This study’s purpose was to utilize a prospective dataset to examine differences in functional brain connectivity in male high school athletes who suffered an anterior cruciate ligament (ACL) injury relative to their non-injured peers.
MethodsSixty-two male high school football players were evaluated using functional magnetic resonance imaging prior to their competitive season to evaluate resting-state functional brain connectivity. Three athletes later experienced an ACL injury and were matched to 12 teammates who did not go on to sustain an ACL injury (controls) based on school, age, height, weight, and year in school. Twenty-five knee-motor regions of interest (ROIs) were created to identify differences in connectivity between the two groups. Between-subject F and t tests were used to identify significant ROI differences using a false discovery rate correction for multiple comparisons.
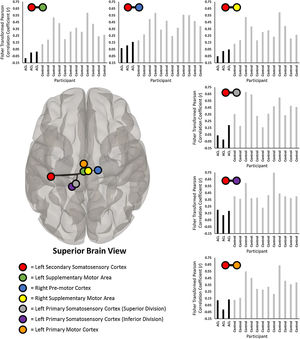
ResultsThere was significantly less connectivity between the left secondary somatosensory cortex and the left supplementary motor area (p = 0.025), right pre-motor cortex (p = 0.026), right supplementary motor area (p = 0.026), left primary somatosensory cortex (superior division; p = 0.026), left primary somatosensory cortex (inferior division; p = 0.026), and left primary motor cortex (p = 0.048) for the ACL-injured compared to the control subjects. No other ROI-to-ROI comparisons were significantly different between the groups (all p > 0.05).
ConclusionOur preliminary data indicate a potential sensorimotor disruption for male football players who go on to experience an ACL injury. Future studies with larger sample sizes and complementary measures of neuromuscular control are needed to support these findings.
Anterior cruciate ligament (ACL) injuries are debilitating knee injuries, with annual direct costs exceeding $2 billion in the United States.1 Nearly 75% of ACL injuries occur via noncontact mechanisms without direct blows to the knee,2 typically attributed to motor coordination errors resulting in tri-planar hip, knee, and ankle motion increasing strain on the ACL.3–7 Recent evidence indicates that ACL injury can induce central nervous system (CNS) changes in cortical electrical activity when measured by electroencephalography8,9 and altered cortical and subcortical blood oxygen dependent level (BOLD) signal activity by means of functional magnetic resonance imaging (fMRI).10–12 While such retrospective information may help guide rehabilitation for ACL injury through brain-targeted approaches,13,14 prospective data pertaining to the state of the CNS contributing to the initial injury could also be beneficial to prevent injury altogether. Video analyses of ACL injury illustrate that complex CNS processing, including the integration of cognitive, visual, proprioceptive, vestibular, and motor systems, are needed to avoid compromising knee positions that result in traumatic injury.15,16 Considering that sensorimotor processing, including brain activity and connectivity, are related to overt motor control, behavioral outcomes, and changes in performance,17–24 prospective CNS data related to ACL injury could supplement injury prevention efforts aimed to leverage neural processes.17,25,26
Limited literature exists detailing neurologic data prior to knee injury.12,27–29 Specifically, decreased neurocognitive function,29 depressed quadriceps activation,28 and altered BOLD signal activity12 have been observed in athletes prior to an ACL injury. Neurocognitive function, however, is an indirect measure of neural function and quadriceps activation does not provide information on what part of the CNS is driving muscle inhibition. Further, the participant investigated previously12 had a prior ACL injury which may have neurologically contributed to the reported prospective second contralateral injury.
A potential complementary approach to understanding CNS function is through the examination of the temporal coherence of the BOLD signal among spatially distinct brain regions at rest (i.e., functional connectivity).30,31 Functional connectivity reflects the degree of BOLD signal co-activation (or lack thereof) during active states,32,33 provides stable indicators of brain function as evidenced through consistent reproducibility of network connectivity,34,35 and has been suggested as a potential neural biomarker for therapeutic care.36 Functional connectivity is capable of distinguishing pathologies such as Alzheimer’s and Parkinson’s disease37,38 and is sensitive to detecting neurological changes resulting from combined cognitive and motor skill training.39 Recently, a prospective functional connectivity analysis was used to identify differences between female athletes who went on to experience an ACL injury compared to matched controls.27 Connectivity between regions of the ‘knee motor network’ (i.e., active brain regions during knee movement) were evaluated and depressed functional connectivity between the left primary somatosensory cortex and the right posterior lobe of the cerebellum (Lobule XIIB) was identified in the ACL injured athletes.27 Although a specific mechanism for ACL injury related to such prospective altered connectivity was not reported,27 tasks with high spatial and temporal sensorimotor coordination demands (scenarios where ACL injury often occurs15,16) increase sensory to cerebellar connectivity40 and such connectivity is critical for error-free movement.41 Sensory-cerebellar connectivity is also involved with timed, spatial orienting tasks,42 thus, lessened connectivity may indicate that this interactive process is disrupted, thereby hindering an athlete’s ability to maintain a safe knee position during dynamic, athletic scenarios.
