There is a poor understanding of the dose–response relationship between years of physical activity and motor and cognitive function. We determined the dose–response effects of physical activity duration in years on motor and cognitive function and their relationship in healthy old females.
ObjectivesTo determine the dose-effects of physical activity duration in years on motor and cognitive function and their relationship in health aging adults.
MethodsWe conducted a retrospective observational study with 201 old (age 69 years; SD=5.9) and 12 young (mean age 21 years; SD=1.9) females, with sub-groups based on number of years of self-reported physical activity. Aerobic capacity, mobility, functional reach, standing balance, global cognition, episodic memory, executive function, and processing speed were assessed with performance-based tests. We analyzed sub-group differences quantitatively and qualitatively and performed regression and mediation analyses to determine predictors and mediators of physical activity effects.
ResultsBased on physical activity of minimal (0.3 y, n=29), short (2.4 y, n=77), moderate (6.2 y, n=36) and long (16.6 y, n=59) duration, physical activity for at least 2.4 years affords old adults benefits in body mass index with peak dose-effects present in aerobic capacity and mobility at 6.2 years without additional benefits after 16.6 years of physical activity. Physical activity for any duration had no effects on functional reach, balance, executive function, episodic memory, and processing speed. Although weakly mobility predicted global cognition and executive function.
ConclusionPerforming physical activity up to 6.2 years on average had favorable effects on body mass index, aerobic capacity and mobility. The data strengthen current recommendations for an active lifestyle in adulthood to prevent aging-related motor and cognitive decline.
Physical and cognitive functions decline with aging. Reductions in cardiorespiratory, musculoskeletal, and neural function are associated with motor and cognitive impairments such as slow gait,1 unstable balance, low memory capacity, and slow information processing. Physical activity (PA) comprises any bodily movements produced by skeletal muscles that results in energy expenditure above resting levels.2
PA reduces and sedentariness increases the risks for morbidity and mortality.3 However, there is a poor understanding whether or not the beneficial effects of PA are cumulative in a dose–response manner especially with respect to the duration of PA expressed in number of years.3
Dosing of PA is a key element of PA prescription and for setting PA guidelines.4 A pooled analysis from six cross-sectional studies revealed that the duration and intensity of leisure-time PA are associated with reduced all-cause mortality in a U-shaped relation.5 Different types of PA are strongly associated with improvements in cardiovascular health, endurance, functional abilities, muscle strength, and postural control. The nature of the dose–response relationship between PA and physiological improvements varies between conditions or health outcomes.6,7 With respect to cognition, old adults who regularly performed 30min of moderate to vigorous (3–7 METS) PA compared with lower levels of PA had 15% higher executive function.8 Perhaps due to differences in methods of assessing PA, for example, objectively9 vs. by self-report10–14 prospective studies reported inconsistent dosing effects of PA on cognition in cognitively healthy older adults.
Especially the dose–response effects of PA on cognition with respect to the duration of PA expressed in number of years remain understudied.15 One possibility is that 10–15 years of PA before age 70 affords cumulative benefits by slowing age-related motor and cognitive decline, and producing a linear dose-effect on motor and cognitive function through underlying mechanistic paths.16,17 Alternatively, it is likely that 10–15 years of PA provides no additional benefits as compared with fewer years of PA if PA frequency and intensity are insufficient to keep pace with the rate of motor-cognitive decline.16,17 The purpose of this study was to determine the dose-effects of PA duration in years on motor and cognitive function and their relationship in healthy aging adults.
MethodsParticipants — This is a retrospective observational study of healthy old females who volunteered for PA classes in the metropolitan area of Londrina, Paraná, Brazil. Volunteers attended classes at local health clubs, schools, churches, and community centers and were invited to participate in the study through advertisements. Active participants (n=189) attended one of five PA classes: (1) stretching (n=89); (2) aerobic/water sports (n=40); (3) ball room dance (n=10); (4) strength training (n=12), and (5) gymnastics (n=38). Some participants also pursued PA on their own in the form of pilates, walking, biking, or swimming. We also examined healthy old females who were sedentary for the year prior to data collection (n=12). A healthy young female comparison group (n=12) was included for qualitative comparison purposes. These young participants engaged in various forms of PA (described in Table 1). The exclusion criteria were <60 years old, physical dependence18 or mental or physical illnesses that could interfere with the assessments. The Universidade Estadual de Londrina (UEL), Londrina, PR, Brazil and National Council of Health, Brazil approved the study protocol (357.369) and each participant gave written consent prior to the start of the study.
