Recent evidence suggests that physical therapy interventions targeting the hips may improve outcomes, including pain and disability, for patients with low back pain (LBP). Currently, there is conflicting data in regard to whether an individual with LBP needs to have a concurrent hip impairment in order to respond to this approach. The purpose of this clinical trial will be to determine the short and long-term effectiveness of physical therapy interventions directed at the lumbar spine only, versus lumbar spine and hip(s), in individuals with a primary complaint of LBP with a concurrent hip impairment.
MethodsA multi-center, randomized controlled trial of 76 adult individuals with a primary complaint of LBP, who also have at least one concurrent hip impairment. Participants will be randomized into the ‘LBP only’ or ‘LBP+Hip’ group. Treatment to the low back in both groups will be a pragmatic approach consisting of interventions targeting the low back without targeting the hip(s). Participants randomized to the LBP+Hip group will also receive a semi-prescriptive set of manual therapy and exercise techniques that target the hips. The primary outcome measures will be the modified Oswestry Disability Index and the Numeric Pain Rating Scale at discharge.
DiscussionThese two treatment strategies are commonly utilized in physical therapy practice, but there is uncertainty which is superior. This trial will also help to provide a better understanding of the role of concurrent hip impairments in LBP.
Trial registrationThis trial has been prospectively registered at clinicaltrials.gov (ID# NCT03550014, https://clinicaltrials.gov/ct2/show/NCT03550014) on June 7, 2018.
Low back pain (LBP) is a leading cause of disability worldwide.1,2 According to the Global Burden of Disease 2010 study, LBP ranked as the top condition for years living with disability accounting for 10.7% of total years lived with disability.2 LBP is the fifth leading reason that patients seek care from physicians in the United States.3 With an increase in patients seeking care, the healthcare expenditures related to LBP have outpaced other medical conditions.4
Over the past several years, studies have suggested providing interventions targeting adjacent regions may provide beneficial effects for the individual's primary region of complaint.5,6 Using this regional approach to LBP, several studies have investigated the role of physical therapy interventions directed at one or both hips for individuals with LBP.7–9 These preliminary studies have suggested that physical therapy interventions targeting one or both hips may improve outcomes for LBP.7–9 In 2011, Burns et al.8 provided a standardized approach of manual therapy and exercise targeting one or both hips in eight consecutive patients with chronic LBP (>6 months). In this small case series, over 60% of patients experienced a reduction in disability and reported a global improvement in their symptoms.8 This approach to treating individuals with LBP was further investigated in a recent randomized clinical trial.10 Bade et al.10 randomized 84 participants to receive either pragmatic physical therapy program targeting the lumbar spine only (LBP only) or to receive pragmatic physical therapy to the lumbar spine plus a prescriptive set of manual therapy and exercise interventions directed at the hip(s) (LBP+Hip).10 At two weeks, significant differences (p<0.05) favoring the LBP+Hip group in terms of perceived recovery and patient satisfaction were identified. At discharge from physical therapy, significant differences (p<0.05) were observed in favor of the LBP+Hip group for perceived disability, pain rating, patient satisfaction and perceived recovery. The effect size for the improvement in perceived disability was 0.36 and for pain rating 0.58, indicating small to medium effects. A possible reason for the small to medium effect sizes was that most of the participants (87%) did not have a concurrent hip problem.10 An intervention targeting the hip might be expected to be more effective in a patient presenting with LBP and a concurrent hip problem. The prevalence of hip problems in patients presenting with LBP is estimated to be 80%, suggesting that interventions targeting the hip(s) might have a greater effect in a typical population of patients with LBP than what has been previously reported.11
ObjectivesThe aim of the current study is to determine the impact of physical therapy interventions directed at the lumbar spine only (LBP only) versus lumbar spine+hip(s) (LBP+Hip) on patient perceived disability and pain rating at discharge and at 12 month follow-up, specifically for individuals with a primary complaint of LBP and with concurrent hip impairment(s). Additionally, we will conduct a pre-planned secondary analysis to determine which possible factors (e.g., body mass index, perceived disability) may explain why some participants achieve a successful outcome following physical therapy interventions and others do not.