While the identified connectivity alteration may provide a neural mechanistic target for interventions aimed to restore sensory-cerebellar connectivity,27 this finding was in a cohort of only female athletes and not directly generalizable to male athletes. When comparing ACL injury differences between sexes, females have an estimated 2–4 times greater incidence rate of ACL rupture compared to males,43,44 but males still constitute a higher absolute number of ACL injuries and exhibit similar lifelong ailments, such as rapid osteoarthritis progression and prolonged disability.45–48 Considering the differences underlying ACL injury risk mechanisms for males and females (e.g., hormones, laxity, etc.),49 combined with potential functional connectivity sex-differences,50,51 we aimed to determine if neural activity may influence ACL injury risk in males. The purpose of this study was to utilize a prospective dataset to examine differences in functional brain connectivity in male high school athletes who subsequently suffered an ACL injury relative to their non-injured peers. We hypothesized that those who experienced an ACL injury would exhibit decreased functional brain connectivity of the knee motor network compared to those who did not experience a traumatic knee injury during their competitive season.27
MethodsParticipantsSixty-two healthy male varsity high-school football players from two large, local, private high schools enrolled in a prospective longitudinal neuroimaging study examining head impacts. All participants enrolled for one year and a subset (n = 32) enrolled for a second year, with MRI testing occurring prior to and following each football regular season. This study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center and each participant signed an informed consent prior to participating. Out of the 62 initial participants, one individual was excluded from imaging testing due to metal orthodontics and one for testing anxiety. From the resulting cohort of 60 participants, three males sustained complete ACL ruptures (n = 3; 16.33 ± 0.58 years, 181 ± 10.97 cm, 107.93 ± 20.42 kg). Two participants experienced ACL ruptures during their first competitive season (participant 1 & participant 2) and one participant’s injury happened in a pre-season camp before the start of the second season (participant 3). All three athletes sustained an ACL rupture to the left leg (with no other significant structural damage), which was confirmed by clinical MRI, and each occurred from player to player contact with no direct blows to the knee.
Videos were available for all injury incidents and reviewed by experts in the field of orthopedic medicine to provide a qualitative biomechanical description of the injury mechanism. Participant 1 was blocking an opposing defender in practice, stepped forward and laterally with his right foot to form a wide base of support, but as the opponent continued pushing forward, participant 1’s momentum and weight shifted backwards on to the left leg resulting in knee valgus and a left ACL rupture. Participant 2 was running with the ball during a competition toward the right sideline, and a defender made lateral contact above participant 2’s left waist, which forced the participant to plant his left leg, go into a valgus positioning, and rupture his left ACL. Participant 3 was shuffling his feet backwards during practice while blocking an opponent, and planted with his left leg in valgus positioning and ruptured his left ACL. All three injuries were biomechanically similar with no opponent(s) making direct contact to the knee before the injury.
Participant 1, participant 2, and participant 3 were matched with twelve athletes who did not sustain an ACL injury (controls) based on school, age, height, weight, and year in school (n = 12; 16.83 ± 0.39 years, 181.75 ± 4.54 cm, 102.97 ± 13.69 kg). The imaging session that preceded the injury was utilized for prospective data analysis for the injured athletes. Preseason data from season 1 was used for participant 1 (57 days between testing and injury) and participant 2 (67 days between testing and injury). Postseason data from season 1 was used for participant 3 as this subject was injured prior to the start of season 2 (243 days between testing and injury). Preseason data from season 1 were used for all the controls as none of these control participants sustained an ACL injury throughout season 1, nor did they report any past history of ACL injury. None of the participants reported other injuries or concussions preceding their ACL injury. Individual demographic data are presented in Table 1.
