Shortwave diathermy (SWD) and microwave diathermy (MWD) are frequently used by physical therapists to treat musculoskeletal conditions. The therapeutic benefits are usually associated with an increase in tissue temperature; however, there is no consensus on the changes in blood flow.
Objectives1) To evaluate the behavior of temperature and arterial blood flow after the application of SWD and MWD to the lower limb of healthy women aged 18–30 and 2) to assess whether changes in limb positioning can influence SWD response.
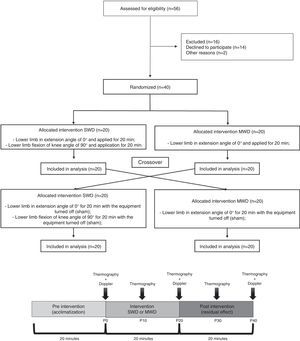
MethodAmong the subjects analyzed, 40 women were eligible to participate in the trial and were randomly allocated to the SWD group or the MWD group. Each group received 20min of diathermy. After receiving the interventions, all patients crossed over to the other group, but the devices were detuned (sham). SWD was applied to the posterior compartment of the thigh and leg, with the knee in 0° and 90° of flexion, and the MWD applied to the posterior thigh. Skin temperature evaluation (digital infrared thermography) and assessment of blood flow velocity (Doppler ultrasound) were performed immediately before and 10 and 20min after the application.
ResultsArterial blood flow increased after SWD diathermy (vs. Sham), but not after MWD diathermy. SWD promoted skin heating at the end of therapy in all areas analyzed, remaining above baseline even 20min after the end of the application. MWD diathermy promoted skin heating in the posterior thigh, reflecting a rise in the temperature of the popliteal fossa area that remained for 10min after the end of the application.
ConclusionThe increase in arterial blood flow velocity depends on the size of the heating area, since it was only observed in the application of the SWD. However, after 20min of application, the position of the lower leg did not affect the heating.
Shortwave diathermy (SWD) or microwave diathermy (MWD) are frequently used by physical therapists for the treatment of musculoskeletal conditions. The mechanisms of action of these devices are related to the increase in tissue temperature. Among their therapeutic effects, SWD and MWD decrease tendinous inflammation and chronic and acute pain and improve function. These effects stand as evidence to justify the use of these methods.1
According to Rabini et al.,2 deep heating promoted by MWD may reduce the intensity of pain and physical function in patients with knee osteoarthritis, and these benefits are maintained up to 12 months after the end of the treatment. As a preventive action, MWD treatment one day before eccentric exercise has a prophylactic effect on muscle damage.3
In 2010, Abib et al.4 reported that, 20min after SWD application, there was an average temperature increase of 2.41°C in the anterior thigh. Verrier et al.5 and Oosterveld et al.6 reported a similar temperature increase in the thigh area, with an average temperature rise of 2.4 and 2.2°C, respectively. However, these authors only observed local temperature under the electrode, disregarding differences in heating in the areas located in the electromagnetic field generated between the electrodes.
Infrared thermography is a noninvasive and highly sensitive method of measuring skin temperature. It provides a safe, painless, non-ionizing examination that determines the degree of distribution of local cutaneous blood perfusion.7 According to Sikdar et al.,8 thermography has become an important tool for measuring temperature in specific areas. Thermography detects alterations in skin temperature affected by the activation of autonomic nervous system pathways and by heat itself, which is conducted from one tissue to another at different depths.9 Studies have shown that the increase in tissue temperature is directly related to the increase in local blood flow.10,11 Blood flow analysis can be based on ultrasound Doppler flowmetry (UDF), which can estimate blood velocity. UDF probe emits the ultrasonic wave to a moving red blood cell and the cell reflects the incident wave. Therefore, the frequency of the reflected wave is altered according to the Doppler principle. This frequency shift is detected and analyzed by the UDF. The UDF monitor shows real-time wave patterns within given time periods, and the UDF unit calculates the blood flow rate, pulsation index, and circulation index. Furthermore, the examiner can listen to pulsation sounds in real time.12
Therefore, we believe the use of SWD and MWD increases the skin temperature and arterial blood flow of the lower limb. This clinical trial is unique and can contribute to a better understanding of the use of SWD and MWD as this has never been investigated in a high-quality trial. Therefore, the aim of this study is to evaluate the behavior of skin temperature before, during, and after the application of SWD and MWD in healthy women and to evaluate the effect of heat on local arterial blood flow using infrared thermography and ultrasound Doppler.
