There is no clear consensus on the anatomical substrates required for recovery from lower limb (LL) impairment after stroke. Knowledge of biomarkers, such as white matter integrity (WMI), could fill this knowledge gap.
ObjectivesTo analyze the associations between WMI of the corticospinal tract (CST) and corticoreticulospinal pathway (CRP) and LL motor impairment after stroke, in terms of synergistic control and muscle strength. It also explores whether any associations depend on time post-stroke.
MethodsIn April 2023, PubMed, Web of Science, and Scopus databases were systematically searched for studies associating WMI of the CST and CRP and LL motor impairment after stroke. Risk of bias was assessed using the Newcastle–Ottawa Quality Assessment Scales.
ResultsNineteen studies were included, 15 about the association between CST and motor impairment, and 4 concerning CST and CRP. Associations were consistently found between higher WMI of the CST and greater muscle strength, but not with synergistic control. There were no clear associations between WMI of the CRP and muscle strength, and associations could not be analyzed for synergistic control. The results could not determine whether the associations are time dependent.
ConclusionsThe results of this review supported using WMI of the CST to understand LL muscle strength after stroke. However, the same cannot be said for LL synergistic control due to the small number of studies. There was no clear evidence of an association between WMI of the CRP and LL muscle strength or synergistic control due to mixed results or a lack of studies.
Stroke incidence is estimated to be 13.7 million cases per year worldwide and is predicted to increase with population aging.1,2 Lower limb (LL) motor impairment after stroke is diverse. It can be disabling for the performance of activities of daily living and may include the loss of muscle strength and synergistic control (i.e., ability to move out of flexor or extensor muscle synergies).3–6 Generally, people after stroke recover approximately 70 % of the initial motor impairments within the first 6 months. The most significant improvements occur in the 8–12 weeks period, after which a plateau is reached.7,8 However, around 35 % of those with more severe initial impairments do not fit this typical recovery pattern.7
The mechanisms driving recovery of muscle strength and synergistic control after stroke are not completely understood despite ongoing research efforts. Most studies have focused on the upper limb and some prediction models for upper limb outcome have been developed.9–12 However, less is known about the mechanisms of recovery from LL impairment. Researching neurological biomarkers, such as white matter integrity (WMI), has been highlighted as a strategy to fill this knowledge gap.7,13 The road to recovery is heterogeneous because people after stroke can have different initial motor impairment. Reliable biomarkers can provide better understanding of these differences.7,10,13
The corticospinal tract (CST) and corticoreticulospinal pathway (CRP) are the two main descending white matter tracts that influence LL function.14,15 The CST is known to act on distal muscle function, while the CRP on proximal muscle function.14,16 Looking deeply into the relationship between WMI of the CST and CRP, and LL motor impairment may shed light on the anatomical substrates required for good outcomes after stroke.7,13
Diffusion magnetic resonance imaging (dMRI) can analyze WMI through three-dimensional reconstruction. The data originating from dMRI can be used to assess WMI of entire white matter tracts or specific regions of interest regardless of their depth within the brain.17 This enables studying the integrity of tracts or regions of interest that are further from the cortical layer, such as the CRP.
There are several studies about WMI of the CST and CRP and their association with motor impairment.16,18,19 So far, to our knowledge, no systematic review has been conducted to give an overview of the current evidence. Therefore, this study aims to answer the following research question: Is the WMI of the CST and CRP associated with LL muscle strength and synergistic control after stroke? Additionally, we hope to address the following secondary research question: Is the association between the WMI of the CST and CRP and LL motor impairment dependent on the time post-stroke? Investigating whether these associations differ in each phase of recovery is specifically important considering the time dependency of stroke recovery.20–22
MethodsProtocol and registrationThis systematic review was registered on PROSPERO CRD42020167167, and it adheres to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA).23
Key terms and definitionsMotor impairment results were categorized as relating to muscle strength or synergistic control. Muscle strength was defined as the ability to perform voluntary muscle contraction around a single joint and can be assessed by the motricity index (MI), medical research council (MRC) scale, and manual muscle testing (MMT).24 Synergistic control was defined as the ability to move out of pathological flexor or extensor synergies, which can be assessed by the Fugl–Meyer Assessment (FMA) tool.5,6 A lower FMA score is interpreted as worse synergistic control. Synergistic control may also be evaluated using other tools, such as electromyography. However, the focus of this study was on clinical measures considering these are widely used in clinical and research practice.