Distribution of physical activities for the old and young sub-groups.
| PA-code | Stretching, n or % of group total | Aerobic/water sports, n/% or group total | Dance, n or % of group total | Gym, n or % of group total | Strength, n or % of group total | None, n or/% of group total | Total, n | Participants who performed additional activities, n |
|---|---|---|---|---|---|---|---|---|
| 0 – minimal | 10/34% | 5/17% | 1/3% | 1/3% | 0/0% | 12/41% | 29 | 5 |
| 1 – short | 43/56% | 13/17% | 6/8% | 4/5% | 11/14% | 0/0% | 77 | 37 |
| 2 – moderate | 14/39% | 8/22% | 2/6% | 10/28% | 2/6% | 0/0% | 36 | 18 |
| 3 – long | 22/59% | 14/24% | 1/2% | 16/27% | 6/10% | 0/0% | 59 | 32 |
| Young | 0.3/3% | 2.5/21% | 0/0% | 2.8/24% | 6.3/53% | 0/0% | 12 | |
| Total, n | 89 | 42 | 10 | 34 | 25 | 89 |
PA, physical activity; minimal, some, moderate, long: duration of physical activity in years.
Protocol — Participants were tested once in a 1.5-h long-session individually by trained researchers. First, participants signed the informed consent document and then performed a block of cognitive and a block of motor tasks. The order of these blocks was randomized. For each participant, different researchers administered the cognitive vs. motor tests.
Operationalization of PA — To operationalize PA, we used a self-constructed questionnaire administered by interview, that asked participants what PA-classes they attended and to estimate the duration of their PA-practice in years. This questionnaire was designed specifically for this study based on previous questionnaires developed to assess physical activity.19 We also asked participants to report any PA outside of the classes. Class durations and attendance were verified by accessing the participants’ records at the participating PA-locations.
Cognitive tests — Global cognition was tested with the Mini Mental State Examination (MMSE, score-range: 0–30).20
Executive function was tested by the three-ring version of the Tower of Hanoi puzzle, which measures planning and problem-solving ability.21 Subjects had to move a stack of rings from one peg to another, minimizing execution time and number of movements. This test has adequate psychometric properties across the lifespan.22
Episodic memory was measured with a mnemonic test used extensively previously.23 Participants observed two lists of objects. First, the test administrator showed one list with 18 objects, which subjects recalled after 90s. A second list of 18 objects was presented to the participant immediately and after a delay of ∼15min during which the test administrator asked the participant personal information. The number of correctly recalled objects (0–18) was recorded.
Processing speed was measured with a computerized, randomized four-choice response time task.24 Participants sat in front of a monitor, with left and right middle and index fingers, respectively, held over the “D”, “F”, “H” and “J” keys of a custom keypad. A visual cue prompted the participant to press the cued key as fast as possible. Participants performed two familiarization and three test trials. We recorded untrimmed response time (ms) with a public domain software (http://okazaki.webs.com/softwaresdownloads.htm).
Motor tests — Functional reach was measured by instructing participants to maintain balance in standing while reaching forward.25
6 Meter Walking Test (MWT) measures aerobic capacity with good reliability, validity, and sensitivity.25,26 Participants walked a rectangular carpeted indoor course of 45.72m with turns marked every 4.57m. We recorded total distance walked in 6min.
Timed Up and Go (TUG) reliably measures leg strength, agility, and dynamic balance. Participants rose from a chair, walked 2.44m, turned, and returned to the seated position.25 Participants performed three trials and the fastest attempt was recorded.
Standing sway, a reliable measure of static balance,27 was measured under four conditions on a portable force platform (AMTI, AccuSway, Watertown, MA). We quantified sway as the dispersion (cm), amplitude (cm), and velocity of the center of pressure (cm/s) (COP) in the anterior and posterior directions and as the area of the COP path (cm2×1000). Participants stood on the platform with feet apart at shoulder width eyes open or closed or in a semi tandem stance with eyes open or closed. Each condition lasted 36s and the average of three trials in each condition was analyzed.
