Type 2 diabetes (T2D) is the most prevalent in the world population, and exercise is one of the main non-pharmacological interventions to treat this health condition.
ObjectiveTo evaluate the effect of a single session of aerobic exercise (AE) and/or resistance exercise (RE) on post-exercise glycemia in individuals with T2D.
MethodsA literature search was conducted in CINAHL, Cochrane Library, EMBASE, Google Scholar, LILACS, MEDLINE/Ovid, SciELO, SPORTDiscus, and Web of Science up to May 2024, randomized and non-randomized clinical trials were included. The risk of bias and the certainty of evidence were assessed using the Cochrane "Risk of Bias" and GRADE tools, respectively.
ResultsInitially, 7210 studies were identified, 26 were included in the systematic review, and 13 in the meta-analysis. A single session of continuous AE (CAE), interval AE (IAE), or RE promoted a significant reduction in glycemia in the first minute after exercise (-1.48 mmol/L [95 % CI:-1.73, -1.23]; -2.66 mmol/L [95 % CI:-3.48, -1.84]; -1.18 mmol/L [95 % CI:-2.15, -0.21], respectively), compared to the control session. This reduction persisted for up to 10 min after the CAE session (-1.61 mmol/L [95 % CI:-2.21, -1.01]) and up to 30 min after the IAE session (-1.11 mmol/L [95 % CI:-1.88, -0.35]). The risk of bias was assessed as uncertain, and the quality of the evidence was moderate.
ConclusionCAE and IAE reduces glycemia for a period of up to 10 or 30 min after its completion, respectively, while a single session of RE reduces glycemia only in the first-minute post-exercise in individuals with T2D.
Type 2 diabetes (T2D) accounts for between 90 and 95 % of diabetes cases worldwide and is characterised by alterations in glucose metabolism that arise when the pancreas can no longer produce insulin at adequate levels and/or when the tissues develop resistance to the action of this hormone, impairing the uptake and storage of glucose by the cells.1,2
Exercise, both aerobic (AE) and resistance (RE), is one of the main non-pharmacological interventions for treating this health condition.1,3 During these exercises, adjustments in hormonal balance occur, leading to a decrease in insulin synthesis and secretion, and an increase in tissue glucose uptake due to greater translocation of the glucose transporter type 4 (GLUT 4) to the cell membrane stimulated by muscle contraction.1,3 After the exercise session, there is a reduction in tissue resistance to insulin action, facilitating glucose uptake by a pathway independent of the insulin cascade.1,3 The glycemic response to exercise can vary depending on the intensity, duration, and type of exercise performed,4 and this response is a clinically relevant outcome in individuals with T2D, as the metabolic changes triggered by exercise directly impact blood glucose levels.2
To date, to our knowledge, there has been no systematic review and meta-analysis conducted to investigate the acute effects of a single exercise session on glycemia in individuals with T2D. Although the systematic review and meta-analysis published by Munan et al.,5 demonstrated improvement in glycemia in response to AE and RE, isolated or combined, in adults with T2D, the acute effects on glycemia were considered from the 24-hour glucose profile assessed after short-term (exercise sessions lasting ≤ 2 weeks) and long-term (> 2 weeks) exercise training and only the 24-hour glucose profile was assessed.
It is essential to understand the acute effects of exercise on glycemia in individuals with TD2 to follow an individual approach in prescribing exercise considering their specific metabolic responses and to prevent glucose-related complications that can occur after exercise.6 In this context, this study aimed to evaluate the acute effects of a single session of AE and/or RE on glycemia in individuals with T2D from glycemia measurement at different times up to 24 h post-exercise.
MethodsStudy designThe protocol of this study was registered in PROSPERO (CRD 42022289985) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search strategyThe literature search was conducted in the electronic databases CINAHL, Cochrane Library, EMBASE, Google Scholar, LILACS, MEDLINE/Ovid, SciELO, SPORTDiscus, and Web of Science, from the inception of the databases until May 8, 2024. The search included studies with human subjects, without language restrictions. The search strategy combined DeCS/MeSH descriptors and their synonyms "Diabetes Mellitus, Type 2″ AND "Exercise" AND "Glucose," as shown in the Supplementary material – Table S.1.
