0001 | ||
| Seated-inclined | −4.79 | −8.07, −1.51 | 0.03 | 0.004 | ||
| Sex | −8.01 | −10.87, −5.15 | 0.11 | <0.0001 | ||
| Vrca% | Constant | 23.93 | 22.39, 25.46 | 0.45 | <0.0001 | |
| Age | −0.08 | −0.11, −0.05 | 0.13 | <0.0001 | ||
| Seated-supine | −8.76 | −10.19, −7.34 | 0.38 | <0.0001 | ||
| Seated-inclined | −6.89 | −8.32, −5.46 | 0.27 | <0.0001 | ||
| Vab% | Constant | 14.88 | 8.31, 21.44 | 0.56 | <0.0001 | |
| Age | 0.29 | 0.22, 0.36 | 0.21 | <0.0001 | ||
| Seated-supine | 28.34 | 24.65, 32.03 | 0.49 | <0.0001 | ||
| Seated-inclined | 11.31 | 7.61, 15.01 | 0.13 | <0.0001 | ||
| Sex | 8.16 | 4.95, 11.38 | 0.09 | <0.0001 | ||
| Veecw (L) | Constant | 11.69 | 9.76, 13.62 | 0.40 | <0.0001 | |
| Age | 0.08 | 0.06, 0.10 | 0.20 | <0.0001 | ||
| Seated-supine | −1.29 | −2.38, −0.21 | 0.02 | 0.02 | ||
| Seated-inclined | −2.90 | −3.99, −1.82 | 0.10 | <0.0001 | ||
| Sex | 4.78 | 3.84, 5.73 | 0.29 | <0.0001 | ||
| Veicw (L) | Constant | 12.07 | 10.12, 14.03 | 0.41 | <0.0001 | |
| Age | 0.08 | 0.06, 0.10 | 0.20 | <0.0001 | ||
| Seated-supine | −1.46 | −2.56, −0.36 | 0.03 | 0.009 | ||
| Seated-inclined | −3.06 | −4.16, −1.96 | 0.11 | <0.0001 | ||
| Sex | 4.98 | 4.02, 5.94 | 0.30 | <0.0001 |
Values are coefficients, confidence intervals, and determination coefficients (R2). VRCp%, percentage contribution of the pulmonary rib cage; VRCa%, percentage contribution of the abdominal rib cage; VAB%, percentage contribution of the abdomen; Veecw, end-expiratory chest wall volume; Veicw, end-inspiratory volume chest wall volume; 95% CI, confidence interval.
The main results of this study were: (1) Aging caused reductions in rib cage contributions to tidal volume that were compensated by increases in abdomen contributions. In addition, increases in Veicw and Veecw with increasing age were observed. (2) Posture influenced all variables of breathing pattern and chest wall motion, except RR and Ti/Ttot. (3) Women presented higher thoracic contribution to tidal volume than men.
Currently, the literature is consistent in suggesting that age does not cause changes in the breathing pattern and chest wall motion of healthy subjects at rest,1,4,7 despite the structural and physiological changes that occur in the respiratory system with aging.23,24 Contrary to results presently available in the literature,1,4,7 our study suggests that chest wall motion is influenced by age. The main alteration of the rib cage related to aging is the reduction of its compliance. This reduction is possibly related to the calcification of costal cartilage and costovertebral joints, as well as the narrowing of the intervertebral spaces.23,25 These changes can explain the average volume reduction observed in the two rib cage compartments – 0.20% in the VRCp% and 0.08% in the VRCa% – for each year of age increase. However, this reduction is compensated by an average increase in 0.29% in VAB%, which explains the absence of influence of age on the variable Vcw found in other studies.1,4,7
In the senile lung, structural changes in connective tissue due to changes in the proportions of collagen and elastin, result in loss of pulmonary elastic recoil, leading to progressive air retention.23,25 These changes may explain the mean 0.08% increase in Veicw and in Veecw for each year of age observed in our study. To our knowledge, this is the first study to provide data on the influence of age in Veicw and Veecw. Other authors have investigated the impact of age on the variables of breathing pattern; however, those studies were performed by inductive plethysmography that does not allow the analysis of these volumes.1,4,7
Previous studies have shown that moving from seated to the supine position reduces the lung compliance and increases static and dynamic resistance and pulmonary elastance.3,26 Barnas et al.11 demonstrated that the change from seated to supine position increases pulmonary elastance by about 24% and lung resistance and total resistance of the respiratory system (defined as resistance of lungs and chest wall components) by around 40%–50%. These findings can explain the reduction of Vcw when changing from seated to inclined and from seated to supine positions. These results were similar to the ones reported by Romei et al.9 concerning the reduction of trunk verticalization. Our results are also in agreement with this study which reported a significantly higher reduction of the Vcw in men with trunk horizontalization compared to women. According to some authors,27,28 lung compliance between men and women is similar. However, to our knowledge, there are no studies that evaluated the influence of posture on the lung compliance of men and women.
Considering the reduction of Vcw from seated to inclined and from seated to supine, a similar VE response was expected, since RR is not influenced by posture and sex. Our results were similar to those found by Romei et al.9 who also observed a greater reduction of VE in men than in women. Kilbride et al.29 showed that at rest the VE in women is lower than in men, and this is entirely due to the lower tidal volume in women.