MethodSubjects, recruitment, and eligibility criteriaA total of 56 female university students were invited, recruited, and screened for eligibility to participate in the study. Among those, 40 subjects between 18 and 30 years of age were selected. All subjects were physically active and had no history of circulatory or nervous disease. Subjects who were taking antipyretics, who had a history of pain, injury, or surgery in the lower limbs, or who were in their menstrual period were not included in the study.
This study was approved by the Research Ethics Committee of Hospital das Clínicas, Faculdade de Medicina da Ribeirão Preto, Universidade de São Paulo (USP), Ribeirão Preto, SP, Brazil (approval number 313.882/2013). All subjects signed an informed consent form. This trial was also prospectively registered at www.clinicaltrials.gov (# NCT01872117).13 The study was conducted between May 2013 and May 2015. The methods of this study were reported using the CONSORT statement.14
Outcome measuresThe primary outcome of the study was change in arterial blood flow velocity and the secondary outcome was skin temperature of the lower limb as a result of the application of SWD with or without knee flexion and MWD without knee flexion.
Randomization and blindingRandomization was generated in tables in Microsoft Excel, which were placed in sealed, opaque envelopes and opened only in the presence of the subject. The allocation was performed by the study coordinator (RRJG), who did not participate in the interventions, assessments, or data collection.
One of the researchers (JGC) conducted the application of MWD and SWD, another (MCQ) captured the thermography images, and the third researcher (ECOG) evaluated arterial blood flow velocity. All researchers were previously trained at the beginning of the study. The fourth researcher (NTAS) blindly processed and analyzed the data. Each researcher was blinded to the steps in which they did not participate.
Sample powerThe procedures for calculating the power of the sample were performed using Ene® software, version 3.0 (Barcelona, Spain). The calculation was based on detecting a minimal difference of arterial blood flow velocity of 10.87mm/s between the diathermy and sham groups, assuming a standard deviation of 8.6mm/s, sample group of 20 subjects, and alpha level of 0.05. Thus, the power of the study was 92%.
ProtocolThe room used for the thermographic examination was illuminated with fluorescent lamps, with no heat generating electrical equipment and no incidence of sunlight or airflow on the participants.15 Participants were instructed to avoid hot baths or showers, topical agents (e.g., creams or powder), physical exercise, and stimulants (e.g., caffeine, nicotine, or chocolate) during the 24h prior to the data collection.16
The subjects were placed, with bare legs, in a prone position on a wooden stretcher, in a temperature-controlled room at 23±2°C and average humidity of 50%, where they remained at rest for 20min in order to stabilize skin temperature, as established by Roy et al.15 After this period, the first assessment was held, followed by the application (or not) of diathermy on the posterior dominant leg, according to group allocation. In the SWD group, the electrodes were placed 15cm from the popliteal line on the hamstring and triceps surae. Felt protectors with 1cm of thickness were placed between the electrodes and the skin to avoid skin burns.17 The MWD emitter was placed on the middle third of the hamstring, 1cm from the skin.
All participants were submitted to the same diathermy application time (20min) and referred the same thermal sensation, moderate heat, reproducing clinical practice. The shortwave device used was Diathermed II (Carci®, São Paulo, SP, Brazil), continuous mode, frequency 27.12MHz with two flexible capacitive electrodes (12×17cm) and power of 240W. The microwave device was Microtherm (KLD®, Amparo, SP, Brazil) featuring a 176mm emitting antenna, frequency of 2.45GHz, and power of 200W. The devices were calibrated prior to the beginning of the study. The average power applied was 60 and 80% of the maximum of the equipment, respectively.
Study designWe implemented a randomized, crossover design (Fig. 1). The application of SWD was performed in four steps: SWD with the power off and knee in extension; SWD with the power on and knee in extension; SWD with power off and 90° of knee flexion; and SWD with power on and 90° of knee flexion. The application of MWD was performed with power off, knee extension; and MWD with the power on and knee in extension. In both cases, randomization guaranteed a minimum 7-day interval between each of the steps.
The subjects randomized to the SWD group were automatically directed to participate in the MWD sham group and the MWD group subjects were also directed to participate in the SWD sham. All subjects were informed that they would participate in two types of application with diathermy. However, one of them would not generate heat.
Skin temperature evaluationA T300 infrared camera (FLIR® Systems, Wilsonville, OR, USA) was used to collect the skin temperature with an accuracy of 0.05°C, emissivity of 0.98. It was fixed on a tripod 1m high at a distance of 1.5m from the subject.
