To assess the association between quality of life, asthma severity, sleep disorders and exercise capacity in children with asthma.
MethodsWe evaluated 45 children with asthma of both sexes aged between 7 and 12 years, diagnosed by a pediatrician/pulmonologist and classified according to the IV Brazilian Guidelines for Asthma Management: severity (intermittent/mild and moderate/severe) and control (controlled, partially controlled and uncontrolled). Quality of life (QoL), presence of sleep disorders and exercise capacity were respectively assessed using the following instruments: Pediatric Asthma Quality of Life Questionnaire (PAQLQ); Sleep Disturbance Scale for Children (SDSC); and six-minute step test (6MST).
ResultsIntermittent/mild and moderate/severe asthma were observed in 51.1% and 48.9% of the children evaluated, respectively. Only 8.89% of the sample had uncontrolled asthma. In the regression model, a better QoL was observed in children with lower asthma severity, lower SDSC total score and lower levels of dyspnea induced by the 6MST (β=−0.395, p=0.003; β=−0.338, p=0.011; β=−0.352, p=0.008; respectively). These factors explained 31% of the PAQLQ total score variation. Other variables (such as cardiorespiratory variables, spirometry, asthma control and number of steps in 6MST) did not predict quality of life.
ConclusionsLower asthma severity (intermittent/mild), fewer symptoms of sleep disorder, and lower exercise-induced dyspnea predicts better quality of life in children with asthma.
Asthma is a chronic disease of the airways characterized by diffuse airflow obstruction, which can be spontaneously reversible or mediated in response to medication.1 The mean worldwide prevalence for this disease is 11.6% among schoolchildren, ranging from 2.4% to 37.6%. In Brazil, the rates are still high, around 20% among children and adolescents.1,2
A common aggravating factor in people with asthma is nocturnal asthma. Such a condition can be diagnosed by episodes of coughing, wheezing, shortness of breath and chest tightness at night or in the early morning. These symptoms disrupt sleep and impair daily activities.3
Another commonly found condition in patients with asthma is exercise-induced asthma. Episodes of exercise-induced asthma are common in children due to the increased physical activity in this age group, and may determine a lower tolerance to physical activity, resulting in a more sedentary lifestyle when compared to healthy children.4,5
Assessing the quality of life (QoL) of children and adolescents with asthma is fundamental given that severe or uncontrolled asthma impairs the quality of sleep, school performance and involvement in physical activities, all of which contribute to reducing QoL.6
A study with adolescents with asthma7 highlighted the six-minute step test (6MST) as an option for assessing exercise capacity because it reflects the discomfort caused by asthma in the practice of daily life activities. Authors of this study verified that responses to the 6MST such as dyspnea and fatigue of the lower limbs are related to the QoL of this population (11–15 years old). However, the same was not investigated in a younger population with asthma, which constitutes a majority among asthmatic patients.
Our study aims to assess the association between QoL, asthma severity, sleep disorders and exercise capacity in children with asthma. The hypothesis for this study is that children with lower severity of asthma (intermittent/mild), fewer symptoms of sleep disorders, and lower exercise-induced dyspnea may predict better QoL in asthmatic children.
MethodsThis study is a cross sectional study that was approved by the Ethics Committee of the Universidade Federal do Rio Grande do Norte (UFRN), Natal, RN, Brazil (protocol number: 876.304).
In the present study, 48 children with asthma of both sexes aged 7–12 years old were included. Children were required to be undergoing treatment for asthma at the time of the study, in a reference center in the city of Natal, Brazil. A physician (pediatrician and pulmonologist) was responsible for the asthma diagnosis and treatment. The classification of severity and asthma control was given according to the IV Brazilian guidelines for management of asthma.2 Moreover, children could not have the following conditions: heart, neuromuscular, rheumatic, musculoskeletal or orthopedic diseases; or any other disorder that could prevent completion of the proposed protocol for this study. Children who had airway infections or acute episodes of asthma during the previous 3 weeks prior to the assessments were also not included.7 All legal guardians gave their written informed consent. Children who declined to participate were excluded from this study.
