The Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) is a biaxial instrument used worldwide for TMD assessment.
ObjectiveTo translate, cross-culturally adapt, and assess the criterion validity and reliability of the Brazilian Portuguese version of the DC/TMD Axis I.
MethodsThe translation and cross-cultural adaptation of Axis I into Brazilian Portuguese followed the recommendations of the International Network for Orofacial Pain and Related Disorders Methodology. For the formal assessment, 117 Brazilians with TMD completed the translated version of the TMD Pain Screener, the Symptom Questionnaire, and the Numeric Pain Rating Scale for orofacial pain intensity assessment. Subsequently, a standardized clinical examination was performed. Fifty-one participants were examined by two raters for interrater reliability, and 53 participated on a second assessment day for intrarater reliability analyses. TMD Pain Screener criterion validity was assessed through sensitivity, specificity, and ROC curve. Kappa and intraclass correlation coefficient (ICC) were used to analyze reliability for categorical and continuous data, respectively.
ResultsThe translated version achieved language equivalency. High accuracy was found for the Brazilian Portuguese version of the TMD Pain Screener short form (AUC = 0.95) compared to the long form, as well as high intrarater reliability (ICC 0.88). Moderate to almost perfect reliability was found for painful TMD diagnoses and disc displacement with reduction.
ConclusionsThe Brazilian Portuguese version of the DC/TMD Axis I is valid and reliable for screening painful TMD and for painful DC/TMD diagnoses.
Temporomandibular disorder (TMD) is a broad term comprising a set of painful and nonpainful dysfunctions related to the temporomandibular joint, the masticatory muscles, and associated structures.1 It mainly affects women between 20 and 40 years old and is prevalent in 31% of adults and elderly and 11% of adolescents and children worldwide.2 In Brazil, the prevalence of TMD was recently reported by a systematic review with meta-analysis: around 33% of the 203 million Brazilians are affected by this disorder.3,4
Since 2014, the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) has been the most popular and used tool for clinical TMD diagnosis in the research field.5,6 It has demonstrated good validity and reliability for diagnosing the most common TMDs, in addition to performing a comprehensive assessment of the patient.5 The DC/TMD comprises two Axes: Axis I is related to physical evaluation, and Axis II to psychosocial factors. Axis I has great value in clinical practice and research because it incorporates a diagnostic algorithm for the clinical diagnosis of the most common TMDs. Axis I includes the TMD Pain Screener, a self-reported instrument for screening painful TMD; the Symptom Questionnaire, which allows the assessment of the signs and symptoms history; demographic data; and a standardized clinical examination.5,7
To systematize the translation, adaptation, and validation process of the DC/TMD versions, the International Network for Orofacial Pain and Related Disorders Methodology (INfORM) published the Guidelines for Establishing Cultural Equivalency of Instruments.8 It describes all required stages, from translation to documentation of the new version, including the test of the measurement properties in the target population. The DC/TMD – originally in English – has been translated into more than 20 languages, with widespread use. However, only a few validation studies are available in the literature.9-12 Most of these studies assessed Axis I interrater reliability, while one also assessed the criterion validity of the clinical diagnoses, comparing it to magnetic resonance imaging.9-11
Besides the need for a standardized and valid method for diagnosing and screening TMD in Brazil, a translated and tested DC/TMD version into Brazilian Portuguese will also provide the equivalence of data between different populations in the world. Thus, the general aim of this study was to translate, culturally adapt, and verify the measurement properties of the Brazilian Portuguese version of the DC/TMD Axis I. The specific aims were to verify the criterion validity of the TMD Pain Screener short form with the long form as a reference, and to analyze the reliability of all Axis I instruments.13,14 The hypothesis was that the translated version would present similar criterion validity and reliability compared to the original version after the cross-cultural adaptation process.
MethodsSampleParticipants were consecutively recruited from social media and through posters at the buildings of Universidade Nove de Julho (São Paulo, Brazil). The examinations were conducted at the Musculoskeletal Research Center, Universidade Nove de Julho.
