Impairments of sensorimotor control relating to head and eye movement control and postural stability are often present in people with neck pain. The upper cervical spine and particularly the obliquus capitis inferior (OCI) play an important proprioceptive role; and its impairment may alter cervical sensorimotor control. Dry needling (DN) is a valid technique to target the OCI.
ObjectivesTo investigate if a single DN session of the OCI muscle improves head and eye movement control-related outcomes, postural stability, and cervical mobility in people with neck pain.
MethodsForty people with neck pain were randomly assigned to receive a single session of DN or sham needling of the OCI. Cervical joint position error (JPE), cervical movement sense, standing balance and oculomotor control were examined at baseline, immediately post-intervention, and at one-week follow-up. Active cervical rotation range of motion and the flexion rotation test were used to examine the global and upper cervical rotation mobility, respectively.
ResultsLinear mixed-models revealed that the DN group showed a decrease of JPE immediately post-intervention compared to the sham group (mean difference [MD]= -0.93°; 95% confidence interval [CI]: -1.85, -0.02) which was maintained at one-week follow-up (MD= -1.64°; 95%CI: -2.85, -0.43). No effects on standing balance or cervical movement sense were observed in both groups. Upper cervical mobility showed an increase immediately after DN compared to the sham group (MD= 5.14°; 95%CI: 0.77, 9.75) which remained stable at one-week follow-up (MD= 6.98°; 95%CI: 1.31, 12.40). Both group showed an immediate increase in global cervical mobility (MD= -0.14°; 95%CI: -5.29, 4.89).
ConclusionThe results from the current study suggest that a single session of DN of the OCI reduces JPE deficits and increases upper cervical mobility in patients with neck pain. Future trials should examine if the addition of this technique to sensorimotor control training add further benefits in the management of neck pain.
Impairments of sensorimotor control relating to head and eye movement control and postural stability are often observed in people with persistent neck pain regardless of the symptoms’ onset.1–3 These impairments, which are thought to be related to altered cervical input and the subsequent changes to sensorimotor integration of combined visual, vestibular, and proprioceptive information, seem to contribute to some extent to the patient's symptoms.1 Thus, identifying and targeting these deficits are recommended as part of the management of neck pain disorders.4,5 Tailored training directed towards head and eye movement control disturbances have shown to improve sensorimotor function as well as patients’ symptoms.4,6–10 However, addressing the local source of altered cervical afferent input (i.e. reduced cervical mobility or impaired muscle function) is thought to be also important for treatment success.4,6–8,10,11
Previous research has shown greater head and eye movement control and postural stability impairments in those people with neck pain presenting with an upper cervical spine dysfunction.12,13 The upper cervical spine contains a great abundance of cervical afferents,14 with the obliquus capitis inferior (OCI) muscle having the greatest density of muscle spindle compared to other cervical muscles.15,16 Moreover, electromyographic research suggests a primary proprioceptive role for the OCI muscle as it contributes more to head and eye movement control than providing a strong directional torque in neck rotation.17 These findings indicate that OCI impairment (e.g., lack of extensibility) may contribute to the above sensorimotor disturbances often observed in people with neck pain.
Due to its deep location in the upper cervical region, dry needling (DN) is probably the only feasible technique for successfully targeting the OCI.18,19 DN has been shown to be an effective technique to reduce pain and disability as well as improve cervical mobility in the short-term in patients with neck pain.20–22 Some research indicates that DN improve muscle function22–25; but studies on the effects of DN on cervical sensorimotor control are lacking. We hypothesized that DN of the OCI improves cervical sensorimotor control in people with neck pain possibly by increasing cervical afferent input. Consequently, the aim of this study was to investigate the short-term effects of a single DN session of the OCI muscle on head and eye movement control-related outcomes and postural stability in people with neck pain. In addition, this study also explored the effects of DN of the OCI on both global and upper cervical mobility.
MethodsTrial designThis study was a double-blind, parallel randomized sham-controlled trial. Research methods and reporting were in accordance with the STRICTA (extension of CONSORT for acupuncture studies) guidelines.26 This study was prospectively registered (NCT03838224).