Individual demographic data for all study participants.
| Participant | Age (yrs) | Height (cm) | Weight (kg) | Grade | School | aInjury side | |
|---|---|---|---|---|---|---|---|
| ACL- injured | Participant 1 | 16 | 167 | 73.8 | 11 | School 1 | Left |
| Participant 2 | 16 | 193 | 130.3 | 11 | School 1 | Left | |
| Participant 3 | 17 | 171.5 | 90.3 | 12 | School 2 | Left | |
| Matched controls | Control 1 | 17 | 177 | 94.1 | 12 | School 1 | N/A |
| Control 2 | 17 | 179.5 | 111.3 | 12 | School 1 | N/A | |
| Control 3 | 16 | 180 | 92.9 | 12 | School 1 | N/A | |
| Control 4 | 16 | 177 | 90.8 | 11 | School 1 | N/A | |
| Control 5 | 16 | 185 | 89.1 | 11 | School 1 | N/A | |
| Control 6 | 17 | 179.5 | 134.5 | 12 | School 1 | N/A | |
| Control 7 | 17 | 183.5 | 99.5 | 12 | School 1 | N/A | |
| Control 8 | 17 | 181 | 86.4 | 12 | School 1 | N/A | |
| Control 9 | 17 | 193 | 108.5 | 12 | School 2 | N/A | |
| Control 10 | 17 | 186 | 113.2 | 12 | School 2 | N/A | |
| Control 11 | 18 | 179 | 105.3 | 12 | School 2 | N/A | |
| Control 12 | 17 | 180.5 | 110 | 12 | School 2 | N/A | |
MR scanning was conducted on a Phillips 3T Achevia scanner (Philips Medical Systems, Best, the Netherlands) equipped with a 32-channel, phase array head coil. First, a high resolution three dimensional T1-weighted anatomical image was acquired in sagittal orientation: TR = 8.1 ms, TE = 3.7 ms; field of view = 256 × 256 mm, acquisition matric = 256 × 256, slice thickness = 1 mm, number of slices = 180. To acquire the BOLD data for functional connectivity analyses, two sequential five-minute resting state fMRI sequences data were acquired with the following parameters: TR = 2000 ms; TE = 35 ms; flip angle = 53°; field of view = 240 × 240 mm, acquisition matrix = 64 × 64, in-plane resolution = 3.75 × 3.75 mm, slice thickness = 5.0 mm, slice gap = 0 mm, 38 slices, ascending slice ordering. Participants were asked to look at a crosshair reflected on a projector and remain still. A total of 150 frames of data were collected in each sequence (totaling 300 frames).
Resting-state connectivity analysesSpatial and temporal preprocessing of fMRI data were carried out using the CONN toolbox (version 17.F, http://www.nitrc.org/projects/conn).52 Spatial preprocessing steps utilized routines from the Statistical Parametric Mapping (SPM) 12 package (Wellcome Institute of Cognitive Neurology, London). Functional volumes were co-registered and resliced to a voxel size of 2 mm³, normalized to the Montreal Neurological Institute (MNI) template brain and smoothed with an 8 mm3 isotropic Gaussian kernel. Temporal preprocessing steps were completed in CONN and including scrubbing of motion-outlier frames (>0.9 mm or >±5 standard deviation in global signal), slice-timing correction, regression of the top five principle components of the BOLD signal from cerebrospinal fluid and white matter compartments using the CompCor method53 as well as zero- and first-order derivatives of realignment parameters, and finally band-pass filtering to a window of 0.008 Hz–0.09 Hz. The two fMRI sessions (˜five minutes each) were temporally concatenated and connectivity values were computed as the Fisher transformed Pearson correlation coefficients between the average residual BOLD time series of pairs of regions of interest (ROIs).
Regions of interestTwenty-five ROIs were adapted from the previously described prospective ACL brain functional connectivity investigation in females.27 The ROIs consisted of various sensory, motor, and cerebellar regions that were active in previous task-based fMRI investigations of knee flexion and extension movements.12,54 To note, this approach27 resulted in two ROIs centered within the left postcentral gyrus (i.e., left primary somatosensory cortex) that were previously referred to as the left primary sensory cortex (MNI: -12 -36 72)12 and left primary sensorimotor cortex (MNI: -16 -40 64),54 respectively, with the former anatomically superior (and more anterior/closer to the midline) relative to the latter. For clarity, we henceforth refer to the left primary sensory cortex as the ‘left primary somatosensory cortex (superior division)’ and the left primary sensorimotor cortex as the ‘left primary somatosensory cortex (inferior division)’. See Table 2 from Diekfuss et al.27 for a listing of the MNI coordinates for all 25 ROIs.