Statistical analysis — Participants (n=213) were classified into five groups. Four old sub-groups were formed based on the number of years attending a PA class. Group 1: minimal PA (range: 0.0–0.99 years); Group 2: some PA (1.0–4.99 years); Group 3: moderate PA (5–10 years): Group 4: high PA (>10 years), and Group 5: healthy young adults (mean: 4.5 years). We compared demographic, cognitive, and motor performance data between the old participant sub-groups with a one-way analysis of variance (ANOVA) followed by Tukey's posthoc contrasts. We computed Hedges’ g effect sizes for group differences. We qualitatively compared the old with the young adults to examine in how far the old adults reached the younger adults’ levels of cognitive and motor function. We performed tests for normality using the Shapiro–Wilk test. Subsequently, due to non-normal distribution, we log-transformed the executive function data. We performed forward moving step-wise multiple regressions to predict cognition from motor variables and motor function from cognitive variables. For the regression analyses, we used only variables that correlated significantly with each other, resulting in the following predictors for cognitive function: PA-years, age, mass, height, BMI, education, TUG, functional reach, 6MWT with or without CoP measures to predict MMSE (with CoP) and the temporal element of executive function, episodic memory, and processing speed without CoP. With regard to cognitive predictors of motor function: MMSE, executive function, episodic memory, and processing speed were regarded as possible predictors of functional reach, TUG and 6MWT. We set the probability of F-to-enter<0.05, and reported R2 change as fit indicator. We performed conditional process analyses to determine mediation among motor variables that predicted cognitive variables.28 Analyses were performed using SPSS version 22.0. The significance level was set at p<0.05.
ResultsTable 2 summarizes participants’ demographic, cognitive, and physical characteristics. In the four sub-groups of old adults and the young comparison group, mean PA duration was respectively 0.3, 2.4, 6.2, 16.6 and 4.5 years (p<0.001). The frequency (2.7, SD=1.46 per week) and duration (54.6, SD=13.19min) of each session and duration of absence from PA due to illness or other reasons (2.5, SD=7.15 months) were similar between sub-groups.
Demographic, cognitive, and physical function characteristics of groups formed based on years of physical activity experience.
| Variable | Old, n=201 | p | Posthoc, p<0.05 | Effect size g [95%CI] | Young, n=12 | |||
|---|---|---|---|---|---|---|---|---|
| 0 – minimal | 1 – short | 2 – moderate | 3 – long | |||||
| n | 29 | 77 | 36 | 59 | 12 | |||
| Age, y | 68.6±5.0 | 68.6±6.3 | 68.1±5.1 | 70.8±5.9 | p=0.09 | 21.3±1.9 | ||
| Mass, kg | 73.9±13.5 | 65.3±10.6 | 64.6±10.5 | 64.4±11.0 | p=0.01 | 0 vs. 1, 2, 3 | 1 vs. 0: g=0.75 [0.31–1.18]; 2 vs. 0: g=0.77 [0.26–1.28]; 3 vs. 0: g=0.79 [0.33–1.25] | 65.1±13.7 |