ParticipantsForty participants with either traumatic or idiopathic neck pain were recruited from the metropolitan area of Valencia (Spain) through poster advertisement between March and June 2019. Volunteers were screened for potential eligibility via phone prior to the baseline assessment. Participants were included if they were 18–65 years old and had neck pain as defined by the International Association for the Study of Pain ≥3 months in the last year with a current pain intensity ≥30/100 mm on a visual analogue scale (VAS) and neck disability ≥10/50 on the Neck Disability Index (NDI).27,28 Participants also had to exhibit impaired cervical joint position error (JPE) in at least one direction of neck rotation determined by a cut-off value of 4.5°.29 Participants were excluded if they had a history of head trauma, cervical fracture, stenosis, surgery, or neurological signs/deficits suggesting nerve root compression as well as known or suspected vestibular pathology and vertigo or dizziness from ear or brain disorders or sensory nerve pathways (e.g. BPPV). Additionnaly, pregnancy, bleeding disorders, use of anticoagulant medication, or needle phobia as well as previous experience with DN treatment in the upper cervical region were reasons for exclusion.
The study was approved by the Ethical Committee at the University of Valencia, Spain (reference number H1542206264486)) and all procedures were performed in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to participation. Data collection and treatment took place at the department of physical therapy, University of Valencia
InterventionsBoth interventions were performed by the same physical therapist with 3 years of DN experience. A 40 × 0.32 mm sterile needle with guided tube (AGU-A1041P, Agu-punt S.L., Spain) was used for DN whereas the Park sham device (DongBang, AcuPrime, UK), a blunted needle which appearance is similar to a DN needle, was used for sham needling.19,30 This sham needle has been previously validated and used as control in previous DN trials in patients with neck pain.31,32 For the DN intervention, the needle was inserted into the OCI muscle following a previously described and validated approach.19 The needle was inserted perpendicular to the skin at a mid-point between the spinous process of C2 and the transverse process of C1 (supplemental online material). Then, the needle formed an angle of 45° with the spinous process of C2 and the transverse process of C1 and was directed into a postero-anterior direction towards the anatomical location of the OCI with a slight inferior angle of 10° until reaching the C2 vertebra lamina. Once the first local twitch response was obtained, the needle was rapidly moved up and down within the muscle using the “fast-in and fast-out” technique described by Hong33 for a total of 12 insertions. As previous DN studies evaluating changes in muscle function, DN was performed bilaterally.23,34 A similar procedure to DN was adopted for the sham intervention to replicate an authentic clinical experience and maintain credibility and participants' blinding.35 Contextual clues associated with DN such as skin's cleaning, needle insertion, and manipulation (simulation in sham needling), and haemostatic compression after procedure were therefore identical in both interventions.
OutcomesCervical JPE was the primary outcome. Secondary sensorimotor related outcomes were cervical movement sense, the smooth pursuit neck torsion test (SPNT), and standing balance. Global cervical rotation range of motion (ROM), the flexion-rotation test (FRT) and pain intensity were also evaluated as secondary outcome measures. Measurements were taken at baseline, immediately post-intervention, and at one-week follow-up. Outcome assessment was blinded to treatment allocation.
Head and eye movement control-related measuresCervical JPE has been suggested to evaluate cervical propioception or kinesthesis.36 This was measured using a laser-pointer mounted onto a lightweight headband with the participants sitting blindfolded 90 cm away from a wall.29,36 Participants were asked to slowly perform full head rotation to limit vestibular input and return to the neutral position as accurately as possible and verbally indicate when they felt they were back in the neutral position.37 The examiner manually repositioned the participant's head after each trial to realign the laser-pointer with the starting position. The difference between start and end positions was measured in centimeters and converted into degrees.29,36 Six trials to each side were performed and the mean was calculated to reduce the vulnerability to outliers.38,39 The impaired side (i.e. right or left) was taken for the analysis when JPE ≥ 4.5° was only found in one direction and the mean was calculated when both sides were ≥4.5°. A moderate-good (0.71) between-day reliability has been reported for this procedure and the minimal detectable change (MDC) is −0.51°.40,41
Cervical movement sense was evaluated using the zigzag pattern (see Werner, Ernst, Treleaven, Crawford42), which is proposed to examine cervical proprioceptive or kinaesthetic afferent input as well as visuomotor function.43 Participants were sitting with the laser-pointer attached to their forehead 100 cm away from the pattern. They were asked to trace the main bold band of the pattern “as accurately as possible” in a clockwise direction to start and end in the center of the pattern.42 One familiarization trial was allowed. The test was filmed and the number and magnitude of errors and time were analyzed using SMIPlayer.42 This procedure has shown excellent intra-rater reliability (>0.90).42,43
The clinical assessment of the SPNT proposed by Daly, Giffard, Thomas, Treleaven44 was used to evaluate oculomotor control. Patients were sitting on a swivel chair. The examiner, who was at 1 m distance, moved a pen horizontally across the patient's visual field in a range of 40° at a speed of approximately 20°/s. Participants performed first the test in a neutral position (trunk and neck forward); followed by 45° trunk torsion to each side with the head fixed by the examiner. The examiner carefully observed the pursuit of the patient's eyes in each position and rated according to the score described by Della Casa and colleagues45 and adapted by Daly et al.44 The test was positive when more saccadic eye movements (excluding the outer limits and directional changes) were detected in either left and or right torsion when compared to neutral.