The RR was not influenced by sex or change of position. Our results are in agreement with previous studies that also did not observe any influence of posture8,9 or of sex7,9 on RR. Concerning Ti/Ttot, no literature was found concerning the influence of posture on this variable. However, the values observed in the present study are similar to the ones reported by Tobin et al.30 (0.42±0.03) in the supine position, as well as by Parreira et al.7 (average of 0.39) in healthy individuals aged 20–80 years.
The variables Veicw and Veecw presented a significant reduction when moving from the seated to inclined position in both sexes and from seated to the supine position, but only in males. The reduction for both positions was more pronounced in males. The decrease of Veicw could be associated with the reduction observed for Vcw from the seated to supine position which was more pronounced in males. In addition, the lower Veicw observed in the supine position could be explained by the increase in pulmonary elastance and lung resistance.11 On the other hand, we can hypothesize that the reduction of Veecw from the seated to supine position may reflect the smaller residual functional capacity in the supine position.31
Regarding chest wall motion, our results are also in agreement with previous studies, which showed by different methods that in the upright or seated position, the relative contribution of the rib cage to tidal volume is higher than in the supine position, and consequently the contribution of the abdomen to tidal volume is lower in the most upright position of the trunk.1,3,5,8–10 The increase in VAB% when moving from seated to inclined, from seated to supine, and from inclined to supine positions in both men and women is explained by the elastic properties of the rib cage and abdomen. During quiet breathing in the standing posture, the abdomen is as compliant as the rib cage. In the seated position, the weight of the abdominal contents distends the abdominal wall, and consequently the elastance of the diaphragm and the abdomen becomes larger, thus reducing the contribution of this compartment to the tidal volume. In the supine position, only the abdomen varies its static characteristic by the increase of its compliance.32
Regarding the effect of sex, women presented a higher thoracic contribution than men, similar to the results found by Romei et al.9 Binazzi et al.33 by considering esophageal and gastric pressures demonstrated that women have a greater contribution of rib cage muscles than men. By using chest X-rays, these authors observed that women have a lower radial dimension of RC in relation to height than men and a greater inclination of the ribs. The reduction of VRCp% with trunk horizontalization occurred between all positions, except in women during the transition from seated to inclined. Consequently, probably due to this factor women showed a greater reduction of this variable in the change of position from inclined to supine positions than men.
The VRCa% was also reduced with trunk horizontalization, except in the change from the inclined posture to supine. Priori et al.8 and Magalhães et al.34 also observed a significant reduction of this variable in healthy individuals, when comparing the sitting position with the supine. However, in these studies, the authors evaluated the responses between supine and seated positions only, thereby preventing a comparison with the results obtained in the present study regarding inclined positions. On the other hand, Romei et al.9 analyzed the rib cage response as a whole (VRCp%+VRCa%) and results for VRCa% with the trunk horizontalization were not presented.
The action of the diaphragm on the rib cage has two components. The first is related to the apposition zone. During inspiration, the increase in intra-abdominal pressure is transmitted through the attached diaphragm to expand the lower part of the rib cage. Its magnitude depends on the size of the apposition zone and on the magnitude of intra-abdominal pressure. The second component is related to the insertion of the muscles in the lower ribs. When the diaphragm contracts, it exerts a cranially oriented force on the lower ribs that has the effect of raising them and turning them outwards. However, for this mechanism to work correctly, the muscle fibers of the diaphragm must be oriented cranially, and the abdominal contents should effectively oppose diaphragm descent. If the resistance is small, the dome of the diaphragm descends easily during inspiration; at the same time, the apposition zone decreases in size, and the abdominal pressure increases only slightly, causing the two action components of the diaphragm to have a small action on the rib cage.35,36 Priori et al.8 demonstrated by means of ultrasonography that the movement of the diaphragm apposition zone of healthy individuals does not differ between the seated and supine positions. Nonetheless, in the supine position, the resistance offered by the abdominal contents to the descent of the diaphragm dome is smaller than that in the seated position. In this way, the diaphragm has a small action on the rib cage, thus reducing the VRCp% and the VRCa%.
One limitation of the present study is the fact that volume values were obtained indirectly, without association with a direct measurement by a pneumotachograph. Therefore, they cannot be used as absolute values. In addition, the sample consisted mostly of women, and the imbalance of the number of participants in the age groups did not allow a more detailed study of the effect of age on variables of breathing pattern and chest wall motion. However, the sample size had sufficient power to study the average influence of age between 21 and 85 years. Another limitation is the fact that the positions were not randomized in order to optimize data collection time regarding to the process to place the markers and to calibrate the system.
In summary, the results of the present study suggest that posture, sex, and age influence the breathing pattern and the chest wall motion of healthy individuals at rest. The sitting position can benefit patients who present with reduced tidal volume for different reasons, such as pain, postoperative lung volume changes, pulmonary collapse or any other restriction. Regarding respiratory rate and duty cycle, any of the studied positions produced a significant reduction or increase of these parameters.
The rib cage contribution to tidal volume is greater in vertical postures, whereas the abdomen contribution is greater in horizontal postures. The demonstration that the degree of contribution of the compartments is dependent on posture could be useful for orientation of positioning in patients with specific expansion reduction in some compartments, such as cardiac and bariatric postoperative surgery.
The reduction of the rib cage contribution to the tidal volume with aging may encourage the development of strategies to minimize the volume loss of this compartment. Future studies to investigate whether interventions aiming to increase rib cage expansion would minimize this loss may be relevant.
Conflicts of interestThe authors declare no conflicts of interest.
The work was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant 309990/2017-3 and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The funding sources had no role in the design, conduct or reporting of this study.