The Comprehensive Pain Management editorial series discussed several aspects around persistent pain, with the ambitious aim to contributing to the paradigm shift that is happening in the medical field and move from a biomedical and structural approach, to a broader, patient-centred approach to health and disease.1-4
The editorials published in the series focused on profiling and phenotyping patients with persistent pain based on their clinical presentation,5 as well as the importance of multimodal interventions including education and behavioural strategies.6 They highlighted the importance of lifestyle factors such as sleep,3 physical activity,7 stress,2 and diet.8 The editorial series was a much-needed initiative, summarising the most relevant findings from the latest research on the topic and represents a significant step forward towards a better management of people with persistent pain.
This last editorial will discuss the biological mechanisms that might both underlie persistent pain and provide the biological substrate linking persistent pain with lifestyle factors. Epigenetic mechanisms are particularly interesting for this aim. Epigenetics represent a set of mechanisms that are able to regulate gene expression and biological functions.9 Most of the human epigenome is relatively stable across the lifespan, with the exception of the epigenetic reprogramming occurring during embryonic development.10 However, epigenetic modifications at some loci in the genome are responsive to environmental challenges and lifestyle such as smoking, adverse childhood experiences, sleep disturbances, diet, and physical activity.11 This makes them a suitable biomarker for many diseases but also complex traits – like chronic pain.
Epigenetics in health and diseaseCancer was the first disease where epigenetics, and in particular DNA methylation (the adding of methyl groups to cytosines in the DNA, often leading to gene silencing), was systematically studied. In the last decades, widespread DNA hypo-methylation accompanied by focal hyper-methylation of tumour suppressor gene promoters has emerged as one of the hallmarks of cancer development and progression.12 This laid the foundation for the broader field of epigenetics in human health and disease. Research rapidly expanded to other conditions, including neurological disorders, autoimmune diseases, and metabolic syndromes.10
A second relevant field worth mentioning where epigenetics has been significantly applied is aging, and particularly biological age.13 Biological age is meant to estimate the general health of one's biological functions relative to your chronological age. Biological age is based on a set of physiological markers, thought to be representative of one's bodily functions and health, and is proposed to represent one's true aging process.
Although the term biological age has been used since the beginning of the century, its modern conceptualisation and its use in predicting health and lifespan became clearer with advances in epigenetics and the development of so-called epigenetic clocks.14 Epigenetic clocks consist of mathematical models that estimate biological age using DNA methylation levels at a set of genomic loci (typically 300 to about 1000 loci are used in the models). They have been validated in several studies, and they now can serve as reliable biomarkers of one's health status.15 A recent study compared epigenetic clocks to other promising biomarkers of biological age such as telomere length, transcriptomics, proteomic, and metabolomics biomarkers, and found that the epigenetic clock was the most accurate estimator of biological age.16
Epigenetic clocks have attracted considerable attention for their accuracy and potential to predict morbidity and mortality and thus represent a turning point in aging research.15 In addition, epigenetic clocks can be applied outside the aging field and serve as biomarker for many diseases, complex traits such as chronic pain, but also to examine the influence of environmental and lifestyle factors. Together with DNA methylation of specific functional genomic regions, they can indeed be used as the main outcome measure in trial studying the effect of smoking, physical activity, stressful events, sleep, and diet on health.
The link between epigenetics and lifestyle factorsDNA methylation has consistently been shown to be linked to several health aspects and diseases and is already used as a biomarker for smoking, obesity, and type-2 diabetes.17-19 Smoking is a particularly meaningful example. Smoking induces arguably the strongest effect among the lifestyle factors studied so far. DNA methylation is consistently found altered in thousands of methylation sites in current smokers. Most of these alterations – yet not all of them – go back to normality between 1 and 5 years after smoking cessation.