Assessments were performed over two days. In addition, children wore an accelerometer at home for 7 days to determine their physical activity levels. On the first day, the Sleep Disturbance Scale for Children questionnaire (SDSC) and two spirometric evaluations (before and after bronchodilator – 15min between them) were applied. On the second day of evaluation, the 6MST was performed twice with a 30-min interval between them. The Pediatric Asthma Quality of Life Questionnaire (PAQLQ) was applied and the accelerometer was installed on the same day.
Anthropometric measurementsA mechanical weight scale and a stadiometer (Model 110-CH, Welmy, Brazil) were used to assess height and body weight. Growth and nutritional status were assessed using the World Health Organization software, WHO Anthro Plus.8 Respiratory rate was assessed by counting the number of breaths in one minute.
Lung function assessmentSpirometry was assessed on the first day of assessments. It was performed using a handheld KOKO® digital spirometer (Longmont, the United States), which followed the recommendations of the European Respiratory Society (ATS and ERS).9 Spirometry was performed before (pre) and after (post) bronchodilator, according to the ERS.9 The obtained values were analyzed according to the reference values proposed by Mallozi.10
Pediatric Asthma Quality of Life Questionnaire (PAQLQ)The PAQLQ asks children to think about their activities during the previous week. The questionnaire is validated for Brazil6,11 and has 23 questions across 3 domains (symptoms – S, activity limitation – AL and emotional function – EF). The activity domain contains 3 ‘patient-specific’ questions, and in order to answer these questions the child chooses the three activities of their daily life that are most affected by asthma. The questionnaire was interviewer-administered and was responded to on a 7-point scale (7=not bothered at all, 1=extremely bothered). Scores were calculated in total and by domain. The PAQLQ total score was the mean of all 23 responses.11 The PAQLQ showed satisfactory reliability (Cronbach's alpha: 0.93 for total score; ranging from 0.72 to 0.88 for the individual domains).11
Sleep Disturbance Scale for ChildrenThe SDSC assesses the frequency of sleep disorders symptoms in the previous six months by discriminating them into categories of transient or persistent. It is an instrument composed of 26 items validated for Brazil,12 which distinguishes six groups of sleep disorders through analysis of the corresponding partial scores (disorders of initiating and maintaining sleep, sleep breathing disorder, disorders of arousal, sleep–wake transition disorders, disorders of excessive somnolence, and sleep hyperhidrosis).12 The Brazilian-Portuguese version of the SDSC showed satisfactory reliability (Cronbach's alpha: >0.55).12
This instrument was applied by an interview with the children's legal guardians between pre and post bronchodilator spirometry. The score was derived by the sum of the item scores for each domain, and the sum of all scores (total score). In order to determine the presence of sleep disorder, the scores were compared to those obtained by the validation study for the Brazilian population.12
Six-minute step testThe six-minute step test (6MST) was performed in accordance with the standards of previous studies7,13 on the second day of assessments. The cadence was free, and a 20cm step was used (upper limbs had no support).13 Peripheral oxygen saturation, heart rate, dyspnea and fatigue of the lower limbs were assessed at each 2-min interval.
Maximal heart rate (HRmax) was obtained by the following formula: 210−(0.65×age in years).14 Blood pressure and respiratory rate were assessed at rest and immediately after the test. The performance on the test was determined by the total number of steps (up and down). If one leg had gone up at the end of the test, it was counted as ½ step. Each child performed two tests with a 30-min interval between them. The second test was administered only if dyspnea and vital signs were similar to the ones at baseline. Otherwise, the interval between the tests was increased. The best test was selected for data analysis.
A 10-point Borg category-ratio (CR10) was used to assess perceived dyspnea and fatigue of the lower limbs. Heart rate and peripheral oxygen saturation (SpO2) were assessed using a pulse oximeter (OxyWatch Pulse Oximeter MD300C631, China). Blood pressure was measured by a digital sphygmomanometer (Visomat® Handy IV, UEBE Medical GmbH, Germany).