Inclusion criteriaBrazilian men and women between 18 and 70 years old15; with 15 points or more on the short form Fonseca Anamnestic Index16; with Brazilian cultural identity (born in Brazil; with at least one parent born in Brazil; Brazilian Portuguese as the first language; and self-identified as a member of Brazilian culture)8; and with the cognitive ability to answer questionnaires as assessed with the Mini Mental State Examination (cut-off score > 23)17 and Cloze Test (≥60% correct answers).18
Exclusion criteriaParticipants who used: analgesics during the last 24 h prior to the examination; muscle relaxants or steroids in the last seven days prior to the examination; non-steroidal anti-inflammatory drugs (NSAIDs) in the last three days prior to the examination; anti-depressants or anxiolytics unless the dose was stable for at least 60 days. Additional exclusion criteria were illiteracy; pregnancy; fibromyalgia; neurological disease; TMD treatment history in the last six months; facial trauma in the last two months; ongoing dental treatment; wearing dentures; and TMJ surgery in the last six months.15
Study designThis study was conducted according to the Guidelines for Establishing Cultural Equivalency of Instruments, the Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN), and the Guidelines for Reporting Reliability and Agreement Studies (GRRAS).8,13,14 The validation process included the phases of translation, cross-cultural adaptation, and testing of the measurement properties of the Brazilian Portuguese version of the DC/TMD Axis I.
The present study is part of a larger study that received approval from the institutional review board of Universidade Nove de Julho (certificate number: 29,673,920.9.0000.5511) and is in compliance with Helsinki Declaration. All study participants signed an informed consent form.
InstrumentsDC/TMD Axis IAxis I consists of the TMD Pain Screener, Demographics (with demographic data), Symptom Questionnaire, and Clinical Examination.
The TMD Pain Screener is a six-question questionnaire used to assess pain-related TMD. Scores range between 0 and 7 points, where the cut-off for a positive score is 3 points. The short version consists of the first three questions of the original version with a cut-off of two points for a pain-related TMD.7,19
The Symptom Questionnaire assesses the history of pain in the face and headache in the temporal region, closed and open locking of the jaw, and jaw joint noises.19 Responses to this questionnaire combined with findings in the DC/TMD clinical examination results in 11 possible TMD diagnoses according to the diagnostic algorithms (Appendices – Table 1).5 The clinical examination covers, in addition to assessment of familiar pain on palpation or mandibular movements, measurements of mandibular range of motion such as pain-free opening, maximum unassisted opening, maximum assisted opening, and horizontal movements.19
Pain intensity during the last weekA validated 11-point Numeric Pain Rating Scale was used to assess orofacial pain intensity.20 Participants were asked to choose a number between 0 and 10 that represented the average of their pain intensity in the face in the last seven days, considering 0 as “no pain” and 10 as “the worst pain imaginable.”
Translation and cross-cultural adaptation into Brazilian PortugueseThe translation and cross-cultural adaptation of Axis I of the DC/TMD into Brazilian Portuguese followed the Guidelines for Establishing Cultural Equivalency of Instruments of the INfORM, which included: forward-translation conducted independently by two translators (who were native speakers of Brazilian Portuguese and specialists in orofacial pain, aware of the content of the instrument); synthesis and resolution of discrepancies; back-translation conducted by one English native speaker (unaware of the content and lay to the field); independent review; revision related to disparities; consolidation of all translation; review by an expert committee (including TMD and orofacial pain specialists, doctors of dental science, physical therapists, psychologist, and language translator) and cultural validity revision; and preparation of the pre-final instrument.8 Logs were used for documentation process, according to INfORM recommendations, and the team leader completed the records at the end of each phase.8,21
After an independent review of the translation process by the INfORM, the pre-final version was posted on the INfORM website, followed by a formal assessment in a Brazilian sample.
Formal assessmentTwo physical therapists with more than eight years of experience each were trained and calibrated in DC/TMD by self-instruction.22-24 They downloaded the instructional video and read the DC/TMD documentation to learn and memorize all mandatory commands.25 In addition, a calibration examination with feedback by a previously calibrated researcher (DA) was performed.