Five thermographic images were taken over time. The first image (P0) was taken immediately before the start of application, the second (P10) was taken 10min after the start of application (at which time the electrodes were quickly removed from contact with the skin surface for approximately 30s), and the third (P20) immediately after the end of the 20min of application. Two more photos were collected to evaluate the residual effect 10 and 20min after the end of application (P30 and P40), respectively, with the individual at rest in the same position. The generated images (800×600 pixels – 180×140mm – 72dpi) were standardized between 23 and 40°C and processed in FLIR ThermoScan software (FLIR® Systems, Wilsonville, OR, USA). In order to standardize the analysis of the thermographic images, rectangular areas were determined, as established by Dibai-Filho et al.,16 showing excellent reliability (with an ICC of 0.94). The areas were analyzed on hamstring (50×30mm) and triceps surae electrodes (40×30mm) as well as in the popliteal fossa electrode (15×30mm), which served as a reference being positioned equidistant from the popliteal line with a distance of 10mm between frames. In all areas, the highest skin temperatures were selected.
Assessment of the blood flow velocityA portable Doppler ultrasound (NicoletVersalab®, Madison, USA) with 4MHz transducer (gain level 3, range from −3 to +6kHz), coupled to the skin with sterile water soluble gel and at 45° inclination against its flow,18 was used to collect the data of the popliteal arterial blood flow at P0, P20, and P40 (Fig. 1). The skin of the subjects was marked with a demographic pencil to ensure transducer positioning in all data collection.
Data analysisThe Shapiro–Wilk test was used to check the distribution of the data. The Kruskal–Wallis test and post hoc Dunn were used to compare the data regarding the velocity of arterial blood flow between groups and the Friedman test was used for intragroup comparisons. Two-way ANOVA and Tukey's post hoc were used for between-group ad inter-group comparisons. Data were processed using SPSS 17.0 (IBM, Chicago, IL, USA) and the significance level was set at 5% (p<0.05).
Clinical effect size, as described by Cohen's d,19 was used to evaluate the heating effects of both types of diathermy on skin temperature and the velocity of the arterial blood flow immediately after application, i.e., between P0 and P20. Effect size is classified as small (less than 0.2), moderate (close to 0.5), or large (above 0.8).
ResultsThe subjects were equally distributed into two groups: shortwave diathermy (SWD) and microwave diathermy (MWD). Both groups had homogeneous anthropometric characteristics regarding age (SWD 21.05 (2.06); MWD 21.65 (2.62) years; p=0.5150), weight (SWD 55.89 (9.12); MWD 59.69 (11.08) kg; p=0.2446), height (SWD 01.62 (0.05); MWD 1.65 (0.06) m; p=0.2022) and BMI (SWD 21.19 (3.04); MWD 21.81 (3.46) kg/m2; p=0.5792).
The blood flow velocity in SWD 0° was higher than SWD 90° and MWD 0° and SWD 90° was higher than MWD 0° after the end of the application (P20). Comparing the groups SWD 0° and 90° with their respective SHAM groups, a significant increase in blood flow velocity was found for all time points after the end of the application (P20 and P40). After SWD, the effect size of the increase in blood flow was large in both leg positions. After MWD, however, the effect size of blood flow velocity was considered small despite the increase in skin temperature (Table 1). No correlation was found between the temperature variation and arterial blood flow (p=0.093) despite the changes in blood flow velocity.
Blood flow velocity of the popliteal artery (mm/s) presented as median (first and third quartile) and within and between-group differences presented as mean (95% Confidence Interval for Difference) before, during, and after the application of shortwave and microwave diathermy on lower limb and effect size (n=20).