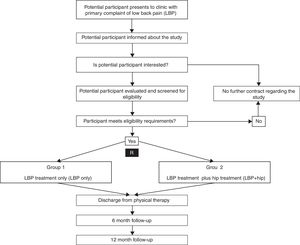
Trial designThis multi-center clinical trial was designed in accordance with the CONSORT guidelines for randomized trials12 and will compare two parallel interventions (LBP only versus LBP+Hip) for patients presenting to physical therapy for treatment of LBP. The primary outcomes will be the modified Oswestry Disability Index (ODI) and numeric pain rating scale (NPRS) at discharge from physical therapy care and at 12 months after enrollment in the study. Fig. 1 illustrates the trial design.
MethodsTrial setting & recruitmentWe estimate that we need to recruit from six physical therapy centers across a range of geographic regions.10 The pragmatic design of the trial and large geographic area represented will ensure the generalizability and applicability of the study findings. The physical therapy sites that will participate in recruitment of participants in the trial are Bellin Heath Systems, BSR Physical Therapy, Franciscan Health Physical Therapy, Kinetic Physical Therapy, OSF Healthcare System and Temple University.
The research team will assess all recruitment sites for feasibility of being able to deliver the trial interventions. Each clinical site will have a site coordinator and at least one additional licensed physical therapist who will be trained in the study procedures. The pre-recorded training session will include instruction in the administrative aspects of the study (e.g., informed consent, subject recruitment) and specific training in the performance of the examination and treatment procedures, including the manual therapy techniques and the exercise program. The purpose of this training will be to ensure the participant screening examination is performed consistently across sites and that treating therapists fully understand the interventions for each group. The physical therapists involved frequently use the examination and treatment procedures in this study as part of their usual clinical practice, so the training is designed to facilitate consistency in delivery across practitioners. Each participating physical therapist will also be provided with a detailed Manual of Standard Operations and Procedures (MSOP) that describes all the study procedures in detail. Each participating physical therapist will complete an assessment of their understanding of these procedures and complete an experience survey. This survey will include questions regarding years of clinical experience and training and experience with manual therapy, including experience with the specific manual therapy techniques used in this study. We will also record each therapist's entry-level degree (MPT, DPT, etc.), post-professional academic degrees (MS, DSc, PhD, etc.), and post-professional specialty certification (OCS, FAAOMPT, etc.).
Eligibility criteriaConsecutive patients presenting to the participating physical therapy centers seeking care for LBP will be informed about the study. If the patient is interested in the study, they will be evaluated and screened for eligibility requirements by a licensed physical therapist. At baseline evaluation, each participant will be over the age of 18 years and have a primary complaint of LBP (>2/10 on the NPRS) that is causing at least moderate levels of disability (>20% on ODI). In addition to a primary complaint of LBP, each participant will have to exhibit a concurrent impairment(s) at the hip(s) that includes reported groin pain reproduced during the examination of the hip(s), decreased hip range of motion (ROM) or hip strength, or a positive special test for hip pathology. Participants will be ineligible for participation if they have contraindications to physical therapy, recent severe trauma to the lumbar spine, positive neurologic examination, recent spinal surgery or total hip arthroplasty. A full list of inclusion and exclusion criteria is presented below:
Inclusion criteria:
- •
Age over 18 years
- •
Modified Oswestry Disability Index (ODI) ≥20%
- •
Numeric Pain Rating Scale (NPRS) of ≥2 points
- •
Primary complaint of low back pain with at least one hip impairment in one or both hips
Methods for identifying a concurrent hip problem (at least one of these items needs to be positive in one or both hips):
- •
Patient self-reports concurrent pain in the groin (verbally or via body diagram)
- •
Pain reproduced during examination of the hip(s)
- •
Decreased range of motion
- •
Flexion (supine) ≤110°
- •
External rotation (prone) ≤30°
- •
Internal rotation (prone) ≤30°
- •
External rotation (supine at 90° hip flexion) ≤30°
- •
Internal rotation (supine at 90° hip flexion) ≤30°
- •
Extension (prone) ≤10°
- •
Decreased muscle strength (assessed by manual muscle testing)
- •
Hip flexors
- •
Hip abductors
- •
Hip extensors
- •
Positive special tests (one or both hips)
- •
FADDIR
FABER
Exclusion criteria:
- •
Contraindications to manual therapy
- •
Severe trauma to the lumbar spine or hip(s) in the last 6 weeks
- •
‘Red flag’ symptoms including:
- •
Tumor
- •
Metabolic disease
- •
Rheumatoid arthritis or other systemic rheumatologic disorders
- •
Acute fracture
- •
Bowel/bladder dysfunction
- •
Prolonged history of corticosteroid use
- •
Evidence of central nervous system involvement
- •
Two or more positive neurologic signs consistent with nerve root compression:
- •
Diminished or absent muscle stretch reflexes of lower extremity
- •