Postural stabilityStanding balance evaluation consisted of one 30 s trial for each of four test conditions; firm and soft surface (high-density 9 cm thick foam rubber) with eyes open and eyes closed. These test conditions were selected because they are suggested to examine static balance disturbances due to cervical proprioceptive dysfunction rather than vestibular function and for their ability to demonstrate altered stability in people with neck pain.46,47 A force platform (Dinascan/IBV, Biomechanics Institute of Valencia, Spain) with a plate (600 × 370 × 100 mm) comprised of four force transducers was used. Participants were requested to take a comfortable standing position (feet shoulder-width apart in an angle of 20°) and look at an eye level reference point located 2 m in front. Signals were recorded with 40Hz-frequency by an amplified analogue-to-digital converter. The center of pressure displacement data were obtained in antero-posterior (AP) direction using NedSVE/IBV analysis software (Biomechanics Institute of Valencia, Spain).
Cervical mobilityA CROM device (Performance Attainment Associates, USA) was used to evaluate both tests of cervical mobility. To measure global cervical rotation ROM, patients were sitting on a chair, with the back supported on the backrest and the shoulders relaxed with the arms resting on their thighs. Then, they were requested to perform an active complete pain-free cervical rotation.48
The FRT was used to evaluate the rotation mobility of C1-C2 with the participants lying supine on a plinth.49 The examiner first passively pre-positioned participants’ neck in maximal full flexion and then rotated the head to each side. The end of the movement was determined either by a firm resistance felt by the examiner or participant's pain. Each of the cervical mobility test was repeated twice to each side and the mean of both repetitions and sides was calculated for the analysis.48 An excellent between-day reliability has been reported for the global cervical rotation ROM and the FRT (>0.9) and the MDC is 7.6° and 7.0° respectively.48,50,51
Neck pain intensityCurrent neck pain intensity was scored at baseline and at one-week follow-up using a VAS/100 mm with “no pain” on the left side and ‘‘maximum pain ever experienced’’ on the right side. Neck pain intensity was not assessed at post-intervention since post-needling soreness may have biased patients’ perceptions on pain. The VAS has shown an excellent between-day reliability and the minimal clinically meaningful change for patients with neck pain is 24 mm.52,53
Sample sizeSample size was determined using G*Power 3.1.9.2. and calculated based on a significance level of 0.05 and a power of 80% to detect a difference of 2° in cervical JPE based on previous data.6 Following these criteria, at least 16 participants were required per group; and so, 40 participants in total were included, accounting for a drop-out rate of 20%.
Randomization and blindingFollowing the baseline assessment, patients were randomly assigned to each group. Randomization was achieved using a computer-generated sequence of numbers, created prior to data collection by an independent researcher, which were concealed in sealed and opaque envelopes. The therapist, who was blinded to baseline assessment results, opened the envelope and proceeded according to the group allocation. Outcome assessment and data analysis were conducted by a researcher who was blinded to participant's treatment allocation. Participants were blinded to group allocation and were instructed to not reveal any treatment experience to the outcome assessment examiner. Participants blinding was evaluated at one-week follow-up with a written form and the Bang's blinding index (BI) with a 2(DN and sham) x 3(DN, sham, and do not know) format was calculated.54
Statistical analysisStatistical analysis was performed using SPSS statistics V25.0 and conducted according to an intention-to-treat approach. Inferential analysis including parametric and chi square tests were used to examine baseline between-group differences in patient's characteristics. Linear mixed-models with repeated-measures analysis, random effect models, and restricted maximum likelihood were used to model the intervention effect over time for primary and secondary outcome measures. We modeled the random effects of individuals and fixed effects of group (DN and Sham), time (baseline, post-intervention, and one-week follow-up), and group x time. All randomized participants were included in the analysis because the linear mixed-model estimates values for missing data.55 Pairwise comparisons with Bonferroni adjustment were used when interaction effect group x time or time was significant and change scores (compared with baseline) for post-intervention and one-week follow-up were calculated to examine if MDC was exceeded. Percentages of positive SNPT per group per timepoint were reported.