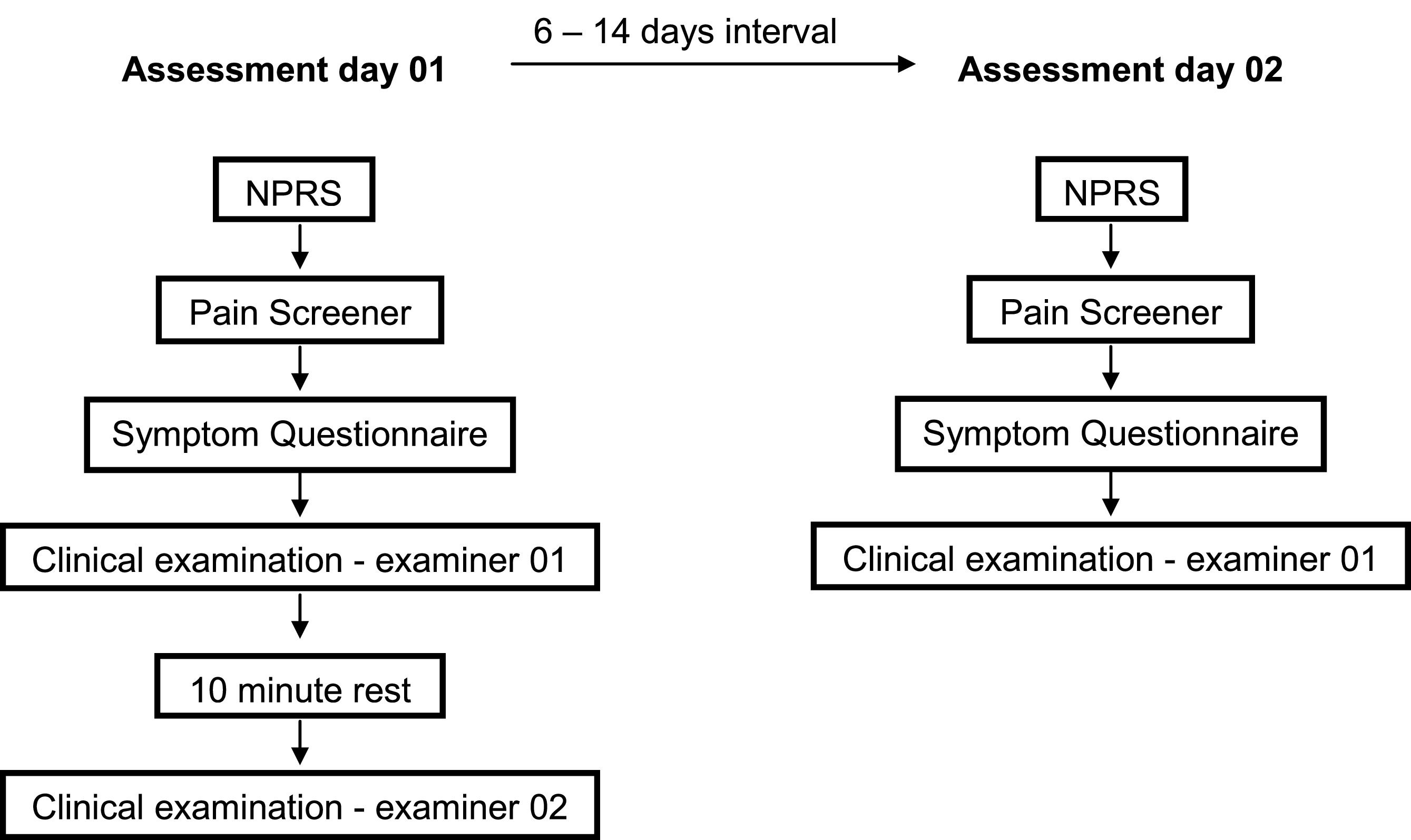
All potentially eligible participants answered a screening questionnaire to assess the inclusion and exclusion criteria, including the cognition tests and the short form Fonseca Anamnestic Index for TMD assessment.16-18 After this first stage, the included individuals reported their pain intensity during last week for the assessment of orofacial pain, answered the Axis I's instruments of the DC/TMD on paper – Demographics, TMD Pain Screener and Symptom Questionnaire – and subsequently underwent clinical examination by examiner 01. After 10 mins of rest, an independent and identical clinical examination was performed by examiner 02, who was blinded to the results of the first examination (Fig. 1).23 This 10-minute interval was defined based on the Guidelines for DC/TMD Training and Calibration.23
In the clinical examination, the mandibular range of motion was measured using a digital caliper (Starrett®, São Paulo, BR), and palpation calibration – as recommended for the clinical Axis I examination protocol – was made with the assistance of a digital algometer (DD-200 model, Instrutherm®, São Paulo, Brazil).24 The calibration was performed immediately before each palpation site, which ensured a more precise finger pressure (1 kg or 0.5 kg) in each specific tissue, according to the clinical form.24
For the assessment of intra-observer agreement, an interval from six to 14 days between the assessment days was considered long enough to prevent recall and short enough to guarantee the participant's condition stability.13 The second assessment day was conducted in the same location and conditions as the first day, and only participants with a maximum of 2 points of difference (plus or minus) in average pain intensity during the last week from the first evaluation were included.20 The participants answered the same questionnaires – pain intensity, TMD Pain Screener, and Symptom Questionnaire – and underwent an independent clinical examination performed by examiner 01.
Data analysisThe descriptive data are presented as median and interquartile interval for continuous data, and frequencies and percentages for categorical variables. The Kolmogorov-Smirnov analysis was used to test data normality.