Muscle weakness in any lower extremity myotome
- •
Diminished or absent sensation in any lower extremity dermatome
- •
Spinal surgery in the last 6 months
- •
Total hip arthroplasty
- •
Currently pregnant or post-partum ≤6 months
- •
History of osteoporosis
- •
History of cancer within the last 12 months
- •
Inability to understand the English language
Participants in both groups will attend in-person physical therapy sessions on an outpatient basis. The frequency of physical therapy sessions will be at the discretion of the treating clinician, but the recommendation is 2–3 sessions per week. Each treatment session will be with a licensed physical therapist and will last between 45 and 60min. The duration of the entire episode of care will be at the discretion of the treating physical therapist. All participants, regardless of group allocation, will receive a pragmatic approach to treatment directed at the lower back. One group (LBP only) will receive a pragmatic set of physical therapy interventions which would be based on the impairments identified at the baseline physical examination. The treating physical therapist will prescribe initial exercises and/or perform manual therapy techniques directed at the lumbar spine only. These initial interventions will be progressed by the treating physical therapists throughout the duration of care to match the recovery and functional demands of the participant. The dosage of all interventions will be at the discretion of the treating physical therapist and can be adjust at the discretion of the treating physical therapist. The other group (LBP+Hip) will receive the pragmatic interventions directed at the low back (as described above for the LBP only group) plus a semi-structured approach to the treatment directed at the hip(s). The semi-structured approach to the hip interventions will include manual therapy and exercise that have been used in other studies.7,9 The LBP+Hip group will receive at least 2–3 hip manual therapy techniques from a pre-determined list each session, as well as a two-phase hip exercise program. The hip manual therapy techniques have been utilized in other studies in the management of individuals with a primary complaint of LBP.8,10 Additionally, they were commonly cited techniques from a current survey regarding clinical practice habits of physical therapists in the United States for LBP.13 The dosage of the manual therapy interventions is up to 2× for thrust and 2–3 bouts of 30–60s for non-thrust manual therapy. The two-phase strengthening exercise program is based on maximum voluntary contraction data of key musculature in the hip and gluteal region.14,15 The treating physical therapists will be able to select two flexibility and two strengthening exercises that they deem will be most appropriate to facilitate recovery for the participant. The treating physical therapist will have the participant perform these exercises until they deem they are ready for progression. At that point, the physical therapist will choose two flexibility and two strengthening exercise progressions from the phase 2 list of interventions.
Participants will also be provided with verbal and written education for their LBP, including messaging that ‘pain with movement does not equal harm’; ‘a gradual increase in activity levels should be based on time as opposed to pain level’; and ‘it is safe to exercise and work with back pain.’16
Adherence by the physical therapists to trial interventions will be explained verbally, via pre-recorded training conducted by the primary investigator (SB) and in the detailed MSOP. Additional training sessions will be conducted as needed in person, via phone, or through video-conferencing. After training participating physical therapists will complete an assessment of their understanding of trial elements including informed consent, enrolment, and study procedures. Each clinical site coordinator will be contacted at least monthly by the research team to ensure trial procedures are being implemented appropriately.
OutcomesThe primary outcome measures will be the modified ODI and NPRS at discharge from physical therapy and at 12 months. The ODI and NPRS are commonly utilized self-report measures for individuals with LBP, both in research and in clinical settings. Following eligibility screening and enrollment, the participant will complete baseline demographic information and self-report measures including the ODI, NPRS, Fear-Avoidance Beliefs Questionnaire (FABQ), Patient Health Questionnaire (PHQ-2) and Godin Leisure-Time Physical Activity. After two weeks in physical therapy, the participant will complete the ODI, NPRS, FABQ, the Global Rating of Change (GRoC), and the Patient Acceptable Symptom State (PASS) self-report measures. At discharge from physical therapy, the participant will complete these questionnaires again, as well as the Godin Leisure-Time Physical Activity Questionnaire.
Participant timelineAt baseline, at 2 weeks, and at discharge the participant will complete the relevant questionnaires in person during a physical therapy session but independent of the treating physical therapist. For long-term follow-up, participants will be contacted by the research team either via email or mail to complete the questionnaires at the 6 and 12-month time points. Table 1 outlines the questionnaires and time-points for collection.