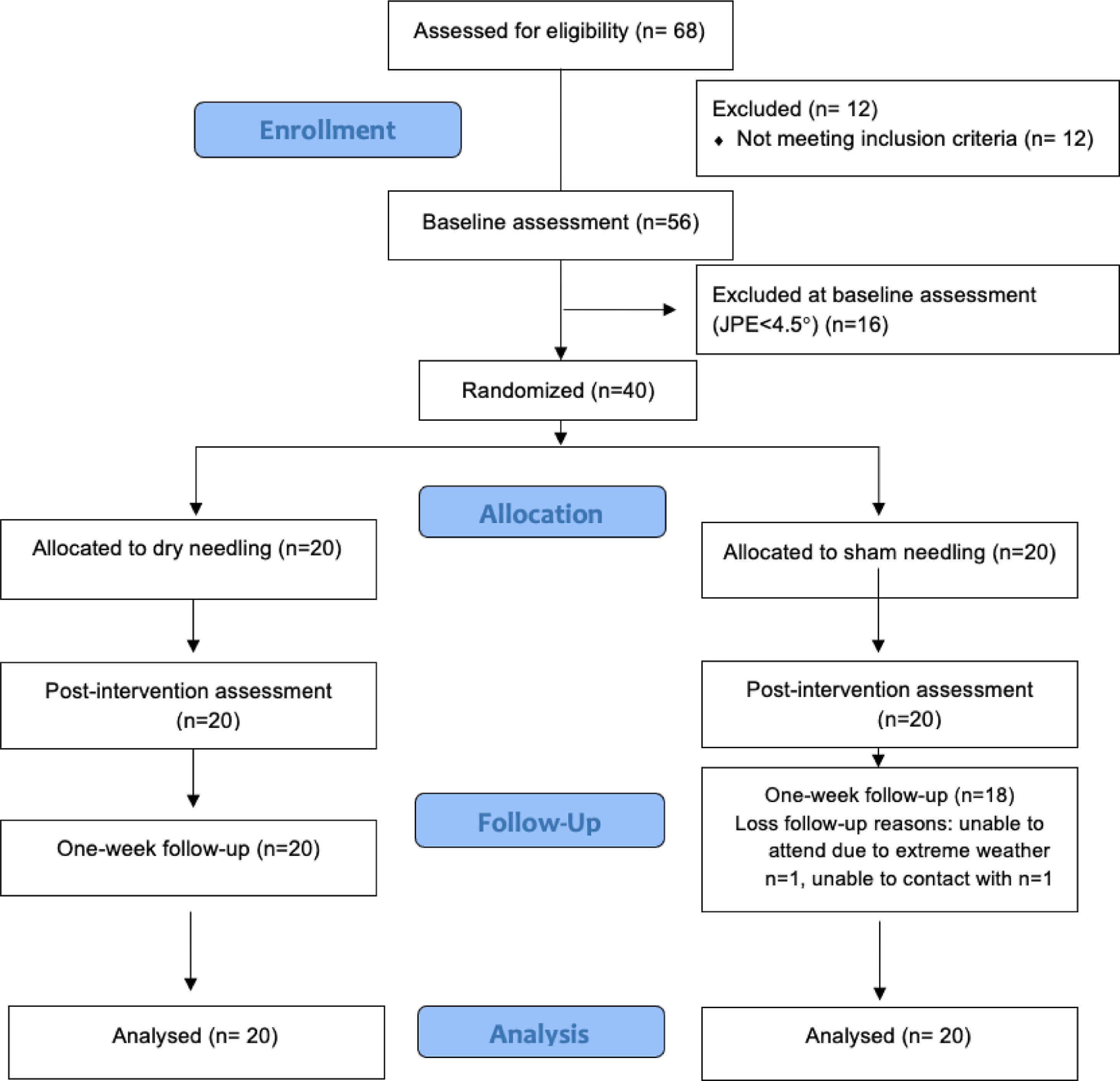
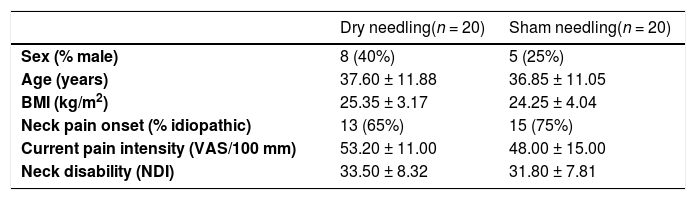
ResultsOf the 68 potential participants who were eligible, 12 were excluded at the screening phone interview, and 16 were excluded at baseline assessment for not presenting with a JPE ≥ 4.5°. The final sample (40 participants) was randomized into the 2 groups (Fig. 1). Groups were comparable at baseline in terms of patients’ characteristics and outcomes (Tables 1and2).