According to COSMIN, criterion validity is defined as the degree to which the tested instrument reflects a ‘gold standard’. When analyzing a short form, the long form of the questionnaire can be considered as the gold standard. Thus, the criterion validity of the short form TMD Pain Screener was verified with accuracy analysis using the long version as the reference, through the analysis of the ROC curve, sensitivity, and specificity. For this purpose, the criterion validity of both versions of the TMD Pain Screener was also previously analyzed using the DC/TMD as the gold standard, through accuracy analysis. The area under the curve (AUC) was considered suitable and sufficient if ≥0.70.13 Acceptable values were defined as ≥0.70 for sensitivity and ≥0.95 for specificity.5
The inter and intrarater reliability of the DC/TMD diagnoses were analyzed using kappa statistics (κ) and interpreted as: <0.00 poor, 0.00 – 0.20 slight, 0.21 – 0.40 fair, 0.41 – 0.60 moderate, 0.61 – 0.80 substantial, and 0.81 – 1.00 almost perfect reliability.26 The 95% confidence interval (CI) was also calculated. Only diagnoses prevalent in at least 5% of the total sample (≥0.05) were considered adequate for the kappa analysis.10,27 Overall agreement (OA) was also calculated and expressed as a percentage, and the cut-off of 75% was considered a good agreement.9,28
The inter and intrarater reliability of the measurements of mandibular range of motion and the TMD Pain Screener scores were analyzed using intraclass correlation coefficient (ICC2,1) and their respective 95% CI. ICC values were classified as excellent (>0.75), moderate (0.40–0.75), or poor (<0.4) reliability.29
SAS for Windows v.9.4 and SPSS for Windows v.21 software were used for data analysis. Missing data were excluded from the analysis.
ResultsTranslation and cross-cultural adaptationThe Translation Logs of each stage were created in separate files as follows: TMD Pain Screener, Symptom Questionnaire, Demographics, Examination-Related Pain Interview, and Examiner Commands. All stages were followed as recommended, and the necessary number of iterations was made to achieve consensus regarding language equivalency.8 The expert panel evaluated the translation for semantic, idiomatic, experiential, and conceptual equivalency; and suggested some modifications when necessary.
The Brazilian Portuguese version is available on the website https://ubwp.buffalo.edu/rdc-tmdinternational/tmd-assessmentdiagnosis/dc-tmd/. The dubbed version of the instructional video following the translated standardized protocol was created and will be published online. The documentation of Brazilian Portuguese translation and adaptation is detailed in the Translation Logs available with the team leader and the INfORM.