The link between DNA methylation and other lifestyle factors is less studied and has yet to be fully elucidated. However, preliminary findings are promising. Regular physical activity has been shown to cause methylation changes in muscle and adipose tissues, particularly in genes involved in energy metabolism and inflammation such as the PPARGC1A gene.20 Intriguingly, a two-year intervention implementing physical activity and a healthy diet in 219 post-menopausal women found significant reduction at the epigenetic clock, showing that a healthy lifestyle can actually slow biological aging.21
Epigenetic changes in genes regulating the stress system seem pivotal in determining differences in gene expression and stress resilience in adulthood.22 Adverse childhood experiences also change DNA methylation in genes regulating the stress response in children, whom then show higher risk of psychiatric disorders in adulthood.23 Early trauma in childhood (but not in adulthood) was associated with reduced DNA methylation near the FKBP5 gene, and with an enhanced risk of developing post-traumatic stress disorder and chronic pain.24,25
Epigenetics changes in chronic painUnderstanding and treating chronic pain remains a challenge for researchers and health professionals worldwide. It has been shown that chronic pain not only decreases a patient's quality of life, but it actually decreases one's lifespan for all-cause mortality.26 Crucially, mortality risk disappears when controlling for lifestyle factors.26 This, together with other research showing that the majority of people with chronic pain report reduced physical activity, poor diet, and sleep and stress disturbances, has naturally directed the attention towards the role of lifestyle factors in chronic pain.27 In the field of pain, epigenetics is still in its infancy. A recent systematic review28 showed that chronic pain is also associated with several changes in DNA methylation of various genes as well as with gene and micro-RNA expression. These genes regulate immune functioning, proinflammatory markers, glutamate and glycine transporters, brain-derived neurotrophic factor, leptin, and histone de-acetylases.28,29 Remarkably, none of these genes relate to the musculoskeletal system.
Epigenetic clocks have also been applied to populations with chronic pain. To date, results are rather conflicting, but sample sizes are small, in the range of 8–25 participants per group, which is a clear limitation. One study, however, explored epigenetic age acceleration in a cohort of almost 4000 participants, with a large group of over a thousand patients with high-impact pain. Epigenetic age was significantly accelerated in patients with high-impact pain versus both patients with low-impact pain and healthy controls.30
Taken together, these observations suggest that chronic pain is associated with changes in several biological systems – from metabolic, immune, and neural functions, to the stress response, as reflected by the widespread changes in DNA methylation found in these patients.
As these same mechanisms can be influenced by lifestyle factors such as physical activity, diet, and adverse childhood experiences, recent discoveries in epigenetics support the role for lifestyle interventions in people with chronic pain, and provide a biological substrate to study the effectiveness of such interventions in people with chronic pain.
The importance of identifying objective biomarkersChronic pain is inherently subjective, and to this date diagnosis is based heavily on patient self-reporting. This is problematic for a number of reasons. The absence of objective tests increases the risk of misdiagnosis, as symptoms of chronic pain overlap with many other autoimmune or neurological conditions. Patients often endure lengthy processes of ruling out other conditions, before receiving a diagnosis, which prolongs suffering and might worsen clinical outcomes. In addition, clinical diagnosis alone does not provide insights into the underlying mechanisms, thus limiting the development of targeted therapies.