Summary of outcome measure collection time points.
| Baseline | 2 weeks | Discharge | 6 months | 12 months | |
|---|---|---|---|---|---|
| Modified Oswestry Disability Index | × | × | × | × | × |
| Numeric Pain Rating Scale | × | × | × | × | × |
| Fear Avoidance Beliefs Questionnaire | × | × | × | × | × |
| Global Rating of Change | × | × | × | × | |
| Patient Acceptable Symptom State | × | × | × | × | |
| Godin Leisure-Time Physical Activity Questionnaire | × | × | |||
| Patient Health Questionnaire-2 | × |
While all the treatments are common in physical therapy practice, data will be collected relating to adverse outcomes in the study using a tally sheet at each data collection site.
Sample sizeThe a priori sample size and power calculations were performed using G-Power 3.1.9.2 (http://www.gpower.hhu.de/). The calculations were based on the minimal clinical improvement of 6 points on the ODI, assuming a standard deviation of 5.40 points, one-tailed, and an alpha level equal to 0.05. This will require a minimum sample size of 32 participants per group.
A total of 76 participants with a primary complaint of LBP whom meet the inclusion/exclusion criteria and consent to participate will be enrolled in the study. This sample size will control for a drop-out rate of 20% (at the 2-week follow-up) and maintain a power of 80% to detect both statistically significant and clinically meaningful changes in self-report measures.
Allocation and blindingRandomization will be conducted a priori using the Microsoft Excel randomization equation (=RAND()). Concealed allocation will be performed by a research assistant (not involved in recruitment or data collection) using a computer-generated randomized table of numbers created for each participating site prior to the beginning of the study. Individual, sequentially numbered index cards with the random assignment will be prepared and placed in sealed opaque envelopes. Secondary to the study design and interventions, blinding of therapists and participants is not possible. Outcome questionnaires will be administered by office staff that will be blinded to group allocation.
Data collection, management & confidentialityOne of the investigators (SB) will conduct an on-site and/or virtual visit at each site prior to data collection to train site coordinators and therapists participating in data collection/treatment in the study procedures. This training will include a discussion on data safety monitoring and subject confidentiality issues. Once data collection begins, the primary investigator will contact each site coordinator by telephone at least monthly. The purpose of these meetings will be to monitor subject recruitment and retention, answer any questions from the therapists, and address any factors that might impact the safety or ethics of the study.
All records pertaining to a subject's involvement in the study will be stored in a locked filing cabinet at each of the sites in which the study is to be conducted. This information will only be accessible to the investigators, the research study staff, and the physical therapists involved in providing the treatment. Each subject will be assigned a Study Identification Number as per the instructions in the MSOP. The initial patient demographic information and completed baseline questionnaires will be scanned and emailed to the primary investigator using a secure password-protected method. The computers utilized will be on secure networks that meet Health Insurance Portability and Accountability Act (HIPAA) standards. The subjects in the study are also seeking physical therapy care at the data collection centers, so patient related information will be kept in accordance with the clinical policies that are required for confidentiality in patient care. After the data are transmitted from the multi-center sites, they will be stored in a locked filing cabinet at Temple University, and then entered into a password-protected database for future analysis. The research team will be responsible for all data analyses, which will be conducted at Temple University. The data will be stored for five years after completion of the study and then destroyed. Procedures will be implemented to ensure accurate recording and timely reporting and entry of data, including the use of standardized data collection forms at all sites. Monthly random checks of 10% of the individual records will also be performed to ensure that the computerized database is identical to the data recorded on the data collection forms.
All subject information will be handled in a confidential manner consistent with other hospital medical records. Subjects will not be specifically identified in any publication or presentation of research results. However, in unusual cases, the research records may be inspected by appropriate government agencies or be released in response to an order from a court of law.
Statistical methodsDescriptive statistics, including frequency counts for categorical variables and measures of central tendency and dispersion for continuous variables will be calculated to summarize the data. Baseline variables will be compared between groups using independent t-tests or Mann–Whitney U tests for continuous data and chi-square tests of independence for categorical data.
Missing data will be handled using methods of multiple imputation including Little's Completely at Random Test. Intention-to-treat analysis was performed to assure all patients were analyzed per their initial group allocation using expectation maximization.17
All drop-outs and the reason for dropping out of the study will be reported. An a priori alpha level of 0.05 will be used for all analyses. All data will be reviewed to ensure the assumptions have been met to proceed with parametric analysis. If the assumptions are violated, appropriate non-parametric tests will be utilized.