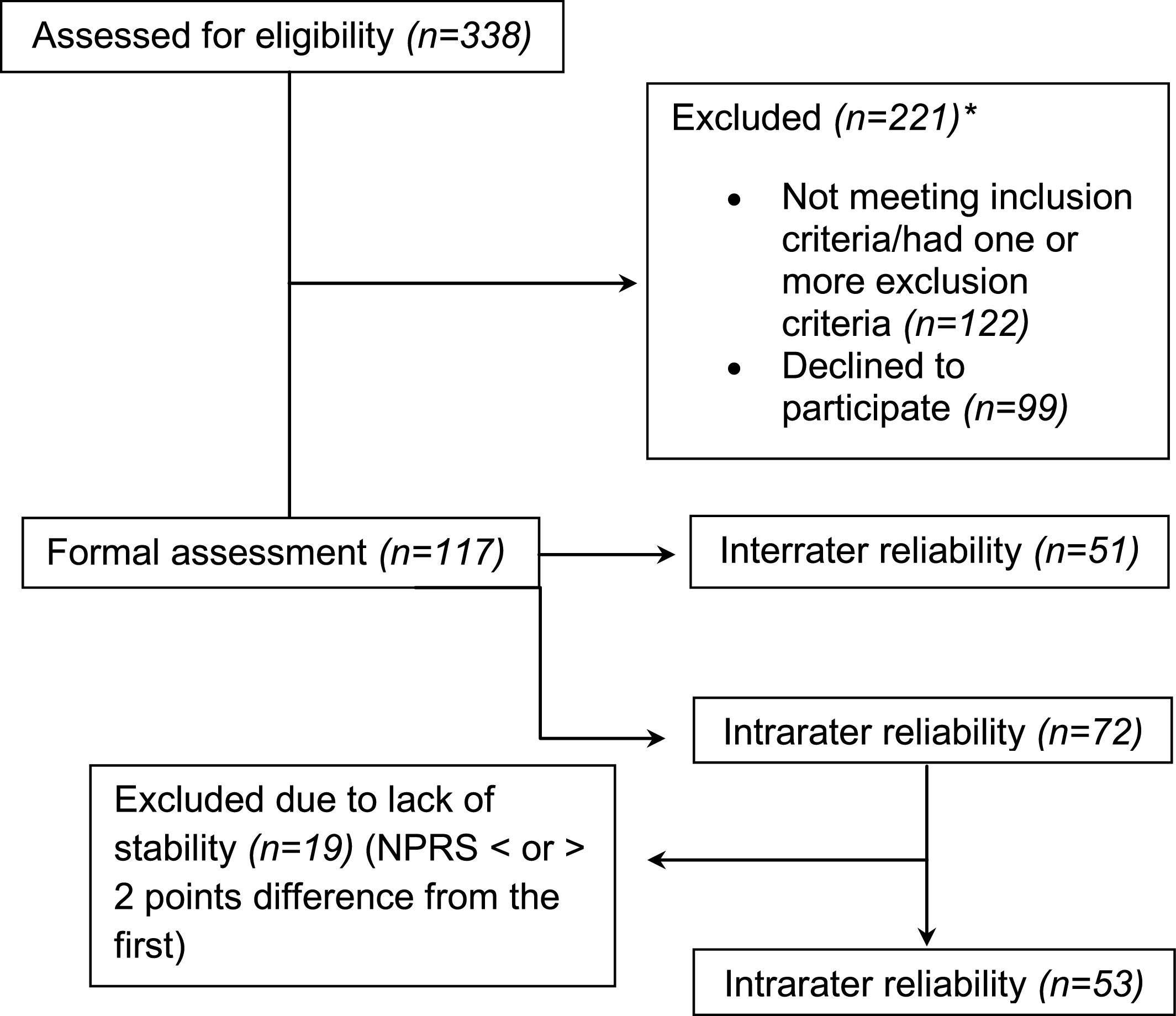
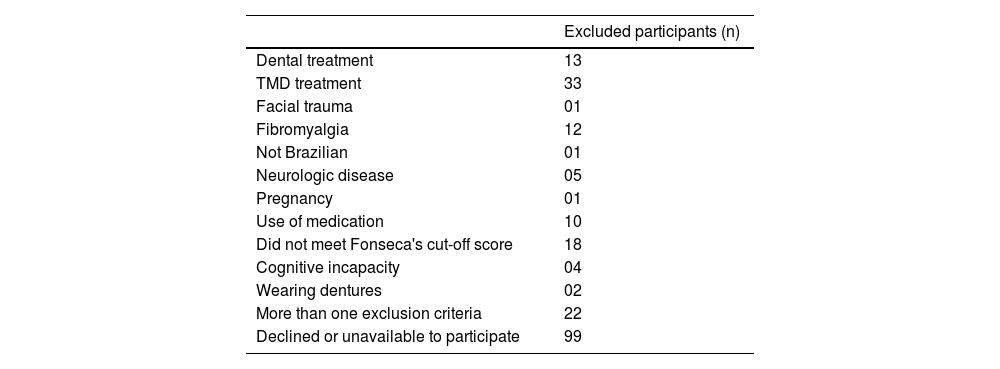
Formal assessmentThree hundred and thirty-eight participants completed the screening questionnaire. One hundred and twenty-two were excluded for not meeting inclusion criteria or for having one or more exclusion criteria, and 99 declined to participate (Fig. 2). The specific reasons for excluding participants are presented in the Appendices – Table 2.
Flow diagram. NPRS, numeric pain rating scale. *Specific reasons for excluding participants are presented in the Appendices – Table 2.
The total sample comprised 117 participants with a median age of 33 years (IQR: 25.5 – 41.5), median orofacial pain intensity of 6.0 (IQR: 3.5 – 7.5), 76.1% women, 0.9% with basic school, 27.4% with a high school degree, and 65% graduated college. A subgroup of participants (n = 51) was recruited to the interrater reliability study and another subgroup (n = 72) to the intrarater reliability study. Of this sample, 19 participants were