Data analysesWe first conducted an omnibus F test for each of our 25 ROIs to identify which ROIs exhibited significant connectivity differences with one or more of the other 24 ROIs between the ACL-injured and matched controls. In other words, we used one-way ANOVAs to identify ‘seed’ ROIs that exhibited a significant group effect in their connectivity patterns. These tests were carried out using an alpha level of p < 0.05 and we applied a false discovery rate (FDR) correction for multiple comparisons.55 Post-hoc independent-samples t-tests were applied to each pairwise connection between any significant seed ROIs and each other ROI to determine specific differences in connectivity between the ACL-injured and matched controls. Alpha level was also set at p < 0.05 with multiple comparison error corrected using the FDR approach.55 Congruent with Diekfuss et al.,27 we conducted additional Mann–Whitney U tests for any significant post-hoc findings to account for potential data non-normality due to the small and uneven sample sizes.
ResultsThe omnibus test revealed significant connectivity differences between the ACL-injured and control participants for the left secondary somatosensory cortex and all other target ROIs, F (3, 11) = 10.46, p = .037. Post-hoc analyses revealed significantly decreased connectivity between the left secondary somatosensory cortex and the left supplementary motor area (SMA), t (13) = −4.19, p = 0.025, U = 0.0, p = 0.004, right pre-motor cortex, t (13) = −3.76, p = 0.026, U = 0.0, p = 0.004, right SMA, t (13) = −3.58, p = 0.026, U = 0.0, p = 0.004, left primary somatosensory cortex (superior division), t (13) = −3.42, p = 0.026, U = 2.0, p = 0.017, left primary somatosensory cortex (inferior division), t (13) = −3.32, p = 0.026, U = 0.0, p = 0.004, and left primary motor cortex, t (13) = −2.91, p = 0.048, U = 1.0, p = 0.008 for the ACL-injured relative to the controls. See Fig. 1 for a graphical display and individual connectivity data for each significant finding.
Significantly less prospective connectivity between the left secondary somatosensory cortex (red circle) and six sensorimotor-related regions of interest (ROI; identified by colored circles in legend) between the participants with ACL injury and control participants. Each bar chart represents the individual fisher transformed Pearson correlation coefficients between the average residual BOLD time series between the left secondary somatosensory cortex and the respective ROI (noted by colored circles above each bar chart). All ROI-to-ROI results demonstrate significantly less connectivity for the ACL-injured participants (noted as ‘ACL’ in bar charts) relative to the controls. Brain is superior view. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We prospectively examined the functional brain connectivity at rest in male high-school football players who went on to sustain an ACL injury compared to their non-injured counterparts. This study supports a prior investigation of ACL injury in high school female soccer players27 by corroborating a potential neural predisposition to injury, but with unique differences in this male football sample. Congruent with our hypothesis and previous findings in female high school athletes, depressed knee motor network functional connectivity was also observed for male athletes prior to the occurrence of ACL injury. The depressed connectivity between knee-specific sensorimotor regions in females was between the right cerebellum and left primary somatosensory cortex,27 however, ACL-injured male athletes exhibited prospective decreased connectivity between the left secondary somatosensory cortex (SII) and the left and right SMA, right pre-motor cortex, left primary motor cortex, and the left primary somatosensory cortex (superior and inferior divisions). The findings from this report supplement the novel, yet growing body of evidence illustrating differences in CNS function that may predispose athletes to ACL injury.27–29
The SII enables the integration of sensory inputs bilaterally through afferent pathways56 and is involved in sensorimotor integration for movement monitoring and online corrections through tactile information from muscle contractions.57,58 SII provides feedback to the primary motor cortex to ensure precision with voluntary motor control and the premotor cortex and SMA help to integrate sensory information and plan movements.59 The integration of sensory and motor input is critical in highly complex and dynamic environments such as sporting fields of play where athletes must respond to stimuli with timely coordinated movements to avoid compromising high-injury risk positions.60 Lower extremity and trunk neuromuscular control dysfunction are commonly accepted risk factors for non-contact ACL injury that have been investigated extensively.7,61,62 Loss of neuromuscular control of the knee and\or trunk was apparent in our video evidence of the three ACL injury events, with no direct contact to the knee (i.e., players ruptured their ACL when pivoting or backpedaling in response to contact). Truncal sway contributes to worsened knee abduction and valgus collapse and predicts ACL injury with high sensitivity, specificity, and accuracy.7,61,63–65 Although the preliminary nature of the results in the present study precludes definitive conclusions, the depressed connectivity between SII and other brain regions responsible for sensorimotor coordination add to previous evidence of supraspinal contributors to ACL injury risk.27,29
Our results share similarities to those previously described in a prospective fMRI study of female high school soccer athletes,27 which reported depressed functional connectivity between the primary somatosensory cortex and the cerebellum in athletes who went on to ACL rupture. Depressed connectivity between sensory and cerebellar regions was not observed in the present study, but connectivity between sensory and motor regions reflect similar processes.41 The different connectivity alterations in these two populations may be due, in part, to sex differences. Research has robustly shown sex differences in anatomical connectivity (i.e., white matter volume),66–68 structural connectivity (i.e., white matter tractography),69,70 and functional connectivity using both position emission tomography71 and resting-state fMRI.72,73 Sex differences in neuronal communication may reflect observable cognitive and behavioral differences74 that could explain our divergent results observed in males.