| Height, m | 156.4±5.5 | 155.4±6.1 | 156.1±6.5 | 155.3±7.0 | p=0.82 | 165.5±8.2 | ||
| BMI, kgm2 | 30.3±5.8 | 27.0±4.1 | 26.6±4.1 | 26.7±3.8 | p=0.01 | 0 vs. 1, 2, 3 | 1 vs. 0: g=0.71 [0.27–1.15]; 2 vs. 0: g=0.74 [0.24–1.25]; 3 vs. 0: g=0.78 [0.33–1.24] | 23.6±3.5 |
| Education, years | 4.8±3.6 | 6.2±4.9 | 6.5±4.7 | 7.1±4.9 | p=0.19 | 15.1±1.6 | ||
| PA duration, y | 0.3±0.3 | 2.4±1.0 | 6.2±1.3 | 16.6±9.6 | p≤0.01 | 0.1 vs. 2.3 | N/A | 4.5±4.6 |
| PA frequency per week | 2.83±1.64 | 2.84±1.47 | 2.45±1.21 | 2.41±1.54 | p=0.27 | 3.8±1.3 | ||
| PA duration per session, min | 56.5±11.22 | 54.7±12.23 | 53.9±9.89 | 53.9±18.12 | p=0.85 | 105.8±90.7 | ||
| PA absence, month | 1.1±1.87 | 3.0±9.33 | 3.3±7.9 | 1.64±3.66 | p=0.42 | N/A | ||
| Functional reach, unitless | 0.14±0.06 | 0.17±0.04 | 0.16±0.04 | 0.16±0.05 | p=0.09 | 0.19±0.04 | ||
| 6MWT, m | 367.9±52.1 | 396.3±74.1 | 428.9±54.5 | 406.7±76.4 | p=0.01 | 0 vs. 2 | g=1.14 [0.60–1.65] | 510.6±36.4 |
| TUG, s | 8.4±1.4 | 7.7±1.2 | 7.2±1.4 | 7.5±1.9 | p=0.01 | 0 vs. 2 | g=0.85 [0.34–1.36] | 5.8±0.7 |
| CoP AP dispersion, cm | 0.27±0.09 | 0.26±0.08 | 0.26±0.08 | 0.27±0.13 | p=0.44 | 0.20±0.06 | ||
| CoP ML dispersion, cm | 0.27±0.11 | 0.27±0.09 | 0.26±0.10 | 0.29±0.14 | p=0.49 | 0.19±0.05 | ||
| CoP AP amplitude, cm | 0.51±0.15 | 0.49±0.13 | 0.49±0.15 | 0.53±0.20 | p=0.52 | 0.37±0.09 | ||
| CoP ML amplitude, cm | 0.55±0.18 | 0.55±0.17 | 0.54±0.17 | 0.57±0.20 | p=0.53 | 0.38±0.10 | ||
| CoP AP velocity, cm/s | 2.05±0.67 | 1.95±0.62 | 1.95±0.71 | 2.17±0.97 | p=0.23 | 1.43±0.41 | ||
| CoP ML velocity, cm/s | 2.18±0.75 | 2.19±0.76 | 2.20±0.81 | 2.36±0.94 | p=0.41 | 1.47±0.44 | ||
| CoP area, cm2×1000 | 9.1±0.48 | 8.1±0.54 | 8.2±0.47 | 9.3±0.59 | p=0.33 | 7.9±0.39 | ||
| MMSE | 24.3±3.2 | 24.9±3.3 | 25.4±3.0 | 25.2±2.8 | p=0.46 | 28.3±1.4 | ||
| Executive function, sa | 61.5±43.7 | 67.6±65.3 | 65.8±61.2 | 62.2±43.5 | p=0.97 | 24.9±14.9 | ||
| Executive function, number | 14.2±7.7 | 14.1±7.9 | 15.2±7.3 | 15.5±7.7 | p=0.79 | 10.6±2.9 | ||
| Episodic memory, number | 21.7±5.7 | 21.7±5.6 | 24.7±5.7 | 22.4±5.8 | p=0.06 | 29.4±3.9 | ||
| Processing speed, s | 1.3±0.4 | 1.4±0.6 | 1.3±0.5 | 1.4±0.7 | p=0.48 | 12 | ||
Minimal, some, moderate, high: duration of physical activity in years; PA, physical activity; Functional reach, reach span in m divided by body height in m, unitless; 6MWT, six-minute walk test; TUG, timed up-and-go test; CoP, center of pressure; AP, anterio-posterior direction; ML, medio-lateral direction; MMSE, mini mental state examination; N/A, not applicable.
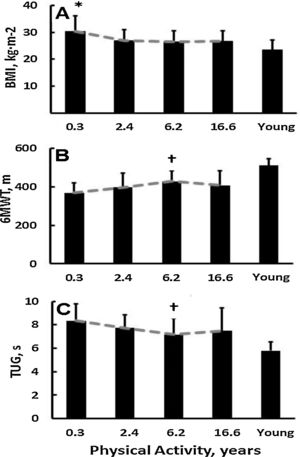
Age and height of the old sub-groups were similar (p>0.05). ‘Minimal PA’ participants were 9kg heavier than the other groups combined (p<0.05). PA duration had a dose-effect on BMI: the ‘minimal PA’ sub-group reached Class I obesity, the remaining three old sub-groups were overweight (25–30m/kg2), and the young comparison group's BMI was normal (Fig. 1A, Table 2). Education was similar (p>0.05) in the four old sub-groups.