Physical activity level – ActiGraph GT3XPhysical activity level was assessed by a triaxial accelerometer ActiGraph GT3X (Actigraph LLC, USA). The device was worn on the right hip.15 Children wore the GT3X over a seven-day period. They additionally received an activity diary for registering periods with and without the device. Data were analyzed by the Actilife Lifestyle – Desktop software and recorded using 60-s epoch periods.15 Valid days were considered as those with more than 10h of wearing the device. Wearing time was defined subtracting the amount of time the accelerometer was not worn from 24h. Interruption in wearing time was the same as defined by Troiano et al.16 The cut-off points used to determine the physical activity level were those proposed by Romanzini.17
The use of GT3X is reproducible, showing good reliability between accelerometer intensity and walking speed with an intraclass correlation coefficient of 0.96.18
Data analysisData were analyzed by the Statistical Package for the Social Sciences (SPSS) software version 17.0 with a significance level of 5%. Data normality was verified by the Kolmogorov–Smirnov test. Descriptive statistics were expressed as mean and standard deviation, median and interquartile range or as percentage.
We considered the similar study of Basso et al.7 to estimate the sample of the present study. Thus, in using a sample calculation for a correlation of −0.54 (between PAQLQ and Borg Scale), it was determined that a sample size of 45 patients would be enough to detect a correlation with a power of 80%, a significance level of 0.05, and coefficient of determination of 0.15.
One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to compare PAQLQ scores according to levels of asthma control. Non-paired Student's t test was used to compare the obtained means for PAQLQ scores between the levels of asthma severity and to compare the means for PAQLQ between those with and without sleep disorders.
Pearson's correlation test was performed according to parametric distribution to assess the relationship between QoL and the variables of: SDSC total score; the total number of steps; cardiorespiratory variables (peripheral oxygen saturation, dyspnea and lower limb fatigue at the end of the 6MST); and spirometric variables. The magnitudes of the correlations were classified as follows: very low, ≤0.25; low, 0.26–0.49; moderate, 0.50–0.69; high, 0.70–0.89; and very high, 0.90–1.00.19
The Pearson correlation test investigated the presence of confounding variables and of multicollinearity in order to avoid overlap between independent variables in the regression model. The regression analysis was performed with the total score and with the 3 domains of the PAQLQ.
Finally, considering those variables that had p<0.10 of significance in the Pearson, ANOVA and independent Student's t tests performed previously, a multiple linear regression analysis (backward method) was performed between the variables to assess which were capable of independently determining QoL. The PAQLQ scores were considered as the dependent variable. The variables with p<0.05 were considered and retained in the final model.
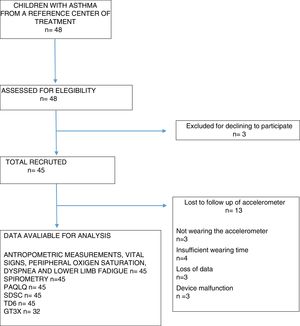
ResultsA total of 48 children aged 7–12 years old of both genders (27 males) were eligible to participate in the study. However, 3 children declined to participate and were then excluded, thus totaling a sample of 45 children with asthma. From the total sample, 13 children did not have their physical activity levels recorded due to the following reasons: not wearing the accelerometer; insufficient wearing time; loss of data or device malfunction (Fig. 1).
The results of demographic, anthropometric and spirometric characteristics, time of diagnosis, medications used for asthma control, and physical activity levels are shown in Table 1.
Characterization of the children.
| Analyzed variables | Value |
|---|---|
| Total sample | 45 |
| Boys (n) | 27 |
| Age (years) (mean/SD) | 9.0 (1.5) |
| Weight (kg) (mean/SD) | 33.8 (8.6) |
| Height (m) (mean/SD) | 1.4 (0.1) |
| BMI (kg/m2) (mean/SD) | 18.0 (3.1) |
| Percentile (%) | |
| Underweight | 13.3% |
| Eutrophic | 55.6% |
| Overweight/obese | 31.1% |
| Lung function (% of predicted) | |
| FEV1 (mean/SD) | 88.7% (19.9) |
| FVC (mean/SD) | 98.0% (15.7) |
| FEV1/FVC (mean/SD) | 88.7% (17.3) |
| Time of diagnosis (years) (mean/SD) | 5.0 (3.2) |
| Medications used for asthma control, N | |
| Short-acting beta-agonists | 21 |
| Beta-agonist+corticosteroids | 21 |
| Only corticosteroids | 1 |
| No medication | 2 |
| Physical activity level, N (%) | 32 (71.1%) |
| Sedentary | 6 (13.3%) |
| Low physical activity level | 26 (57.8%) |
Mean/SD, mean and standard deviation; BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; FEV1/FVC, FEV1/FVC ratio.