Eligibility criteria and study selectionThe search was structured according to the PICOS criteria: 1) Population: men and/or women diagnosed with T2D (≥ 18 years of age); 2) Intervention: a single session of AE and/or RE; with AE involving large muscle groups in rhythmic or dynamic movements, characterized as continuous (CAE) or interval (IAE).8 In comparison, RE involves the use of muscular strength to move a weight, is a brief activity, and engages isolated muscle groups, including isometric, concentric, or eccentric contractions against a body segment load or an external load.8 3) Control: no exercise or any type of exercise that did not meet the characteristics of the intervention and that was performed by individuals with T2D; 4) Outcome: blood glucose levels in response to a single session of AE and/or RE, with glycemia measurements taken before and within a period of up to 24 h after the session; 5) Study type: Randomized controlled trials, including parallel or crossover designs with a washout period exceeding 72 h, and non-randomized clinical trials.
The exclusion of duplicate articles and the evaluation of titles and abstracts of the articles retrieved from the databases were performed using the Ryyan9 tool, and the eligible articles were stored using the Mendeley Desktop software.
Screening and data extractionTwo independent reviewers (JAA, CVO) read the studies in their entirety and systematically extracted data from the included articles. Disagreements were resolved by a third reviewer (LPS) and the data were analyzed using qualitative and quantitative syntheses, where possible meta-analysis.
The data were extracted using a standardized spreadsheet developed by the authors using Microsoft Excel software, and any missing data were requested by email from the authors of the studies. The following data were extracted: 1) characteristics of the study population (age, sex, body mass index (BMI), time elapsed since diagnosis, medications, glycated hemoglobin, diabetes mellitus (DM) complications, comorbidities, smoking, and level of physical activity); 2) aspects of the intervention; and 3) outcome of interest (pre- and post-exercise glucose values). Additionally, information regarding any changes in diet and medication use before the session was also extracted.
Risk of bias and certainty of the evidenceThe studies' risk of bias was assessed independently by two authors (JAA, APDBB) and they were classified as "low", "uncertain", or "high" risk.10 The Cochrane "Risk of Bias" tool (RoB 2) was used to assess the risk of bias in randomised clinical trials with a parallel design and the "Revised Cochrane Risk of Bias” tool for randomized trials (RoB 2) - Additional considerations for crossover trials" was used to assess the risk of bias of randomized and non-randomized crossover design studies.10 In the present study, funnel plot asymmetry was not performed to assess publication bias in the meta-analyses because the meta-analyses presented included fewer than 10 studies.11
The certainty of the evidence and the strength of the recommendations found in the meta-analysis for the investigated outcome were assessed according to GRADE and was categorised as "high", "moderate", "low", or "very low".12
Data synthesis and analysisFor studies that reported glucose values in mg/dL, the values were converted to mmol/L, considering that 1 mmol/L is equivalent to 18.02 mg/dL.13 Studies with different glucose measurement interventions and/or post-exercise session time points, as well as non-randomized clinical trials (quasi-experimental) and those where data extraction was not possible, had their results qualitatively analyzed. Data were combined for meta-analysis using a minimum of two randomized clinical trials assessed as clinically homogeneous, considering the type of exercise performed and the timing of post-intervention outcome (glucose) measurement. Statistical analyses were conducted following the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions.11 When applicable, dispersion data were converted from standard error to standard deviation.
The meta-analysis was conducted using Stata 17.0 software (STATA Corp., College Station, TX, USA), considering the DerSimonian and Laird random-effects model. For each study, the mean, standard error or standard deviation, and sample size were extracted. The mean differences and 95 % confidence intervals (CI) were calculated. Effect measures were derived from post-exercise glucose values, and studies were analyzed separately based on the type of exercise performed. A significance level of 5 % was established.
Statistical heterogeneity between studies was quantified using Cochran's Q test and inconsistency (I²),14 and the estimates of the variation in heterogeneity (τ²) to assess the variance between studies, it is a measure of clinical relevance.15 The statistical significance was defined as a P < 0.05 in Cochran's Q test, as for the inconsistency test, I² >75 % was considered to indicate high heterogeneity, I² between 25 % and 75 % to indicate moderate heterogeneity, and I² <25 % to indicate low heterogeneity.14 The results were represented in forest plots.
ResultsLiterature search and screeningThe literature search identified 7210 studies. After removing duplicates, 5793 were screened by title and abstract, of which 77 articles were assessed for eligibility. After searching for and reading the full texts, 51 studies were not eligible for the following reasons: two of them included participants without T2D (participants); seven did not assess the acute effect of exercise, five involved interventions that were not AE or RE, and 12 had a washout period shorter than 72 h (intervention); nine did not include a control group (control); two were excluded due to translation issues (unable to translate); two were literature reviews (study design); and 12 were conference abstracts.
Twenty-six studies were included in this review, with 13 of them (52 %) being grouped through meta-analysis techniques (Fig. 1).