Patient's characteristics.
Data are mean ± standard deviation or frequency (proportion).
BMI, body max index; NDI, neck disability index; VAS, visual analogue scale.
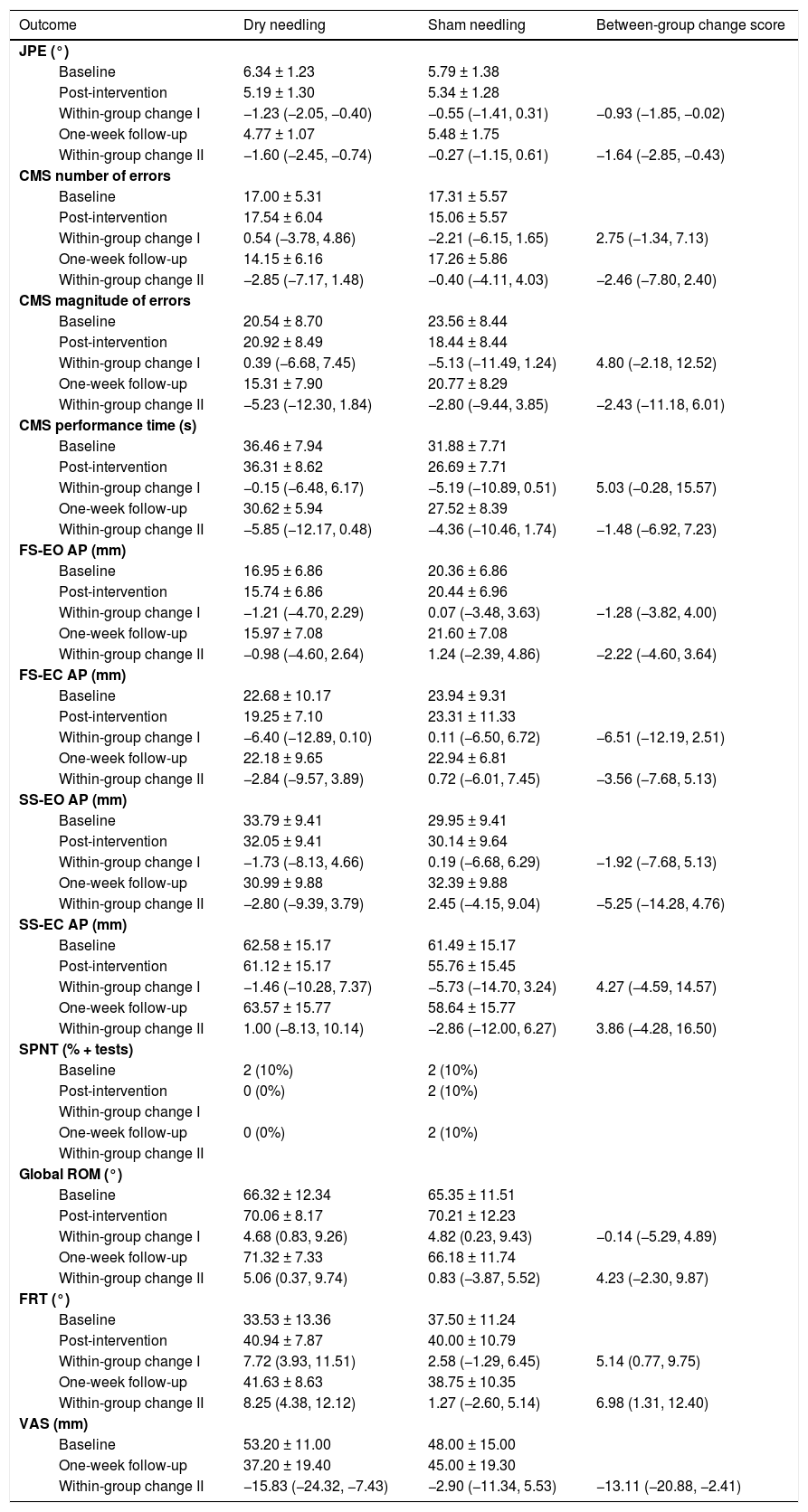
Outcome data.