Dose-dependent responses to years of physical activity in old adults. (A) Body mass index (* different from all other, p<0.05); (B) six-minute walk test († different from 0.3, p<0.05); (C) timed up-and-go test († different from 0.3 p<0.05). In each panel PA improved old adults’ scores toward the values of a healthy young reference group (young reference group was not included in sub-group analyses). Vertical lines denote +1 standard deviation.
Motor function. Functional reach was similar in the PA sub-groups (p>0.05). There was a dose-effect of PA duration on 6MWT: the ‘moderate’ PA group walked longer distance compared with the ‘minimal’ PA sub-group (p<0.05; Fig. 1B, Table 2). The ‘minimal’ PA sub-group needed approximately 1s longer to complete the TUG than the ‘moderate’ PA sub-group (p=0.014). The CoP measures did not differ between the PA-subgroups (all p>0.05).
Cognition. MMSE scores were similar across the old sub-groups (p>0.05). The old sub-groups produced similar scores (p>0.05) on the time and the number of movement elements of the Tower of Hanoi puzzle. Episodic memory scores were lower in the ‘short’ compared with the ‘moderate’ PA subgroup but this was not significant (p=0.06). PA duration had no dose-effects on processing speed (p>0.05).
Demographic and motor predictors of cognition, mediation analysis — Age negatively correlated with MMSE (n=201, r=−0.22, p=0.001). Education correlated with MMSE (r=0.47, p=0.001). TUG (r=−0.17, p=0.07), functional reach (r=0.16, p=0.010), and 6MWT (r=0.14, p=0.020) weakly predicted MMSE. Of these variables, education (r2=0.22, p=0.001, model 1) in combination with TUG (r2=0.23, p=0.045, model 2) only entered a forward moving stepwise multiple regression to predict MMSE (p≤0.001, model 2) and explained in combination 23% of the variation in MMSE. COP measures (dispersion, amplitude, velocity, area) and direction (AP, ML) did not predict MMSE (all p>0.05). Of age, PA duration, BMI, functional reach, 6MWT, TUG, and education, age (r2=0.06, p=0.001, model 1) in combination with 6MWT (r2=0.09), p=0.009, model 2) only predicted the temporal element of executive function (p<0.001, model 2) and explained in combination 9% of the variability in temporal element of executive function.
From the same predictors used in the previous models, education (r2=0.15, p=0.001, model 1) in combination with TUG (r2=0.20, p=0.001, model 2), and age (r2=0.22, p=0.029, model 3) predicted episodic memory (p≤0.001, model 3) and explained in combination 22% of the variability in episodic memory.
TUG (r2=0.21, p=0.001, model 1) in combination with age (r2=0.24, p=0.006, model 2), and education (r2=0.26, p=0.031, model 3) predicted processing speed (p≤0.001, model 3) and explained in combination 26% of the variability in processing speed.
Cognitive predictors of motor function and education — Of MMSE, executive function, episodic memory, and processing speed only this latter entered the forward moving stepwise regression model and weakly predicted functional reach (r2=0.05, p=0.002). Only processing speed predicted 6MWT (r2=0.14, p<0.001). Processing speed (Model 1, r2=0.26 p<0.001) predicted TUG and episodic memory further increased this prediction (Model 2, r2=0.27, p<0.001).
Distribution of PA-activities across old and young sub-groupsTable 1 describes the distribution of PA-activities across old and young sub-groups.
Old versus young adultsAs shown in Table 1, participants in the higher PA-groups were more similar regarding body weight, functional reach, walking speed, mobility and MMSE to the young adults compared with participants in the ‘minimal’ PA-group, although a dose–response relationship could not necessarily be discovered. Episodic memory scores were closest to young adults’ scores in the ‘moderate’ PA group compared with the other groups.