| Time | Within group differences | ||||||
|---|---|---|---|---|---|---|---|
| P0 | P20 | P40 | P20–P0 | P40–P0 | P40–P20 | ||
| 0° | SHAM | 60.5 (56; 69.2) | 54 (50; 60)*† | 55 (47; 61)*† | − 6.33 (−9.65 to −3.00) | −6.28 (−9.78 to −2.78) | 0.05 (−2.92 to 3.02) |
| SWD | 55 (47; 61) | 80.5 (70.7; 87) * | 65 (58.7; 72.7)*‡ | 23.02 (19.69 to 26.34) | 10.83 (7.33 to 14.33) | −12.18 (−15.15 to −9.20) | |
| 90° | SHAM | 60 (64; 66.2) | 58 (46; 66.2)¥ | 51.5 (46; 62)*¥ | −3.90 (−7.22 to −0.57) | −8.25 (−11.75 to −4.74) | −4.35 (−7.32 to −1.37) |
| SWD | 54.5 (46; 62) | 74.5 (61; 82)*† | 60.5 (53.5; 70)*‡ | 20.75 (17.42 to 24.07) | 9.03 (5.53 to 12.53) | −11.72 (−14.69 to −8.74) | |
| 0° | SHAM | 59 (52; 66) | 55 (49.7; 60.2)* | 54 (47; 61)* | −4.00 (−7.32 to −0.67) | −5.22 (−8.71 to −1.71) | −1.22 (−4.19 to 1.75) |
| MWD | 54 (47; 61) | 54 (47.7; 57.5)†¥ | 52 (46; 58.2)*†¥ | −1.85 (−5.17 to 1.47) | −2.30 (−5.80 to 1.20) | −0.45 (−3.42 to 2.52) | |
| Between-group differences | Effect size | ||||
|---|---|---|---|---|---|
| P20 | P20 | P40 | P20–P0 | ||
| SWD 0°–SWD 90° | SWD 0°–SHAM | ||||
| 0° | SHAM | 5.73 (−0.11 to 11.58) | 23.06 (17.21 to 28.91) | 10.83 (5.32 to 16.34) | −0.70 (−1.34 to −0.06) |
| SWD | 1.97 (1.18 to 2.68) | ||||
| SWD 90°–MWD 0° | SWD 90°–SHAM | ||||
| 90° | SHAM | 20.81 (14.96 to 26.66) | 16.40 (10.55 to 22.25) | 9.03 (3.52 to 14.54) | −0.31 (−0.93 to 0.32) |
| SWD | 1.76 (1.00 to 2.45) | ||||
| SWD 0°–MWD 0° | MWD 0°–SHAM | ||||
| 0° | SHAM | 26.55 (20.70 to 32.40) | −3.06 (−8.91 to 2.78) | −2.30 (−7.81 to 3.21) | −0.41 (−1.03 to 0.23) |
| MWD | −0.24 (−0.85 to 0.39) | ||||
SHAM, Group equipment turned off; SWD, Shortwave diathermy; MWD, Microwave diathermy; P0, Pre-application; P10, 10min of application; P20, immediately after application; P40, 20min after the end of application; 0°, 0° knee flexion; 90°, 90° of knee flexion.
p≤0.05 in relation to time in the same group: * vs. P0; ‡vs. P20. p≤0.05 in relation to group at the same time: † vs. SWD 0°; ¥ vs. SWD 90°.
The SWD promoted heating in all areas analyzed after the first 10min of application, especially the hamstrings with the limb in 90° of flexion. A greater increase in temperature was found in that position compared to 0° of flexion. However, after 20min of application, the position of the lower leg did not affect temperature (Table 2). After the application ended, there was a very gradual decrease in temperature, which remained high even 20min after the end of the application compared to pre-application and sham values (Tables 2 and 3). The MWD promoted cutaneous heating of the hamstring, reflecting an increase in the temperature of the popliteal fossa, which remained high until after the end of the application. In addition, the temperature of the hamstring area remained high for 20min after the end of the application compared to sham (Table 2). When analyzing the effect size of heating immediately after the diathermy, a large effect was found in all areas that received the application (Table 3, Fig. 2).
Mean between-group differences (95% confidence interval for difference) in skin temperature (°C) before, during, and after the application of shortwave and microwave diathermy to the lower limb (n=20).