Characteristics of included studiesOf the 26 studies included in this review, 13 investigated CAE vs control,16-28 six investigated IAE vs control,29-34 three investigated RE vs control,35-37 three investigated CAE vs IAE vs control,38-40 and one study41 investigated CAE vs IAE vs AE followed by RE vs RE followed by AE vs control. For the studies by Bellini et al.41 and Marcotte-Chénard et al.,34 we considered the comparison of each exercise session with the control session and not the comparison between exercise sessions.
The studies included were published between 1997 and 2024, and most of them were conducted in the USA (24 %), followed by Canada (15 %), China (11 %), Denmark (11 %), the Netherlands (11 %), Brazil (8 %), Italy (8 %), Iran (4 %), Portugal (4 %), and the United Kingdom (4 %). Three studies reported no adverse effects from the exercise session investigated.28,32,40 In the other 23 studies16-27,29-31,33-39,41 the occurrence or non-occurrence of adverse effects of the exercise session investigated was not reported. Concerning adherence to the exercise session investigated and the control session, in all the studies the experimental protocol was completed in full by the participants and the statistical analyses were performed per protocol.
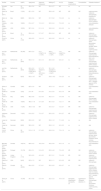
Characteristics of study participantsThe total sample was made up of 438 participants aged between 21 and 73 years, with the mean BMI ranging between 22.2 ± 2.3 and 37.0 ± 5.7 kg/m2; with the majority being male (290 men, 133 women, and 15 not reported). When reported, the average time elapsed since T2D diagnosis ranged from 1.8 ± 1.0 to 11.6 ± 1.9 years, and the mean value of glycated hemoglobin (A1c) varied between 6.0 ± 0.3 % and 10.4 ± 3.0 %. Regarding diabetes complications, 15 studies reported that participants did not have complications,16,18-20,22-26,28,29,33,34,38,41 and this information was not reported in 11 studies.17,21,27,30-32,35-37,39,40 Seven studies reported no diabetes comorbidities,16,23,24,33,35,38,41 and this information was not available in 18 studies.16,18-22,25-27,29-32,34,36,37,39,40 The sociodemographic and clinical characteristics of participants from each study are described in Table 1.
Characteristics of participants in the studies included in the systematic review.
Abbreviations: A1C, glycated hemoglobin; BMI, body mass index; DPP4, dipeptidyl peptidase 4; IT, insulin treated; N (M/F), number of individuals (male/female); NIT, non-insulin treated; NR, not reported; NS, not specified.
*medication use was maintained during the days of exercise or control sessions.
Exercise sessions lasted from 1031 to 60 min.21,23,27,32 Most of the studies that evaluated CAE performed a single exercise session at moderate intensity,16-19,21-24,26-29,38-40 only one study performed the exercise session at low intensity25 and one study conducted the exercise session with high intensity.17 Among the studies that evaluated a session of IAE, only one study conducted a single session with moderate intensity,32 while the remaining studies performed the exercise at high intensity.30-34,38-40 Of the four studies that assessed a single session of RE, three of them conducted the exercise session with moderate intensity,36,37,41 and only one study conducted a session with both low and high intensities.35 Regarding the time of day, the majority of interventions were conducted in the morning (68 %).16,17,21-24,27-30,32-35,38-41
Glucose was measured from interstitial fluid in 13 studies,18,21,22,23,25-27,29-31,34,35,40 from blood collected through a venous catheter in eight studies,16,19,24,32,33,36-38 and from capillary blood measured using a glucometer in five studies.17,20,28,39,41 Regarding pre-exercise diet, 92 % of the included studies17-31,33-41 standardized the diet ingested on intervention days, with one study not reporting whether there was standardization of the diet,16 and one study did not standardize the diet ingested by participants pre-exercise.32 The characteristics of the investigated exercise sessions are described in the Supplementary material (tables S.2.1, S.2.2, and S.2.3).
Synthesis of resultsOf the 13 studies that assessed the effect of a session of CAE on blood glucose,16-28 ten of them reported a significant reduction in post-CAE glucose,16,17,19-26 with no difference in three studies.18,27,28 Of the six studies that investigated a session of IAE vs. control session,29-34 two of them found a significant reduction in post-IAE glucose,29,31 with no difference in four studies.30,32,33,34 Of the three studies that investigated a session of CAE vs. IAE vs. control session,38-40 two of them showed that both CAE and IAE sessions significantly reduced post-exercise glucose,38,39 and in one study there was no significant change in glucose in response to any of the investigated sessions.40 The three studies that investigated RE vs. control sessions did not find a significant reduction in post-exercise glucose.35-37 In the study that investigated a single session of CAE vs. RE vs. CAE followed by RE vs. RE followed by CAE vs. control session,41 the CAE and RE sessions, both individually and combined, significantly reduced post-exercise glucose compared to the control session.