Data are mean ± standard deviation, frequency (proportion) or mean difference (95% confidence interval).
AP, antero-posterior displacement of center of pressure; CMS, cervical movement sense; FRT, flexion rotation test; FS-EC, firm surface eyes closed; FS-EO, firm surface eyes open; JPE, joint position error; ROM, range of motion; SPNT, smooth pursuit neck torsion test; SS-EC, soft surface eyes closed; SS-EO, soft surface eyes open; VAS, visual analogue scale.
Within-group change I (baseline – post-intervention).
Within-group change II (baseline – one-week follow-up).
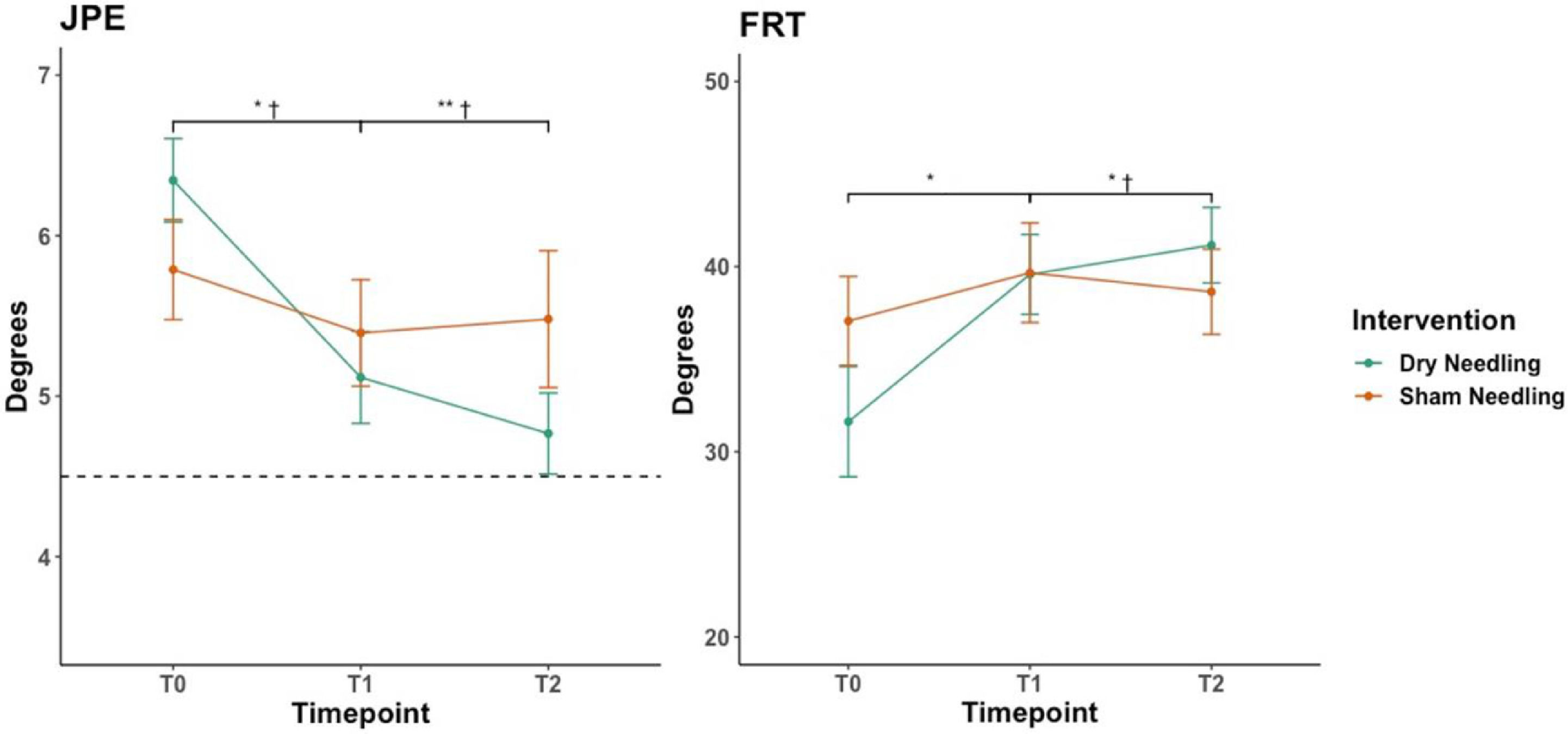
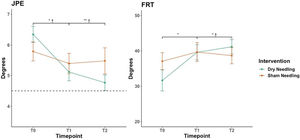
Table 2 summarizes the results of each outcome assessment for DN and sham groups, as well as within- and between-group changes. The linear mixed-models revealed a significant time-by-group interaction for JPE (p = 0.034), where the DN group showed a greater decrease of JPE compared to the sham group at post-intervention (mean difference [MD]= −0.93°; 95% Confidence Interval [CI]: −1.85, −0.02) and at one-week follow-up (MD: −1.64°; 95%CI: −2.85, −0.43) (Fig. 2). No time-by-group interaction was found for cervical movement sense number (p = 0.075) and magnitude (p = 0.123) of errors as well as performance time (p = 0.159). Additionally, no time by-group interaction was observed for AP displacement in any standing balance conditions; firm surface with eyes open and eyes closed (p = 0.566 and p = 0.232, respectively) and soft surface with eyes open and eyes closed (p = 0.386 and p = 0.659, respectively).