| SWD 90°–SHAM 90° | SWD 0°–SHAM 0° | MWD 0°–SHAM 0° | SWD 90°–SWD 0° | MWD 0°–SWD 90° | MWD 0°–SWD 0° | ||
|---|---|---|---|---|---|---|---|
| P10 | HS | 5.46 (4.53 to 6.39)* | 4.32 (3.39 to 5.24)* | 6.58 (5.65 to 7.51)* | 0.97 (0.04 to 1.89)* | 0.79 (−0.13 to 1.72)* | 1.76 (0.83 to 2.69)* |
| FP | 6.21 (5.08 to 7.33)* | 6.28 (5.16 to 7.40)* | 0.74 (−0.37 to 1.86) | 0.65 (−0.47 to 1.77) | −6.19 (−7.31 to −5.06)* | −5.54 (−6.66 to −4.41)* | |
| TS | 5.66 (4.48 to 6.85)* | 4.82 (3.64 to 6.01)* | −1.13 (−2.31 to 0.05) | 0.68 (−0.50 to 1.87) | −6.76 (−7.95 to −5.58)* | −6.08 (−7.26 to −4.89)* | |
| P20 | HS | 5.03 (4.10 to 5.95)* | 4.83 (3.90 to 5.76)* | 6.05 (5.12 to 6.97)* | 0.22 (−0.70 to 1.14) | 0.29 (−0.63 to 1.22) | 0.51 (−0.41 to 1.44) |
| FP | 5.99 (4.77 to 7.21)* | 6.20 (4.97 to 7.42)* | 2.03 (0.80 to 3.25)* | 0.58 (−0.63 to 1.80) | −4.68 (−5.90 to −3.45)* | −4.09 (−5.31 to −2.87)* | |
| TS | 6.24 (5.15 to 7.34)* | 5.99 (4.89 to 7.08)* | −0.66 (−1.76 to 0.43) | 0.45 (−0.64 to 1.55) | −7.49 (−8.58 to −6.39)* | −7.03 (−8.13 to −5.94)* | |
| P30 | HS | 2.93 (2.16 to 3.70)* | 2.71 (1.94 to 3.48)* | 3.08 (2.31 to 3.85)* | 0.23 (−0.53 to 1.00) | 0.02 (−0.74 to 0.79) | 0.26 (−0.51 to 1.03) |
| FP | 2.77 (1.92 to 3.61)* | 2.79 (1.94 to 3.63)* | 0.69 (−0.15 to 1.53) | 0.60 (−0.24 to 1.44) | −2.54 (−3.39 to −1.69)* | −1.94 (−2.79 to −1.09)* | |
| TS | 3.32 (2.16 to 4.48)* | 3.32 (2.16 to 4.47)* | −0.72 (−1.88 to 0.43) | 0.11 (−1.04 to 1.27) | −4.03 (−5.19 to −2.87)* | −3.92 (−5.07 to −2.76) | |
| P40 | HS | 2.49 (1.68 to 3.3)* | 2.19 (1.38 to 2.99)* | 2.77 (1.96 to 3.58)* | 0.23 (−0.57 to 1.03) | 0.20 (−0.60 to 1.00) | 0.43 (−0.37 to 1.23) |
| FP | 2.34 (1.55 to 3.13)* | 2.01 (1.22 to 2.80)* | 0.35 (−0.43 to 1.14) | 0.94 (0.15 to 1.72)* | −2.48 (−3.26 to −1.69)* | −1.54 (−2.32 to −0.75)* | |
| TS | 3.22 (2.07 to 4.37)* | 3.29 (2.13 to 4.44)* | −0.63 (−1.78 to 0.51) | 0.07 (−1.08 to 1.22) | −3.75 (−4.90 to −2.60)* | −3.68 (−4.83 to −2.53)* | |
SHAM, Group equipment turned off; SWD, Shortwave diathermy; MWD, Microwave diathermy; P10, 10min of application; P20, immediately after application; P30, 10min after the end of application; P40, 20min after the end of application; HS, Hamstring; PF, Popliteal Fossa; TS, Triceps surae; 0°, 0° knee flexion; 90°, 90° of knee flexion.
Skin temperature (°C) and intragroup differences before, during, and after the application of shortwave and microwave diathermy on lower limb and effect size (n=20).