The primary aim (effects of treatment on pain and disability) will be examined with a 2×3 mixed model analysis of variance (ANOVA) with treatment group (LBP only versus LBP+Hip) as the between-subjects variable, and time (baseline, 2 weeks, discharge, 6 months and 12 months) as the within-subjects variable. Separate ANOVAs will be performed with ODI and NPRS as the dependent variable. For each ANOVA, the hypothesis of interest is the 2-way interaction (group×time). The other outcome measures (e.g., FABQ, PHQ-2, and Godin Leisure-Time Physical Activity) would be entered into the models as co-variates.
LimitationsFirst, the treating therapists and participants cannot be blinded to group allocation and this may inadvertently influence the verbal and non-verbal interactions. To help address this limitation, all therapists will be educated that both treatment approaches have resulted in successful outcomes for individuals with LBP.5–8,13 Second, therapists are permitted to perform pragmatic interventions to the lumbar spine in both treatment groups as this is reflective of real-world clinical practice in which therapists choose interventions based upon their likely success with a particular patient. To address this the pragmatic nature of the study, the participating therapists will all have undergone previous similar advanced clinical training in the physical therapy interventions utilized and will undergo additional study-specific training prior to the start of the study. Additionally, the semi-structured nature of the hip interventions for the LBP+Hip group is another potential limitation as it lacks strict control. Although therapists will be allowed to make clinical decisions for their patients as they would in routine clinical practice, hip intervention choices will be restricted to only those that have been used successfully in previous trials of individuals with LBP.
A final potential limitation is the non-inclusion of physical examination tests/measures in an effort to decrease loss to follow-up, particularly at the 6 and 12-month time points. Additionally, physical examination tests/measures generally have poor inter-rater reliability and data collection will be performed by multiple individuals across the centers.18,19
HarmsThe risk of harm to subjects is minimal. Common side effects to physical therapy interventions include minor soreness and transient changes in pain levels.20 The risks for trial participants are the same as for individuals receiving similar physical therapy care. There are no other foreseeable risks with participating in this study. Each participant will be seeking physical therapy care and will be required to comply with financial implications set forth by their insurance provider.
Ethics and disseminationResearch ethics approval & consentThe Temple University, Philadelphia, PA, USA, Institutional Review Board (Protocol #24664) and University of Newcastle, Callaghan, NSW, Australia, Human Research Ethics Committee (H-2018-0009) approved the study and all patients will provide informed consent prior to participation in the trial.
Trial resultsData collection is expected to be completed by the end of 2019. Data analysis and manuscript preparation are expected to be finalized by early 2020. The research team intends to perform data analysis with the intent of publishing the results in a peer-reviewed journal and present findings at professional conferences.
AuthorshipIndividuals that provide substantive contributions to the design, conduct, analysis and reporting of the trial will be recognized as authors on the final publication.
DiscussionLBP is a very common symptom that often improves within 6 weeks, however over 60% of individuals may still report pain at 3 and 12 months.21,22 While LBP and its recovery is multifactorial,23,24 there is emerging evidence that indicates some individuals with LBP may experience improvement in pain and disability using a combination of manual therapy and exercise directed at the hip(s).8,10,25 Despite this, the data are conflicting as to whether the individual with LBP will have an improved outcome following the application of hip interventions if they present with a concurrent hip impairment (e.g., decreased range of motion or strength).8,10
Therefore, the aim of this trial is to determine whether individuals with a primary complaint of LBP and a concurrent hip impairment will have better outcomes with physical therapy interventions directed at the lumbar spine only, or with a combination of interventions targeting the lumbar spine plus the hip(s).
It is hypothesized that patients receiving physical therapy interventions directed at both the lumbar spine and hip(s) will experience greater reductions in pain and perceived disability in both the short-term and the long-term compared to those who receive physical therapy interventions targeting the lumbar spine only.
The results of this trial will therefore help further delineate the role of concurrent hip impairments in individuals with LBP and assist in determining the long-term outcomes.
Potential challenges to this study will include difficulties with patient recruitment and patients lost to follow-up, especially over the 12 month long-term follow-up period. To address these challenges, only physical therapy clinics have a large percentage of patients with LBP and have previously collected data for clinical research studies will be used.
Access to dataAll principal investigators (both U.S. and Australia) will have access to the data set. To ensure confidentiality, data dispersed to research team members will be blinded of any identifying participant information.
Conflicts of interestThe authors declare no conflicts of interest.