Regarding asthma severity, 51.1% of the sample was classified as having intermittent to mild asthma, and 48.9% as moderate to severe asthma. Regarding asthma control, 33.33%, 57.78% and 8.89% of the sample were respectively classified as controlled, partially controlled and uncontrolled asthma.
The most restricted activities chosen by children when answering the PAQLQ were: running, climbing and playing soccer. Some children spontaneously affirmed that they were bothered by such activities due to asthma symptoms. However, some of the participants affirmed that despite their wanting to practice physical activity, their parents did not allow them to for fear of exercise-induced asthma.
The presence of sleep disorders was found in 26 children (57.8%), who presented one or more combined sleep disorders. The sleep disorders found were: sleep breathing disorder alone (13 children), sleep breathing disorder combined with sleep hyperhidrosis (5 children), sleep hyperhidrosis alone (6 children), disorders of initiating and maintaining sleep combined with sleep breathing disorder (1 child), and the three disorders combined (1 child).
A total of 8 children stopped when performing the 6MST, reporting fatigue of the lower limbs or dyspnea. They stopped for a mean of 47.75 (SD=26.29)s. Table 2 shows the means and medians for PAQLQ, SDSC scores and 6MST variables.
Mean (SD) and median (min–max) for PAQLQ, SDSC scores and 6MST variables.
| Analyzed variables | Value |
|---|---|
| PAQLQ (mean, DP) (n=45) | |
| Total score | 5.5 (1.2) |
| Symptoms | 5.6 (1.3) |
| Activity limitation | 4.9 (1.4) |
| Emotional function | 5.7 (1.3) |
| SDSC (mean, DP) (n=45) | |
| Total score | 51.8 (14.4) |
| DIMS | 11.9 (4.6) |
| SBD | 6.3 (3.1) |
| SH | 4.9 (3.5) |
| SWTD | 13.7 (4.3) |
| DA | 4.3 (1.7) |
| DES | 9.3 (3.5) |
| Variables assessed at the end of the 6MST (n=45) | |
| Number of steps (mean, DP) | 175.9 (32.9) |
| SpO2 (mean, DP) (%) | 96.9% (6.19) |
| RR (mean, DP) | 19.3 (3.8) |
| HR (mean, DP) | 133.1 (22.1) |
| HRmax (%) (mean, DP) | 65.19% (10.77) |
| SBP (mmHg) (mean, DP) | 119.8 (14.4) |
| DBP (mmHg) (mean, DP) | 74.6 (10.2) |
| BORG – dyspnea (median, min–max) | 5 (1–8) |
| BORG – fatigue of the lower limbs (median, min–max) | 6 (2–10) |
n, number of participants; PAQLQ, Pediatric Quality of Life Questionnaire; SDSC, Sleep Disturbance Scale for Children; DIMS, disorders of initiating and maintaining sleep; SBD, Sleep breathing disorder; SH, sleep hyperhidrosis; SWTD, sleep–wake transition disorders; DA, disorders of arousal; SED, disorders of excessive somnolence; 6MST, six-minute step test; DP, standard deviation; min–max, minimum and maximum interquartile range; SpO2, Peripheral oxygen saturation; RR, Respiratory rate; HR, heart rate; HRmax, percentage of predicted maximal heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The children with intermittent/mild asthma presented higher PAQLQ scores (total and symptoms scores) when compared to children with moderate/severe asthma, as shown in Table 3. Moreover, children with controlled and partially controlled asthma (most of the sample) presented higher PAQLQ scores than those recorded from children with uncontrolled asthma (Table 3). Children without sleep disorders did not present better means for PAQLQ when compared to those who presented the pointed disorder, as shown in the same table.