| Time Mean (Standard Deviation) | ||||||
|---|---|---|---|---|---|---|
| P0 | P10 | P20 | P30 | P40 | ||
| 90° | ||||||
| SHAM SWD 90° | HS | 33.13 (0.97) | 33.59 (0.83) | 33.93 (0.87)* | 33.06 (1.07)†‡ | 32.84 (1.06)†‡ |
| FP | 34.17 (1.01) | 33.85 (0.99) | 33.75 (1.10) | 33.48 (1.20)* | 33.45 (1.12)* | |
| TS | 32.82 (1.36) | 33.03 (1.26) | 33.24 (1.24) | 32.44 (1.57)†‡ | 32.02 (1.55)*†‡§ | |
| SWD 90° | HS | 32.84 (1.06) | 39.06 (0.74)* | 38.96 (1.31)* | 35.99 (0.28)*†‡ | 35.34 (0.47)*†‡§ |
| FP | 33.45 (1.12) | 40.06 (1.04)* | 39.75 (0.72)* | 36.25 (0.32)*†‡ | 35.79 (0.42)*†‡§ | |
| TS | 32.02 (1.55) | 38.70 (0.95)* | 39.48 (0.67)*† | 35.76 (0.29)*†‡ | 35.25 (0.29)*†‡§ | |
| 0° | ||||||
| SHAM SWD 0° | HS | 33.24 (1.33) | 33.77 (0.89) | 33.90 (1.01) | 33.04 (1.10)†‡ | 32.92 (1.24)†‡ |
| FP | 33.50 (0.82) | 33.13 (0.86) | 32.96 (0.91) | 32.86 (1.00)* | 32.84 (0.98)* | |
| TS | 32.92 (1.06) | 33.19 (0.91) | 33.04 (1.30) | 32.32 (1.24)†‡ | 31.89 (1.36)*†‡§ | |
| SWD 0° | HS | 32.92 (1.24) | 38.09 (1.37)* | 38.74 (0.72)*† | 35.76 (0.55)*†‡ | 35.11 (0.52)*†‡§ |
| FP | 32.84 (0.98) | 39.41 (1.32)* | 39.16 (0.60)* | 35.65 (0.52)*†‡ | 34.85 (0.64)*†‡§ | |
| TS | 31.89 (1.36) | 38.01 (1.12)* | 39.03 (0.77)*† | 35.64 (0.87)*†‡ | 35.18 (0.74)*†‡§ | |
| 0° | ||||||
| SHAM MWD 0° | HS | 33.35 (1.01) | 33.27 (0.99) | 33.20 (0.99) | 32.94 (1.01) | 32.76 (1.02) |
| FP | 33.34 (0.86) | 33.13 (0.83) | 33.04 (0.69) | 33.01 (0.71) | 32.96 (0.69) | |
| TS | 33.25 (1.24) | 33.06 (1.33) | 32.66 (1.41) | 32.45 (1.47)*† | 32.13 (1.48)*†‡ | |
| MWD 0° | HS | 32.76 (1.02) | 39.85 (0.92)* | 39.25 (0.86)* | 36.02 (0.43)*†‡ | 35.54 (0.40)*†‡§ |
| FP | 32.91 (0.68) | 33.87 (1.78)* | 35.07 (2.56)*† | 33.71 (1.21)*‡ | 33.32 (0.95)‡§ | |
| TS | 32.13 (1.48) | 31.93 (1.75) | 31.99 (1.33) | 31.72 (1.39) | 31.49 (1.35)*‡ | |
| Mean within-group differences (95% confidence interval) | Effect Size | |||||
|---|---|---|---|---|---|---|
| P10–P0 | P20–P0 | P40–P0 | P40–P20 | P20–P0 | ||
| 90° | ||||||
| SHAM SWD 90° | HS | 0.46 (0.16 to 0.75) | 0.79 (0.48 to 1.10) | −0.29 (−0.63 to 0.05) | −0.30 (−0.51 to −0.09) | 0.87 (0.20 to 1.50) |
| FP | −0.32 (−0.58 to −0.05) | −0.42 (−0.67 to −016) | −0.72 (−1.01 to −0.43) | −0.30 (−1.33 to −0.83) | −0.40 (−1.02 to 0.24) | |
| TS | 0.21 (0.05 to 0.37) | 0.42 (0.20 to 0.63) | −0.79 (−1.16 to 0.42) | −1.21 (−1.49 to −0.93) | 0.32 (−0.31 to 0.94) | |
| SWD 90° | HS | 6.21 (5.62 to 6.80) | 6.11 (5.24 to 6.98) | 2.49 (2.03 to 2.95) | −3.62 (−4.30 to −2.90) | 5.14 (3.77 to 6.30) |
| FP | 6.61 (6.01 to 7.21) | 6.30 (5.77 to 6.82) | 2.34 (1.90 to 2.78) | −3.95 (−4.28 to −3.62) | 6.69 (4.99 to 8.12) | |
| TS | 6.67 (5.92 to 7.42) | 7.46 (6.61 to 8.30) | 3.22 (2.51 to 3.93) | −4.23 (−4.61 to −3.85) | 6.25 (4.65 to 7.60) | |
| 0° | ||||||
| SHAM SWD 0° | HS | 0.53 (0.11 to 0.94) | 0.66 (0.24 to 1.08) | −0.32 (−0.73 to 0.09) | −0.98 (−1.34 to −0.62) | 0.56 (−0.08 to 1.18) |
| FP | −0.37 (−0.60 to −0.13) | −0.53 (−0.74 to −0.32) | −0.66 (−0.88 to −0.43) | −0.12 (−.035 to 0.10) | −0.62 (−1.25 to 0.02) | |
| TS | 0.26 (0.04 to 047) | 0.11 (−0.18 to 0.40) | −1.04 (−1.43 to −0.64) | −1.15 (−0.78 to −0.48) | 0.10 (−0.52 to 0.72) | |
| SWD 0° | HS | 5.17 (4.43 to 5.90) | 5.82 (5.25 to 6.38) | 2.19 (1.70 to 2.67) | −3.63 (−3.93 to −3.32) | 5.74 (4.25 to 7.01) |
| FP | 6.57 (5.78 to 7.36) | 6.32 (5.80 to 6.84) | 2.06 (1.68 to 2.44) | −4.26 (−4.51 to −4.00) | 7.78 (5.84 to 9.41) | |
| TS | 6.12 (5.44 to 6.80) | 7.14 (6.41 to 7.86) | 3.29 (2.78 to 379) | −3.85 (−4.23 to −3.46) | 6.46 (4.81 to 7.85) | |