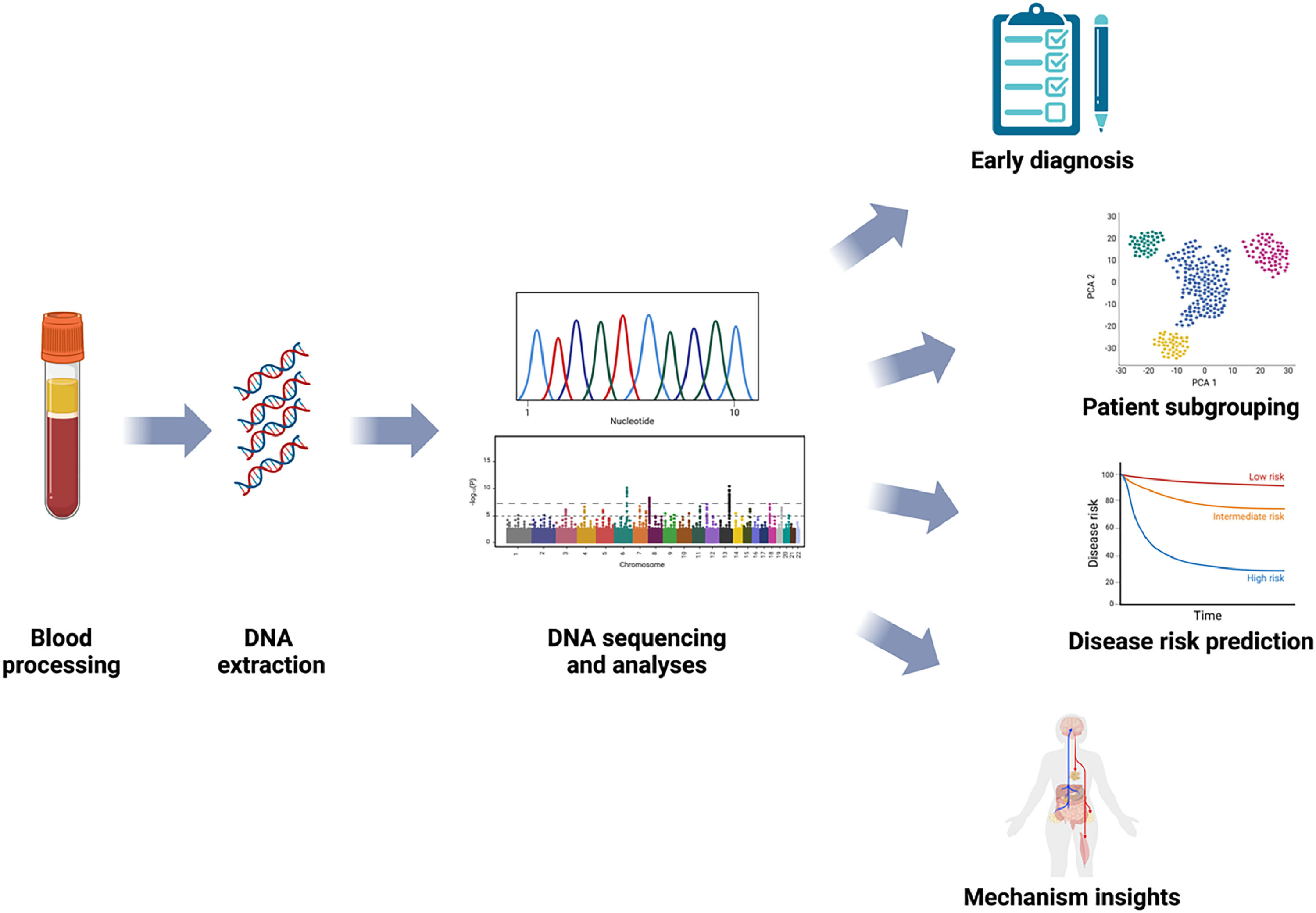
Exploring epigenetics mechanisms would improve our understanding of chronic pain pathophysiology and, as it has already happened in other fields, has the potential to promote diagnosis and subgrouping of complex clinical presentations via the identification of biomarkers. DNA methylation signatures may in fact appear before any clinical presentation can be observed, and profiling the epigenome has the potential to identify diseases at early stages and aid disease risk prediction. This would allow for an early clinical or lifestyle-related intervention to prevent or delay the development of a disease. Epigenetic changes can also serve as targets for personalised treatment approaches from lifestyle interventions to innovative bioengineering applications, as well as assess treatment responses and monitor disease progression (Fig. 1).
By improving diagnosis and identifying accurate personalised treatment at an early stage, epigenetic biomarkers can not only foster better care for people with chronic pain but also reduce the overall burden on healthcare systems by avoiding lengthy and costly diagnostic procedures and ineffective treatment approaches.
Brazilian Journal of Physical therapy. www.paininmotion.be.