Athletes who have experienced an ACL injury have a greater risk of an additional ACL injury75–77 and experience long-term consequences, including physically-disabling osteoarthritis and reduced quality of life.78,79 The current standard ACL prevention method is neuromuscular training,80,81 which are developed based on various biomechanical and physiologic mechanisms underlying injury.82 However, despite extensive research,83 actual ACL injury rates have remained largely stagnant,81,84 warranting novel mechanistic knowledge to augment standard prevention programs. The results from the present study could drive novel biofeedback technologies85,86 to directly engage the identified neuro-therapeutic targets. Preliminary data17 highlight the benefit of congruent, integrated sensory-visual-spatial feedback during motor control for injury-resistant movement patterns to transfer to sport, which has not been considered in prior clinical practice. The current data provide regions with altered connectivity that may predispose an athlete to injury, which could be directly targeted with biofeedback modifications. The SII region identified herein may indicate that for males, biofeedback training could be optimized to increase sensory-spatial feedback during motor training to increase the connectivity between SII and motor regions. For example, adjusting sensorimotor feedback with an external focus (shifting a patients’ focus away from their body and towards the effects of their movement) may increase sensorimotor functional connectivity,87 using vibration or other tactile tools88,89 could increase sensory activity, or stroboscopic training (i.e., completing motor tasks and physical activity with intermittent visual conditions) may reweight motor strategies towards increased sensory-motor processing.10,90
Alternative explanations and limitationsThe above results and discussion of depressed sensorimotor functional connectivity in athletes who experienced an ACL rupture should be interpreted with caution. First, it is interesting to note that the seed which exhibited a significant group effect in its connectivity pattern (SII) was localized to the left hemisphere (and not the right hemisphere), and the ACL-injured participants all injured their left leg. Considering some bilateral findings were present in our results (right and left SMA), one explanation may be that interhemispheric communication is disrupted prior to ACL injury. Interhemispheric communication is vital for bimanual coordination and motor function,91,92 which is of particular importance for stability of lower extremity performance as unilateral ACL injuries reflect bimanual, biomechanical deficiencies.93,94 Although all three of our ACL injured populations injured their left knee, both limbs were involved in the injury incident (stabilization, backpedaling, etc.), indicating a potential neural deficit related to interhemispheric communication contributing to bilateral coordination.
Our video analyses also revealed that all three injury events occurred during dynamic interactions with opponents (unlike the purely non-contact injury reported previously27), which raises the question of whether other, cognitive-related factors may have played a role. For instance, during the presence of stress/anxiety (e.g., during a game or high-intensity practice), participants’ ability to disengage attentional focus from a threat may be compromised (e.g., maintaining a safe knee position when in contact with an oncoming defender).95 Dual-task scenarios (i.e., addition of a secondary cognitive task to a motor task) also produce differing lower extremity biomechanics relative to single-task scenarios96 which interact with an athlete’s attentional focus.97 The ACL-injured participants in our study may have engaged unique cognitive processes (e.g., changes in attentional focus, rapid decision-making, etc.) that we did not investigate, but should be considered when interpreting our connectivity findings. Nevertheless, our intent is to simply inform and augment prevention programs aimed to restore sensorimotor connectivity, and emphasize that decreased connectivity between specific sensorimotor regions is likely only a partial contributor to ACL injury, as our sample size was small and no other corroborating data were obtained (e.g., biomechanical, cognitive, and/or neuromuscular measures).
ConclusionPreseason functional connectivity alterations in sensorimotor networks were present in our sample (n = 3) of male high school football athletes who went on to sustain ACL injuries during their competitive season. Future studies with larger sample sizes, combined with complementary prospective lower extremity kinematic and kinetic analyses, are needed to further confirm the current results. In addition, enhanced video analyses of actual injury events are warranted to better understand the relationship between prospective functional connectivity alterations and motor coordination errors that contribute to ACL injury. If the novel findings of the current report are supported, there is potential opportunity to train identified neuro-therapeutic target and further enhance injury prevention and rehabilitation of ACL injury.
Conflicts of interestThe authors declare no conflicts of interest.