Diffusion MRI can be used to assess WMI based on random proton diffusion signals within biological tissue.25 This assessment can be done using several dMRI metrics: fractional anisotropy (FA) represents water diffusion direction; apparent diffusion coefficient (ADC) represents overall water diffusion; fiber number (FN) is a synonym to all terms referring to the number of neuronal fibers within a region of interest; and fiber volume (FV) covers all terms referring to the volume of a region of interest composed by white matter.25,26 The biological meaning of higher or lower values varies according to the metric. In this review, we refer to “higher WMI” and “lower WMI”. “Higher WMI” will be used when studies give one or more of these results: higher FA, FN, or FV or lower ADC.27–29 Conversely, “lower WMI” will be used when studies give one or more of these results: lower FA, FN, or FV or higher ADC.27–29
The condition of the WMI can also be categorized based on diffusion metrics and thresholds pre-defined by each study to differentiate between preservation, lesion, and post-stroke degeneration. In our results, we used the descriptors: “+”, referring to the preservation of the tract, “−”, referring to lesion of the tract, and “d” referring to degeneration. For example, CST+ means the CST was preserved, CST− means there was a lesion, and CSTd means there was degeneration of the tract from remote structural damage. The statements about preservation, lesion, and post-stroke degeneration are based on the analysis of regions of interest defined by each study. One study also analyzed the origin of the fibers passing through such region of interest.19 The authors divided the WMI of the CST between CST+, CST−, and CST ± M1. The last category (CST ± M1) refers to a preserved CST but composed of fibers originating from cortical areas other than M1 (e.g., primary sensory cortex, posterior parietal cortex, premotor cortex, prefrontal cortex, somatosensory cortex, and others).19
The phases of recovery were defined to allow contextualization of the studies within the course of post-stroke recovery: hyperacute phase is from 0 to 24 h; acute phase from 1 to 7 days; early subacute phase from 7 days to 3 months; late subacute phase from 3 to 6 months; and chronic phase after 6 months.30
Data sources, search strategy and study selectionA systematic literature search (Supplementary online material S1) was conducted in April 2023 using PubMed, Web of Science, and Scopus databases. A hand-search was performed for the reference lists of the included articles. Cross-sectional and longitudinal cohort studies about the association between the WMI of the CST and CRP and LL motor impairment were eligible for inclusion.
The following additional inclusion criteria were used: 1) adults (≥18 years of age) after first ischemic or hemorrhagic stroke; 2) assessment of the WMI of the CST and/or CRP using dMRI; 3) LL motor impairment assessed with clinical tests; 4) studies in English, Portuguese, Spanish, Dutch, French, or German. The following exclusion criteria were used: 1) meta-analyses, systematic reviews, case reports, or expert opinions; 2) not reporting LL motor impairment results separately from upper limb. Three blinded independent reviewers (R. L-C., E.E, and A. v. H.) screened the studies based on title, abstract, and full text. Disagreements were successfully resolved by discussion.
Quality assessmentRisk of bias was assessed independently by two reviewers (R. L-C. and E.E) using the Newcastle–Ottawa Quality Assessment Scales (NOS) for cross-sectional and longitudinal cohort studies (Table 1). The adapted version by Herzog et al. was used for cross-sectional studies.31 A maximum score of nine could be allocated for longitudinal cohort studies and 10 for cross-sectional studies when all criteria were met. Because there are no standard cut-off values, those suggested by McPheeters et al. were used to define the risk of bias and methodological quality (<5: poor, 5–6: moderate, ≥7: good).32 Studies with poor methodological quality were excluded from the written results and discussion to improve the quality of this review, but they are included in the data extraction tables.
Risk of bias.
The following data were independently extracted by two reviewers (R. L-C., E.E): sample characteristics (sample size, sex, age, imaging parameters, and LL motor impairment test) (Tables 2 and 3), and results regarding the association between WMI of the CST and CRP and LL motor impairment (Table 4). Results are reported in a compiled manner to give an organized overview of the findings. Table 4 can be consulted for information about statistical tests and their detailed results.
Sample characteristics.