The reduction in blood glucose persisted for up to 24 h post-CAE, up to 30 min post-IAE, up to 60 min post-RE, and up to 45 min after combined CAE and RE. The mean and standard deviation (SD) values and glucose values from studies that were not included in the meta-analysis are presented in Table S.3, and the reasons for non-inclusion are detailed in Table S.4 of the Supplementary material.
Study quality and risk of biasThe risk of bias was assessed as low in one study,26 uncertain in 22 studies,16-25,27,28,30,33-41 and high in three studies.29,31,32 Although 84 % of the included studies in this systematic review were RCTs,16-28,33,35-41 the randomization method and allocation sequence concealment were not always clearly reported. Only five studies reported the methods used for randomization order,16,26,28,38,40 and only one study reported allocation sequence concealment.26
Considering the nature of the investigated intervention (exercise), it was not possible to blind participants and researchers. On the other hand, the evaluated outcome (glucose) is not influenced by the lack of blinding to the intervention. The results obtained from the Cochrane Risk of Bias tool are illustrated in Fig. 2.
Cochrane Risk of Bias Tool. D1: bias due to the randomization process; DS: bias from period and carry-over effects; D2: bias due to deviations from intended interventions; D3: bias due to missing outcome data; D4: bias due to outcome measurement; and D5: bias due to selective outcome reporting.
Fig. 3 (Panel A) presents the results of the comparison between a CAE session and a control session, showing a statistically significant reduction in glucose of 1.48 mmol/L (26.7 mg/dL) in the first-minute post-CAE. Ten minutes post-session of CAE there was a significant reduction in glucose of 1.61 mmol/L (29.01 mg/dL) (Fig. 3B). There was a decrease in glucose by 0.57 mmol/L (10.27 mg/dL) at the 30-minute mark post-CAE and a reduction of 1.15 mmol/L (20.72 mg/dL) in glucose 60 min after the CAE session (Fig. 3C; Fig. 3D), although the difference was not statistically significant.
Forest plots comparing post-session glucose levels. (A) CAE vs. control session at the first minute. (B) CAE vs. control session at 10 min. (C) CAE vs. control session at 30 min. (D) CAE vs. control session at 60 min. (E) IAE vs. control session at the first minute. (F) IAE vs. control session at 30 min. (G) RE vs. control session at the first minute. (H) RE vs. control session at 30 min. (I) RE vs. control session at 60 min. (J) RE vs. control session at 90 min. (L) RE vs. control session at 120 min.
When comparing IAE vs. control session, a statistically significant difference was observed at both analyzed moments, with a decrease in glucose values of 2.66 mmol/L (47.92 mg/dL) in the first-minute post-IAE (Fig. 3E) and a reduction of 1.11 mmol/L (20.00 mg/dL) 30 min post-IAE (Fig. 3F).]
When comparing RE vs. control session, there was a significant reduction in glucose of 1.18 mmol/L (21.26 mg/dL) in the first-minute post-RE (Fig. 3G). Glucose levels were also evaluated at 30 min post-session (−0.27 mmol/L) (Fig. 3H), 60 min (0.37 mmol/L) (Fig. 3I), 90 min (0.86 mmol/L) (Fig. 3J), and 120 min post-RE (0.57 mmol/L) (Fig. 3L); however, the difference was not statistically significant, and from 60 min post-session, glucose tends to ascent.
At all analyzed time points (CAE vs. control session, IAE vs. control session, and RE vs. control session), the included studies showed low heterogeneity (I²: 0 %).
According to GRADE,12 the comparisons of CAE vs. control, IAE vs. control, and RE vs. control have moderate certainty of evidence (Table 2).
Assessment of the certainty of evidence.
CAE, continuous aerobic exercise; CI, confidence interval; IAE, interval aerobic exercise; RCT, randomized clinical trial; RE, resistance exercise.