DiscussionPA duration in years was dose-dependently related to 6MWT in a robust U-shaped relation: participants with ∼6 years of PA walked 33 and 22m more than participants in the adjacent categories and 61m more than those with minimal PA-years (Fig. 1B, Table 2). Being physically active for longer than ∼6 years did not further increase walking distance-based aerobic fitness. For unknown reasons, the average distances (∼400m) our old and even young (∼511m) participants covered in the 6MWT were much shorter than the 631m ‘reference’ for old females.29 Because 6MWT distance negatively correlated with MMSE, episodic memory, and processing speed, sub-clinical cognitive declines might have contributed to the low 6MWT distances and aerobic fitness in our sample.
PA had a weak dose–response effect on mobility. Only the shortest PA-year sub-group's TUG time (8.4s) differed from the other three age sub-groups (7.2–7.7s). Physically active participants performed diverse PAs, including strength training, but increased leg strength as measured by TUG vs. 6MWT did not further increase the dose-effect of PA-years. Also, TUG requires participants to turn, and therefore has a balance element. The weaker dose-effect of PA on TUG vs. 6MWT may be related to a lack of effect of PA on balance, as PA-duration did not affect the CoP measures (Table 2). Because our participants did not specifically train to improve static balance, this may be the reason for a lack of dose-effects of PA on CoP measures.
Motor predictors of cognition and reversed causality — Our data are consistent with the unfavorable effects of age on cognitive functions (Table 2).30 Education and TUG predicted MMSE weakly (explained variance 23%) and motor prediction of executive function was even weaker. These latter data in particular are in contrast to short-term intervention studies reporting improvements in brain and executive function after exercise interventions, several types of which our subjects also performed,30–32 and in contrast to studies reporting a mediating role of executive function in gait.33,34 Perhaps the divergent results are related to the differences between tests used in the many studies to measure executive function and its constituents. Also, perhaps a lack of any effect of PA-years on executive function in our study reflects a diluting and/or a counter-acting effect between the many types of PA participants pursued. The low correlations between executive function and processing speed (r=0.29, p=0.001) and executive function and episodic memory (r=−0.34, p=0.001) in our sample support this idea by showing weak and non-specific effects of PA-years on elements of cognitive function.
Age, education, and TUG together predicted episodic memory (22%) and processing speed (26%), agreeing with previous behavioral and molecular data linking higher education to better episodic memory and processing speed. Slow TUG has been associated with poor episodic memory, which strengthens the ‘common cause hypothesis’ of correlated decline in motor and cognitive function.16,17 These data are in line with the emerging view that selective aspects of locomotion (pace, rhythm, symmetry, balance) are associated with specific cognitive dysfunctions35,36 and also illustrate that higher leg strength as measured by TUG may promote cognitive function. Furthermore, it was previously hypothesized that the relationship between TUG and episodic memory may be explained by better upright posture of individuals with faster TUG scores.37 Better upright posture promotes mobility and may facilitate dopamine production, which is related to better episodic memory. However, the current study is unable to confirm or reject this hypothesis.
Of the five cognitive measures, processing speed predicted 6MWT and processing speed and episodic memory together predicted TUG, providing some evidence for reverse causality in the link between PA and cognition. Although such a relationship is reported infrequently17 and tends to be weaker,38 as was the case in the present study, it nonetheless complicates the determination of causation direction and temporal course. In total, the data suggest that old adults should engage in physically and cognitively stimulating activities. Unfortunately, the current data do not specify the type of PA that would be the most effective to maintain cognitive and motor function. Regardless of the type, the delivery of PA should be customized according to individual needs and preferences, an approach that would also help keep exercise adherence high.
Limitations — Even though objective measures are preferred, questionnaire-reporting of PA is valid and reliable.39–41 The fixed time schedule of PA-classes at each location helped participants to recall the type and duration of PA-classes they attended over the years. Low and uneven sample sizes in the PA sub-groups prevented us from determining the effects of PA type and intensity on cognition. Due to the cross-sectional design, it is not possible to determine if the dose of PA-years would cause cumulatively greater cognitive benefits or just a differential effect.
ConclusionsPerforming PA up to an average of 6.2 years but no longer had favorable effects on body composition, aerobic capacity and mobility in a dose–response manner. The data strengthen current recommendations for an active lifestyle in adulthood to slow aging-related motor and cognitive decline.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported in part by Fundação Araucária 182/2013, Brazil and from start-up fund 653013 from the University Medical Center Groningen, The Netherlands. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.