Between-group comparison in JPE and FRT throughout the study.
Data are mean and standard error.
JPE, joint position error; FRT, flexion rotation test; T0, baseline; T1, post-intervention; T2, one-week follow-up. Dashed line represent the JPE cut-off value (4.5°)
Post-hoc: * p < 0.05, ** p < 0.01; † ≥ than the (between-group) minimal detectable change.
Significant time-by-group interaction was found for FRT (p = 0.007). FRT showed an increase immediately (MD= 5.14°; 95%CI: 0.77, 9.75) and one-week after DN (MD= 6.98°; 95%CI: 1.31, 12.40) compared to the sham group (Fig. 2). No time by-group interaction was observed for the global cervical rotation ROM (p = 0.196). However, there was a main effect for time (p = 0.002), with both groups showing similar gains inmediately post-intervention.
Finally, time-by-group interaction was found for VAS (p = 0.034). A reduction in pain intensity at follow-up was observed in the DN group compared to the sham group (MD= −13.11 mm; 95%CI: −20.88, −2.41). Two patients reported adverse effects after DN (headache for the following 2,3 days). The BI was 0.65 (95%CI: 0.36, 0.94) in the DN group and −0.50 (95% CI: −0.88, −0.12) in the sham group, which suggests that patients tended to believe they received the true intervention.56 Further details on BI results and interpretation can be found in Table 3.
Blinding assessment and interpretation.
| Assignment | Dry needling | Sham needling | Do not know | Total |
|---|---|---|---|---|
| Dry needling | 15 (75%) | 2 (10%) | 3 (15%) | 20 |
| Sham | 13 (72.2%) | 4 (22.2%) | 1 (5.6%) | 18 |
| Total | 28 (73.7%) | 6 (15.8%) | 4 (10.5%) | 38 |
Data are fequency (proportion).
Bang's blinding index (BI)=0.65 (95% CI: 0.36, 0.94) in the DN group and −0.50 (95% CI: −0.88, −0.12) in the sham group. BI scores range from −1.00 (incorrect guessing; all participants mistakenly guess the other intervention) to +1.00 (correct guessing; all participants correctly guess their allocation) where 0.00 means random guessing.56 In the present study, the BI shows a positive value in the dry needling (DN) group and a negative value in the sham needling group. This scenario is referred as unblinded (DN)/opposite (Sham). Under this scenario, patients tend to believe they received the true treatment regardless of actual treatment received, which reflects patients’ expectations and wish to receive the true intervention.
This study is to our knowledge the first to show that a single session of DN of the OCI can decrease JPE and increase upper cervical mobility in the short-term.
In the present study, OCI was selected for treatment due to its hypothesized contribution to the correct head and eye movement control and the normal function of the upper cervical spine rather than patients’ symptoms.15–17,20,57,58 Evidence supports that tailored interventions targeting impairments of sensorimotor control related to head movement control can decrease neck pain and disability.6,7,9 However, it is suggested that addressing the local source of altered cervical afferent input, such as neuromuscular impairments or reduced cervical mobility, can contribute further to restore normal sensorimotor function; and subsequently, improve patient's complaints.4,6–8,10,11
A decrease in JPE greater than the MDC (−0.93°) was observed immediately after the DN session compared to sham needling; and this was also maintained one-week post-intervention (−1.64°).41 Similar results have been found after other therapeutic modalities targeting cervical neuromuscular impairments such as endurance-strength training,6,11 but conflicting findings have been reported for manual therapy techniques.59,60 By contrast, no short-term gains in postural stability or cervical movement sense were observed after DN. Consistent with the current findings, non-tailored interventions such as manual therapy or exercise training have been shown to be uneffective for this aim11,60; and as this is the first study to our knowledge to evaluate the effect of a non-sensorimotor intervention on cervical movement sense, comparisons with previous findings cannot be made.