Table description– D: design; N: number; SD: standard deviation; CS: cross-sectional; C-R: cohort retrospective; C-P: cohort prospective; NM: not mentioned; I: Infarction; H: Haemorrhage; CST: corticospinal tract; CST+: CST preservation; CST−: CST lesion; CSTd: CST degeneration; CST ± M1: CST preservation without M1 fibers; CST-ant: anterior CST lesion; CST-central: central CST lesion; CST-post: posterior CST lesion; CRP: corticoreticular pathway; CST+/CRP+: CST and CRP preservation; CST−/CRP+: CST lesion and CRP preservation; CST+/CRP−: CST preservation and CRP lesion; CST−/CRP−: CST and CRP lesion; CR: corona radiata; BG: basal ganglia; IC: internal capsule; Mid: midbrain; PVWM: periventricular white matter; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; ICA: internal carotid artery; AcoA: anterior communicating artery; PcoA: posterior communicating artery; MRC: medical research council.
Summary of imaging and motor impairment assessments.
Table description– TPS: time post stroke; SD: standard deviation; NM: not mentioned; CST: corticospinal tract; CST+: CST preservation; CST−: CST lesion; CSTd: CST degeneration; CST ± M1: CST preservation without M1 fibers; CRP: corticoreticular pathway; CP: cerebral peduncle; CR: corona radiata; IC: internal capsule; MRC: medical research council; MI: motricity index; MMT: manual muscle test; FMA: fugl meyer assessment.
Summary of results and conclusions.
Table description– D: design; RoB: risk of bias; Mod: moderate; CST: corticospinal tract; CST+: CST preservation; CST−: CST lesion; CSTd: CST degeneration; CST ± M1: CST preservation without M1 fibers; CRP: corticoreticular pathway;; CRP−: CRP lesion; CRP+: CRP preservation; CST-ant: anterior CST lesion; CST-central: central CST lesion; CST-post: posterior CST lesion; MRC: medical research council; MI: motricity index; MMT: manual muscle test; FMA: fugl meyer assessment; WMI: white matter integrity; FA: fractional anisotropy; FN: fiber number; FL: fiber length; ADC: apparent diffusion coefficient; FV: fiber volume; MD: mean diffusivity
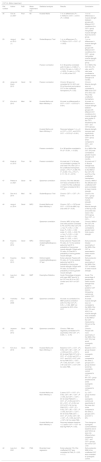
A total of 4145 studies were found after deduplication and hand-search. A total of 48 studies were selected based on title and abstract and 19 were included after full-text screening. Details can be found in the flowchart (Fig. 1).
Study characteristicsEleven cross-sectional and eight longitudinal cohort studies were included, for a total of 781 people (682 with stroke, 99 healthy controls). Diffusion MRI was acquired in the acute phase in two studies,33,34 in the early subacute in ten,19,29,35–42 and in the chronic in seven.18,28,43–47 The WMI of the CST was assessed in 15 studies,18,19,29,33–35,37–40,42,44–47 of the CRP in one,36 and of both tracts in three (Tables 2 and 3).28,41,43
Motor impairment was assessed in the acute phase in seven studies,28,29,33–35,37,40 in the early subacute in six,19,36,38,39,41,42 and in the chronic phase in six.18,43–47 Muscle strength was measured by the MI in 10 studies,18,29,35–37,40,41,43,46,47 MRC in four,28,38,39,44 and MMT in two.33,34 Synergistic control was measured using the FMA in three studies.19,42,45
Risk of biasThree cross-sectional studies were graded as having “moderate”18,33,41 and eight “good” methodological quality.36,38,39,43–47 The scores ranged from five41 to nine.47 Three longitudinal cohort studies were graded as “poor”,34,35,40 three as “moderate”,29,37,42 and two as “good”.19,28 The scores ranged from three40 to seven (Table 1).19,28 The results of the three cohort studies with poor methodological quality can be found in the data extraction tables to provide a complete overview.34,35,40
An important source of bias was samples that were not representative of the population because they included either ischemic or hemorrhagic strokes. From the 19 studies included, only eight included both types.18,19,28,43–47 Another source of bias was lack of comparability, because only 10 studies corrected for key factors such as age, sex, and spasticity.19,28,29,36–39,42,45,47 Only one of the eight cohort studies included specific information about the drop-out rate.34 In addition, no study had multiple dMRI assessments, meaning there are no data documenting recovery of WMI over time on a same group individuals.