| 0° | ||||||
| SHAM MWD 0° | HS | −0.85 (−0.34 to 0.17) | −0.15 (−0.35 to 0.05) | −0.59 (−1.00 to −0.17)) | 0.44 (−.079 to −0.08) | −0.15 (−0.77 to 0.47) |
| FP | −0.21 (−0.38 to −0.04) | −0.30 (−0.54 to −0.06) | −0.38 (−0.72 to −0.04) | −0.08 (−0.36 to −0.20) | −0.38 (−1.00 to 0.25) | |
| TS | −0.18 (−0.18 to −0.20) | −0.59 (−0.87 to −0.30) | −1.12 (−1.53 to −0.70) | −0.53 (−0.82 to −0.23) | −0.44 (-1.06 to 0.19) | |
| MWD 0° | HS | 7.09 (6.43 to 7.74) | 6.49 (5.74 to 7.23) | 2.77 (2.27 to 3.27) | −3.71 (−4.11 to −3.31) | 6.88 (5.14 to 8.34) |
| FP | 0.96 (0.21 to 1.71) | 2.16 (0.93 to 3.38) | 0.40 (0.01 to 0.79) | −1.75 (−2.76 to −0.74) | 1.15 (0.46 to 1.80) | |
| TS | −0.19 (−0.42 to −0.03) | −0.13 (−0.45 to −0.18) | −0.63 (−0.78 to -0.48) | −0.50 (−0.80 to −0.19) | −0.10 (−0.72 to 0.52) | |
SHAM, Group equipment turned off; SWD, Shortwave diathermy; MWD, Microwave diathermy; P0, Pre-application; P10, 10min of application; P20, immediately after application; P30, 10min after the end of application; P40, 20min after the end of application; HS, Hamstring; PF, Popliteal Fossa; TS, Triceps surae; 0°, 0° knee flexion; 90°, 90° of knee flexion.
p≤0.05 in relation to time in the same group and area evaluated: * vs. P0; † vs. P10; ‡vs. P20; §vs. P30.
Thermographic images of the posterior area of the dominant leg. SWD, Shortwave Diathermy Group; MWD, Microwave Diathermy Group; P0, Pre-application; P10, 10min of application; P20, immediately after application; P30, 10min after the end of application; P40, 20min after the end of application.
Our results demonstrate an increase in skin temperature both under and between the SWD electrodes, reaching maximum temperature values 10min after the start of application (P10) and remaining unchanged until 20min after the start of application (P20). These values remained elevated even 20min after the end of application (P40) as shown in Table 3. Lehmann et al.20 applied MWD (2456MHz for 30min) and noted a peak in skin temperature in the first 10min of application.
To elucidate these results, we can mention Guy,21 who showed that when a therapeutic level of energy is absorbed, temperature increases linearly for an initial transitional period lasting about 3min. This period is followed by a non-linear transition period, which typically lasts 7–10min. During that time, energy becomes large enough that blood flow and thermal conduction can dissipate the energy applied, enabling temperature stabilization. These responses may also explain why, when MWD was applied only to the proximal thigh, there was a temperature rise immediately at P20 and P40 in the popliteal fossa. The temperature increase in the triceps surae between P10 and P20 for SWD groups 0° and 90° may be related to lower temperature, given that it is more distal, as observed in the results for P0, thus indicating the need for a longer SWD application time to reach the skin's thermal equilibrium. Delpizzo and Joyner22 explains that diathermy induces the production of heat in a part of the body by using electric currents within the tissue. Johnson and Guy23 used different frequencies of electromagnetic waves (27.1, 433, 918, and 2450MHz) observed that the peak of power density absorption (mW/cm3) is always higher in the skin. These authors further state that the surface heat is excessive and that the patient's tolerance level is dictated by the surface heating. This was controlled in the present study, and subjects reported moderate heat.