Comparison between PAQLQ scores and the groups of asthma severity, asthma control and the presence or not of sleep breathing disorder.
| Variables | Total | PAQLQ (n=45) | ||||||
|---|---|---|---|---|---|---|---|---|
| p value | S | p value | AL | p value | EF | p value | ||
| Asthma severity | ||||||||
| Intermittent/mild | 5.9 (1.0) | 0.03a | 6.1 (1.0) | 0.008a | 5.1 (1.4) | 0.36 | 6.0 (1.2) | 0.11 |
| Moderate/severe | 5.1 (1.3) | 5.0 (1.5) | 4.7 (1.3) | 5.4 (1.3) | ||||
| Asthma control | ||||||||
| Controlled | 6.1 (0.7) | <0.001b | 6.3 (0.8) | <0.001b | 5.2 (1.3) | 0.03b | 6.3 (0.7) | <0.001b |
| Partially controlled | 5.5 (0.9) | 5.5 (1.0) | 4.9 (1.2) | 5.7 (1.1) | ||||
| Uncontrolled asthma | 3.4 (1.9) | 3.3 (2.3) | 3.2(1.5) | 3.7 (1.9) | ||||
| Presence of SBD | ||||||||
| Yes | 5.2 (1.3) | 0.19a | 5.2 (1.6) | 0.10a | 4.8 (1.3) | 0.72a | 5.5 (1.3) | 0.28a |
| No | 5.7 (1.0) | 5.9 (1.0) | 4.9 (1.4) | 5.9 (1.3) | ||||
n, number of participants; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; S, symptoms domain; AL, activity limitation domain; EF, emotional function domain; SBD, sleep breathing disorder.
There were significant correlations between lower SDSC total score and higher PAQLQ scores (T and EF domains), as shown in Table 4. Also, there were significant correlations between higher SpO2 values and higher PAQLQ scores (T and S domains); between lower diastolic blood pressure and higher PAQLQ scores (S domain); lower dyspnea and higher PAQLQ scores (T, S and AL domains); and less lower limb fatigue and higher PAQLQ scores (S domain). However, there was no correlation between the PAQLQ and the spirometric variables or the number of steps on the 6MST (Table 4).
Correlation matrix between the PAQLQ scores and spirometric, 6-min step test and SDSC variables.
| Total | PAQLQ | |||
|---|---|---|---|---|
| Symptoms | Activity limitation | Emotional function | ||
| Variables | ||||
| FEV1% | 0.02 | −0.04 | 0.14 | −0.10 |
| FEV1%/FVC | −0.01 | −0.30 | 0.51 | −0.00 |
| SDSC – total score | −0.31a | −0.28 | −0.15 | −0.36a |
| Number of steps (6-min step test) | −0.11 | −0.13 | 0.03 | −0.14 |
| SpO2 – 6th min | 0.34a | 0.37a | 0.21 | 0.28 |
| RR – 6th min | 0.14 | 0.14 | 0.82 | 0.14 |
| HR – 6th min | 0.17 | 0.15 | 0.64 | 0.19 |
| SBP – 6th min | −0.19 | −0.23 | −0.14 | −0.11 |
| DBP – 6th min | −0.24 | −0.33a | −0.19 | −0.05 |
| Dyspnea – 6th min | −0.35a | −0.36a | −0.30a | −0.26 |
| Fatigue of the LL – 6th min | −0.29 | −0.29a | −0.18 | −0.26 |
n, number of participants; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; FEV1%, forced expiratory volume in the first second (percentage of predicted); FEV1%/FVC, FEV1/FVC ratio (percentage of predicted); 6MST, six-minute step test; DP, Standard; SpO2, Peripheral oxygen saturation; 6-min step test, six-minute step test; RR, Respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LL, lower limbs; SDSC, Sleep Disturbance Scale for Children.