White matter integrity in the early subacute phase: CSTCross-sectionalThe association of WMI of the CST with motor impairment was assessed cross-sectionally in the early subacute phase by three studies.19,38,48 Higher CST integrity was associated with greater muscle strength38,48 and better synergistic control.19 One study used the distinction between CST preservation (CST+), CST preservation without M1 fibers (CST ± M1), and CST lesion (CST−).19 The results showed that those with CST lesions (CST−) had worse synergistic control than those with other CST conditions (CST+, CST ± M1).19
LongitudinalThe association of WMI of the CST with motor impairment was assessed longitudinally by three studies.19,29,37 Two studies assessed WMI of the CST in the early subacute phase but motor impairment at the acute and chronic phases.29,37 The third assessed WMI in the early subacute phase and motor impairment in the early subacute and chronic phase.19
Different CST conditions (CST+, CST−, and CSTd) did not show variations in muscle strength in the acute phase.29,37 People with early subacute CST preservation (CST+) had greater muscle strength at the chronic phase than those with other CST conditions (CST−, CSTd).29,37 Likewise, higher WMI of the CST in the early subacute phase was correlated to greater muscle strength at the chronic phase.29,37 The presence of CST lesion resulted in worse synergistic control compared to CST preservation and CST preservation without M1 fibers at the chronic phase follow-up.19
Two studies analyzed recovery during the first six months after stroke.19,37 People with CST preservation had greater muscle strength recovery than those with CST lesions and degeneration over this period.37 The difference in synergistic control between early subacute and chronic phases was only significant when comparing CST lesions and CST preservation, but not for CST preservation without M1 fibers.19
White matter integrity in the early subacute phase: CST and CRPCross-sectionalThe association of WMI of the CST and CRP with motor impairment was assessed cross-sectionally in the early subacute phase by two studies.36,41 People with preserved CST and CRP (CST+/CRP+), or either CST (CST−) or CRP lesions (CRP−), had greater muscle strength compared to those with both CST and CRP lesions (CST−/CRP−).41 Those with no lesions (CST+/CRP+) had greater muscle strength than those with either CST or CRP lesion,41 particularly for the hip muscles.36 People with CST or CRP lesions had similar muscle strength.41 The WMI of the CRP in the affected hemisphere was positively correlated to greater muscle strength of the hip.36
White matter integrity in the chronic phase: CSTCross-sectionalThe association of WMI of the CST with motor impairment was assessed cross-sectionally in the chronic phase by five studies.18,44–47 Those with CST preservation had greater muscle strength compared to those with CST lesion.18 Those with CST lesion and CST preservation had greater muscle strength of hip flexors, knee extensors, and ankle dorsiflexors than those with CST degeneration.44
Higher WMI of the CST was correlated with greater muscle strength44 and synergistic control.45 In the unaffected hemisphere, there was no correlation between WMI of the CST and muscle strength.46 However, another study found a correlation between the WMI of the CST in either hemisphere and muscle strength.47
White matter integrity in the chronic phase: CST and CRPCross-sectionalThe association of WMI of the CST and CRP with motor impairment was assessed cross-sectionally in the chronic phase by two studies.28,43 In the unaffected hemisphere, there was a positive correlation between WMI of the CRP and muscle strength, but a negative correlation between the CST and muscle strength.43
Only one study found distinct results for each of the diffusion metrics.28 Those with similar WMI of the CST and CRP in either hemisphere measured by fractional anisotropy and apparent diffusion coefficient had different dorsiflexion strength.28 The results concerning fiber volume did not follow the same pattern. Those with similar WMI of the CST in either hemisphere or CRP in the affected hemisphere measured by fiber volume had different ankle dorsiflexion strength.28 However, those with higher WMI of the CRP in the unaffected hemisphere, measured by fiber volume, had greater ankle dorsiflexion strength.28
There was no correlation between WMI of the CST and CRP in either hemisphere measured by fractional anisotropy and apparent diffusion coefficient and ankle dorsiflexion strength.28 There was also no correlation between WMI of the CST and CRP in the affected hemisphere measured by fiber volume and ankle dorsiflexion strength.28 However, there was a correlation between WMI of the CST and CRP in the unaffected hemisphere measured by fiber volume and ankle dorsiflexion strength.28
DiscussionThis review systematically gathered the current evidence on the associations between the WMI of the CST and CRP and, LL strength and synergistic control after stroke, cross-sectionally and longitudinally in different phases of recovery. The literature showed the following main findings: 1) higher WMI of the CST is associated with greater muscle strength and synergistic control when measured cross-sectionally in the early subacute phase; 2) higher WMI of the CST is associated with greater muscle strength and synergistic control recovery from the early subacute to the chronic phase; 3) higher WMI of the CRP is associated with greater muscle strength when measured cross-sectionally in the early subacute phase; 4) no studies have evaluated associations between longitudinal changes in WMI of the CST and CRP and changes in muscle strength and synergistic control.