It could be argued that post-needling soreness may have influenced the result of some of the sensorimotor tests immediately after DN.61,62 However, post-needling soreness normally lasts only few days; so this cannot explain the lack of gains at one-week follow-up and should have also possibly affected the JPE test.63 Previous research has shown no association between cervical JPE deficits and postural stability or cervical movement sense disturbances;4,38,64,65 and factor analysis has recently shown that each of these tests measure unique aspects of the cervical sensorimotor control (i.e. proprioception/kinesthesis or oculomotor control).65 This evidence, together with the current results, suggests that different treatment approaches may be required to target the patient's specific cervical sensorimotor control impairments.4 Thus, while gains in cervical propioception or kinesthesis can be obtained through interventions aiming to restore the normal cervical neuromuscular function; others, such as impaiments in standing balance, may require tailored programs including specific exercises for postural control.
DN of the OCI was also effective in increasing global cervical rotation ROM; but contrary to previous research, these gains did not exceed the MDC.20–22,48 The beforementioned non-clinically meaningful reduction in pain intensity (13.11 mm) may explain this finding.21,22,66 Conversely, previous studies have shown that manual therapy techniques such as sustained natural apophyseal glide (SNAG) or translatoric spinal mobilization are effective to increase upper cervical spine mobility measured with FRT in patients with cervicogenic headache or neck pain.67–69 This is, however, to our knowledge, the first study to report gains in the FRT similar to the MDC one-week after a single session of DN (6.98°) in patients with neck pain.50 Post-needling soreness could explain that the gains in FRT did not exceed the MDC immediately after DN.
So far, current evidence suggests that restoring global cervical rotation mobility does not lead to reductions in cervical JPE following head rotation.60,70 The C1–C2 segment contributes to around half of the total cervical rotation ROM,71 and in vitro research has revealed that C1-C2 rotation is related to the OCI muscle extensibility.72 Thus, a decreased mobility of this segment could affect the normal sensorimotor function of this muscle73,74; and by doing so alter the afferent cervical input and JPE following rotation.13 However, the current study design does not allow to investigate whether or not the decrease in JPE after head rotation was mediated by gains in the FRT and future research should test this hypothesis.75
Future studies should also futher examine the clinical implications of the current research. Current evidence does not support a long-term added benefit of DN to traditional therapeutic modalities for neck pain.76,77 Thus, it should be explored if the addition of DN to tailored sensorimotor training can provide additional gains in sensorimotor control measures. Also, a reduction in pain intensity (which was close to be clinically meaningful) was observed after DN; which is consistent with previous studies targeting the local source of altered cervical afferent input in people with neck pain and impaired sensorimotor function.6–9 However, how improvements in sensorimotor control lead to reductions in pain and disability is still unclear.78
Strengths and limitationsOur study has several strengths. It was prospectively registered and followed an appropriate reporting guideline.26 Similarly, it used a validated DN procedure,19 concealed allocation and blinded outcome evaluation, which improves the internal validity of the study. Another strength of this study was the sham needling protocol, which followed the most recent procedure recommendations published.35 The successful blinding measured with the BI also provides more robustness to the results of this study because poor blinding is associated with outcome bias in favor of DN.79 A further strength of the present research is the clinical rather than laboratory nature of most of the measures; which provides a more relevant message for clinical practice. On the other hand, some limitations should also be acknowledged. The low number of positive SNPT did not allow for any meaningful interpretation of the effects of the intervention on eye movement disturbances. The inclusion of a large proportion of people with idiopathic neck pain in our sample, who exhibit less marked SPNT impairments,14 may be a possible explanation.
ConclusionA single session of DN of the OCI provide short-term improvement of cervical JPE and upper cervical mobility in patients with neck pain. Future studies should explore whether the addition of this technique to sensorimotor control training within a multimodal program add further benefits to other aspects of the cervical sensorimotor control.