The multiple linear regression analysis showed that only asthma severity, the SDSC total score and dyspnea had significant association with the PAQLQ total score (β=−0.395, p=0.003; β=−0.338, p=0.011; β=−0.352, p=0.008; respectively), and explained 31% of the PAQLQ total score variation. The same variables had significant association with the symptoms domain (β=−0.461, p=0.001; β=−0.361, p=0.005; β=−0.319, p=0.013, respectively) and explained 35.6% of this domain variation. Only dyspnea had significant association with the AL domain (β=−0.305, p=0.042), explaining 7.2% of its variation. Regarding the emotional function domain, there was significant association with asthma severity and the SDSC total score (β=−0.299, p=0.038; β=−0.379, p=0.009), explaining 16.2% of its variation.
DiscussionThe findings of the present study suggest that better quality of life (QoL) of children (7–11 years) with asthma is related to lower severity of asthma, fewer symptoms of sleep disorder and lower dyspnea felt after physical activity. These aspects are in accordance with previous studies.7,20–24 In a review on QoL in pediatric asthma,20 the authors affirmed that there is a reduction in the domains of QoL for people with asthma regardless of the severity level, with most of them experiencing restrictions in their lives and also worse health status compared to healthy subjects.
The isolated assessment of the presence of sleep disorders analyzed by the SDSC partial scores did not predict QoL in the children assessed in the present study. However, a lower SDSC total score predicted a better QoL. These findings suggest that fewer symptoms of sleep disorders are associated with better QoL in children with asthma, which is in accordance with previous studies.21–24 When asthma is not well controlled in the pediatric population, the child or adolescent with asthma may have impaired growth, functional status and development, as well as attention deficit disorders, excessive daytime sleepiness, poor school performance, increased school absences and psychological dysfunction, which all affect their QoL.21,23
In the current study, asthma control could not explain the variance of QoL when performing the multiple linear regression analysis, unlike previous studies.22–24 It is assumed that the reason for this finding is due to the fact that most of the sample in the present study consisted of children with good control of the disease, and from the same asthma treatment reference center which used a standardized treatment protocol. Only 8.89% of the children were classified as having uncontrolled asthma and there was no significant difference between controlled and partially controlled asthma.
It was observed that the dyspnea felt after the 6MST was negatively associated with QoL of children with asthma. In studies assessing patients with chronic pulmonary diseases, dyspnea has been identified as the major limiting factor on the general health status.25,26 In the present study as well as in the study by Basso et al.,7 there was no significant relationship between the QoL of children with asthma and the 6MST performance, as measured by the number of steps during the test. However, the relationship between QoL and performance was recently observed by Andrade et al.,27 who used another submaximal test, namely the six-minute walk test. These tests differ given that the 6MST requires more of the lower limb muscles to overcome gravity and shift the center of mass, which thereby contributes to a poorer performance on this test when compared to the six-minute walk test.26
The findings of this study showed that the spirometric variables did not have significant correlation with QoL. Many authors have affirmed that the FEV1 values poorly reflected the daily experiences of patients and do not evaluate the impact that asthma causes in the pediatric population.6,7,9,20 Pereira et al.28 affirmed that QoL involves multidimensional aspects (physical, emotional and social), so it is difficult to estimate it by objective parameters such as spirometric variables. For the same reason it is assumed that the cardiorespiratory variables evaluated after the 6MST also had no significant correlation with QoL.7
In addition, during the PAQLQ interview the children reported that despite having asthma, they would like to do more exercise. However, they were discouraged by their parents due to their parents’ fear of exercise-induced asthma. Williams et al.29 showed that there are some factors that limit the participation of children with asthma in physical activity: limited concepts about themselves because of the disease, concern by parents and caregivers about the risk of physical activity, and family values regarding physical activity. These factors may have interfered in the physical activity level and in the exercise capacity of the children included in the study.
Limitations of this study included the impossibility to assess the level of physical activity of all the children included, and the fact that we were not able to include physically active children. Children with asthma can present high rates of exercise capacity when they have adequate physical exercise levels.30 Thus, it is suggested that future studies also include physically active children to investigate the relation between physical activity and exercise capacity levels in those circumstances, as well as the relationship between these rates and QoL. A more varied sample of children with asthma can help to elucidate other predictive factors for a good quality of life.
Financial supportNo financial support was provided for this study.
Conflicts of interestThe authors declare no conflicts of interest.