The findings of the quantitative analysis revealed that a single CAE, IAE, and RE promoted a significant reduction in blood glucose levels in the first-minute post-exercise. This reduction persisted for up to 10 min after the CAE session and up to 30 min after the IAE session in individuals with T2D. These findings have clinical implications in the management of T2D and the prevention of diabetes complications because lower blood glucose levels lead to an improvement in metabolic, inflammatory, and lipid markers in adults with diabetes, and physical exercise can provide significant benefits such as improved insulin sensitivity, glucose uptake by the muscle and, consequently, a reduction in glycaemic levels.42
In this review, studies were grouped for the conduct of meta-analyses based on the similarity between the characteristics of interventions and the timing of blood glucose assessment after a single exercise session or control session. Despite the rigorous systematization of the search conducted in scientific databases and statistical analyses revealing low statistical heterogeneity among the studies, they exhibited clinical heterogeneity. This indicates differences in important variables related to the intervention and participants' characteristics, which may impact their clinical applicability.43
Among the intervention variables that exhibited clinical heterogeneity, it is noteworthy to highlight the time interval between the last meal and the commencement of exercise in the included studies, ranging from 30 min to 5 h. Additionally, some studies conducted sessions with individuals in a fasting state. Recent studies suggest that, for individuals with diabetes, emphasis should be placed on increasing energy expenditure after the largest meal of the day.6,44 It is considered that the optimal time for engaging in exercise is approximately 30 min to an hour after a meal, aiming to offset the post-meal glucose peak.44 This occurs because the ingested glucose would be utilized as an energy substrate, preventing post-meal hyperglycemia. It is well-documented in the literature that physical exercise is effective in reducing post-meal hyperglycemia, with a rapid glucose recovery when performed within 60 min after a meal. Beyond this period, the response of each individual becomes more personalized.6,45
Regarding participants' characteristics, clinical heterogeneity was observed in the time since the diagnosis of diabetes, comparing individuals recently diagnosed with those dealing with more advanced stages of the disease. The latter may present complications or damage to organs and tissues that are not present at the onset of the disease. Additionally, there was also clinical heterogeneity in BMI, as comparing a lean individual with an obese one would be inappropriate, as this condition directly influences the endocrine profile and performance during exercise.46 Despite these clinical heterogeneities, it was possible to group the studies, and statistical heterogeneity was absent.
The ability of exercise to regulate glucose levels appears to be associated with the intensity of the activity.47 The higher the intensity, the greater the rate of glucose uptake by the muscles. When performed at moderate to high intensities, exercise is considered an efficient influencer capable of reversing many factors associated with T2D, leading individuals to healthier conditions.48 In the present review, the majority of studies that assessed CAE and RE conducted sessions at moderate intensity, while studies investigating IAE implemented high-intensity sessions. This suggests that the exercise sessions in the included articles were conducted within an intensity range considered desirable. Furthermore, it is relevant to highlight that all studies analyzed in this meta-analysis involved structured and supervised exercise sessions, underscoring the importance of correctly performing the exercises to prevent the occurrence of severe hypoglycemia.42
Although AE has traditionally been recommended for individuals with T2D, international guidelines, starting in the 2000s, also began to include RE as part of diabetes treatment.1,2 Recent studies have highlighted the potential of RE to contribute to glycaemic control.4,49 In the present review, it was observed that a single RE session resulted in a reduction of approximately 1.17 mmol/L in blood glucose. In addition, it was noted that from 60 min after the RE session, glycemia tended to increase. These results should be interpreted with caution due to the limited number of studies included that evaluated the effect of post-session RE on glycemia. It is important to emphasize that, despite the beneficial effects of RE, a potential challenge for its implementation is the requirement for specific professionals, equipment, and facilities, which may impact people's accessibility to this type of exercise.50
Worldwide, diabetes guidelines recommend that individuals with diabetes should not go more than two consecutive days without doing some kind of physical exercise, to reduce insulin resistance and improve glycaemic levels.1,3,8 The clinical implication of this guideline suggests that, within this period, the sensitizing effect of insulin is not lost, resulting in a potential reduction in hyperglycemia and the need to adjust medication after exertion.48 According to the Brazilian Diabetes Society Guidelines,51 individuals on insulin therapy should perform RE before AE in the same session to minimize the risk of unwanted hypoglycemia after exercise. As shown in this study, the risk of undesired hypoglycemia after exercise is lower after an RE session compared to AE, due to the lower reduction in glycaemic levels after the RE session.
This systematic review and meta-analysis have some limitations in the included studies, such as the limited number of studies assessing post-exertion glycemia for each type of exercise, hindering the performance of statistical analyses at more post-session time points for each type of exercise. Additionally, the studies were assessed as having moderate certainty of evidence, with bias risk being the factor that downgraded the evidence.
ConclusionA single exercise session was able to act directly on glucose levels with a significant reduction in the first-minute post-CAE, IAE, and RE, and this reduction lasted for up to 10 min after the CAE session and for up to 30 min after the IAE session in individuals with T2D. However, the risk of bias in most studies is uncertain, and new studies with high methodological quality are needed.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no competing interest.