The main findings point to an association between higher WMI of the CST and greater muscle strength after stroke. However, the small number of studies addressing synergistic control does not allow for a conclusion. This may be due to most studies reporting synergistic control of the lower and upper limb combined, making them ineligible for this review. The neural control of the lower and upper limbs is known to differ, making an analysis of the neural substrates of motor outcome separately more appropriate.49
A consensus regarding the WMI of the CRP, muscle strength, and synergistic control is not possible due to conflicting results and a general lack of studies. The sparse and conflicting evidence could be explained by a methodological obstacle to assess the CRP. The regions of interest corresponding to the CRP have to be manually delineated on the images during post-processing. In comparison, the selection of the CST can be done based on existing templates, such as the Johns Hopkins University (JHU) atlas.43,50 We argue that the focus on the CST alone may result from pragmatic reasoning, pointing towards an important knowledge gap on anatomical biomarkers of post-stroke outcomes. Therefore, future studies should investigate the associations of the WMI of both the CST and CRP with LL motor impairment.
A trend linking higher WMI in the early subacute phase with greater muscle strength and synergistic control was observed. This was found for early subacute cross-sectional and longitudinal studies that assessed WMI in the early subacute phase and related these scores to motor impairment outcomes in the chronic phase. The study of anatomical substrates that can predict subsequent motor impairment post-stroke has the ultimate goals of informing clinicians and increasing the efficiency of decision-making in rehabilitation. The data gathered suggest that the condition of the CST evaluated in the early subacute phase may contribute to such prediction models for LL recovery. However, the current evidence is inconclusive. In addition, because no study had repeated measures of WMI, longitudinal studies with repeated measures of both WMI and motor impairment are needed. Such studies may help explain recovery of motor impairment over time and, more specifically, the time dependency of the associations between WMI and LL motor impairment post-stroke.
Results could not explain in detail how the WMI of the CST and CRP separately influence synergistic control or muscle strength of the LL. Only one study had a sample divided into groups without any lesion, with CST or CRP lesions, or with both lesions.41 Both tracts contribute to LL motor function differently, with the CST having a more distal action and the CRP more proximal.14,16 For the upper limb of non-human primates, for example, the CST has been related to fine adjustments while the reticulospinal tract (downstream part of the CRP) is related to overall strength.51 Investigating more in depth how the WMI of the CST and CRP post-stroke separately influence LL motor function might help to differentiate between possible outcome phenotypes.
Because only a single a study reported that people with an injured CST have worse synergistic control in comparison to preserved CST with or without absence of M1 fibers, firm conclusions cannot be drawn.19 However, this study does indicate that future research should focus on more than only assessing the CST itself. Detailed information about the origin of damaged or preserved fibers within white matter tracts or whole-brain WMI could also help to explain why some patients have better outcomes than others.
This study contains certain limitations. First, we were only able to include 19 studies. This may be explained by the inaccessibility of MRI measurements for clinical research. Second, we excluded studies with poor methodological quality from the written results and discussion. This decision was made to increase the quality of our systematic review, but it meant excluding three of the 19 studies. Third, we did not include neurophysiological techniques to assess the functional integrity of white matter tracts, such as transcranial magnetic stimulation.52 However, focusing on WMI assessed by dMRI allows for the analysis of deeper brain regions and tracts in comparison to TMS.53
ConclusionWe analyzed the association between WMI of the CST and CRP, and LL muscle strength and synergistic control, after stroke. The relevance of WMI of the CST to LL muscle strength outcomes has been reinforced by the associations found in this review. Results point to an association between higher WMI of the CST in the early subacute phase and greater muscle strength and synergistic control assessed in the chronic phase.
More research is needed into the association between WMI of the CST and synergistic control considering the small number of studies. No consensus was reached concerning the association between WMI of the CRP and LL muscle strength or synergistic control due to inconsistent results or lack of studies, respectively. In addition, this review was not able to answer whether the association between WMI of the CST and CRP and LL motor impairment is time dependent. To fully answer both research questions, longitudinal studies with repeated measures of WMI of CST and CRP, muscle strength, and synergistic control are needed.
The authors declare no competing interest.
Jonas Schröder has received a doctoral grant from Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek FWO - 1S64819N). LAETITIA YPERZEELE has received funding from the University of Antwerp, Belgium (DOCPRO project ID: 44607). WIM SAEYS has received funding from the University of Antwerp, Belgium (DOCPRO project ID: 40180).
PROSPERO registration no. CRD42020167167.