Another aspect to be discussed is related to the joint position during application of SWD. It was thought that the position of the knee could change the tissue resistance to the transition of the electromagnetic field and consequently skin temperature. Indeed, it was observed only after 10min of SWD application, in the SWD 90° of flexion group. The homogeneity of skin temperature at P20 may be related to heat dissipation by the tissue, as well as by increased arterial blood flow, as observed in this study.
Heinonen et al.24 analyzed the change in blood flow and intramuscular and skin temperature of the calf after heating the area using a device with water heated to 47–50°C for a sufficient time to raise intramuscular temperature. They observed that the skin temperature of the calf increased about 8°C, resulting in an increase of 4°C in intramuscular temperature. It should be emphasized that skin temperature will be higher than intramuscular temperature, therefore the therapist must be aware of the risk of burns, given that the sensation of heat is personal, varying according to the experiences of each individual.
Wyper and McNiven25 showed that after applying MWD for 16.7min to the anterior thigh, there was an increase in muscle blood flow compared to rest values. The fact that we found no increase in blood flow in the application of MWD may be related to the methodology used, as in our study the data collection was performed in a more distal area (popliteal fossa) against the heated area (hamstrings). Instead, an increase in the blood flow of the popliteal artery in the groups receiving the SWD can be seen. This may be related to the area covered by the electromagnetic field produced by the SWD, since the electrodes were positioned in the central portion of the hamstring and calf, unlike the MWD, which generated the electromagnetic field only on the hamstrings, indicating a relationship between increasing the velocity of blood flow and the heating area.
When diathermy is applied to human tissue, the thermal stress causes a cessation of the neural activity of vasoconstriction, resulting in the increase of cutaneous vascular conduction.26 Kellogg et al.27 and Minson et al.28 state that the increase in cutaneous vascular conduction, which occurs across the heating surface tissues, is due to nitric oxide-dependent mechanisms. The cholinergic sympathetic nerve is the main mechanism involved in thermoregulatory vasodilation, but evidence suggests that this mechanism is not the only one involved in the vasodilator response at the moment of local heating.29 Supplementing that answer, Minson et al.28 report that the cutaneous vasodilation that occurs during heating results mainly from the release of nitric oxide (NO), which has been proved essential for the attenuation of the cutaneous vasoconstrictor response.30,31 Minson et al.28 also accept this mechanism, arguing that at least two independent mechanisms contribute to the increase in cutaneous blood flow during local heating: a quick response of the vasodilator system mediated by axon reflexes and a slower one, derived from the vasodilator system dependent on local NO production response.
A limitation of the present study may be the assessment of healthy young subjects in order to avoid any complications in sensitivity to heat or change in blood flow due to some pathology. However, our results suggest that new clinical trials are needed to assess these variables in individuals with autonomic or vascular diseases and correlate the responses to diathermy. A key point to mention is the need for studies that analyze deep heating to find a correlation with heating surface and arterial blood flow.
The present findings indicate that professionals can obtain better responses for arterial blood flow and skin heating if they consider the type of diathermy and the application area, often neglected in clinical practice. We recommend that physical therapists consider the following: 1) heating remained constant between the P10 and P20; 2) skin temperature was still elevated at P40; 3) temperature was higher in the areas under the MWD and SWD electrodes; 4) the popliteal fossa area showed a higher temperature increase in the SWD; 5) the MWD was unable to increase the temperature in the whole posterior portion of the lower limb when applied to the hamstring; 6) knee position interfered with the skin temperature of the hamstring after 10min of SWD application; 7) the velocity of the popliteal artery flow increased with SWD and remained unchanged with MWD and did not return to the baseline even 20min after the end of application. Although the temperature and blood flow increase, these variables do not correlate.
ConclusionSWD increased skin temperature and arterial blood flow velocity of the lower limb, regardless of its position, after 20min of application. Although MWD increased skin temperature, it was not able to change arterial blood flow.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a PhD scholarship and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the grants (2013/07227-0 and 2013/07732-7).
Clinical trial registration number: NCT01872117 - https://clinicaltrials.gov/ct2/show/NCT01872117