excluded due to lack of condition stability - which is considered inappropriate for reliability analysis overtime - resulting in 53 participants for the intrarater study (Fig. 2).13 The average duration between assessments was 9 days (SD 3).
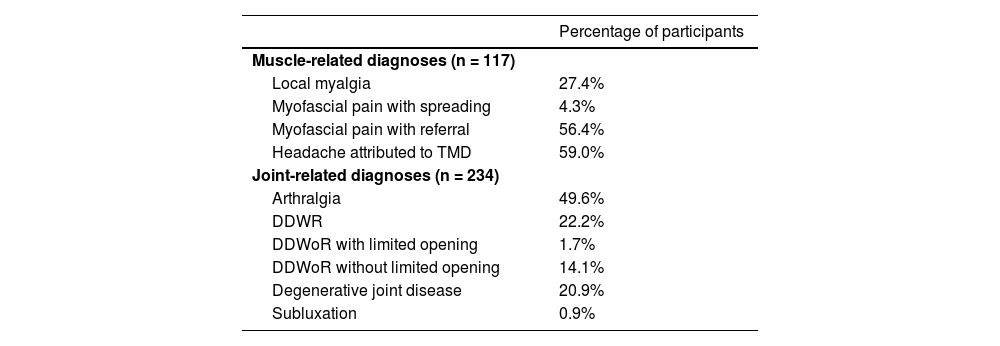
TMD Pain ScreenerOne hundred and seventeen individuals with TMD answered the TMD Pain Screener, pain intensity, and Symptom Questionnaire, and underwent clinical examination. According to DC/TMD diagnostic algorithms, 104 participants were diagnosed with pain-related TMD and 13 with nonpainful TMD. The specific diagnoses are listed in the Appendices – Table 3.
Both long and short versions were compared to the DC/TMD for criterion validity assessment through accuracy analysis. The long version's total score demonstrated a sensitivity of 0.92, specificity of 0.46, and an AUC of 0.87. The short version showed a sensitivity of 0.90, specificity of 0.53, and an AUC of 0.73 with the pain-related DC/TMD diagnoses as the reference.
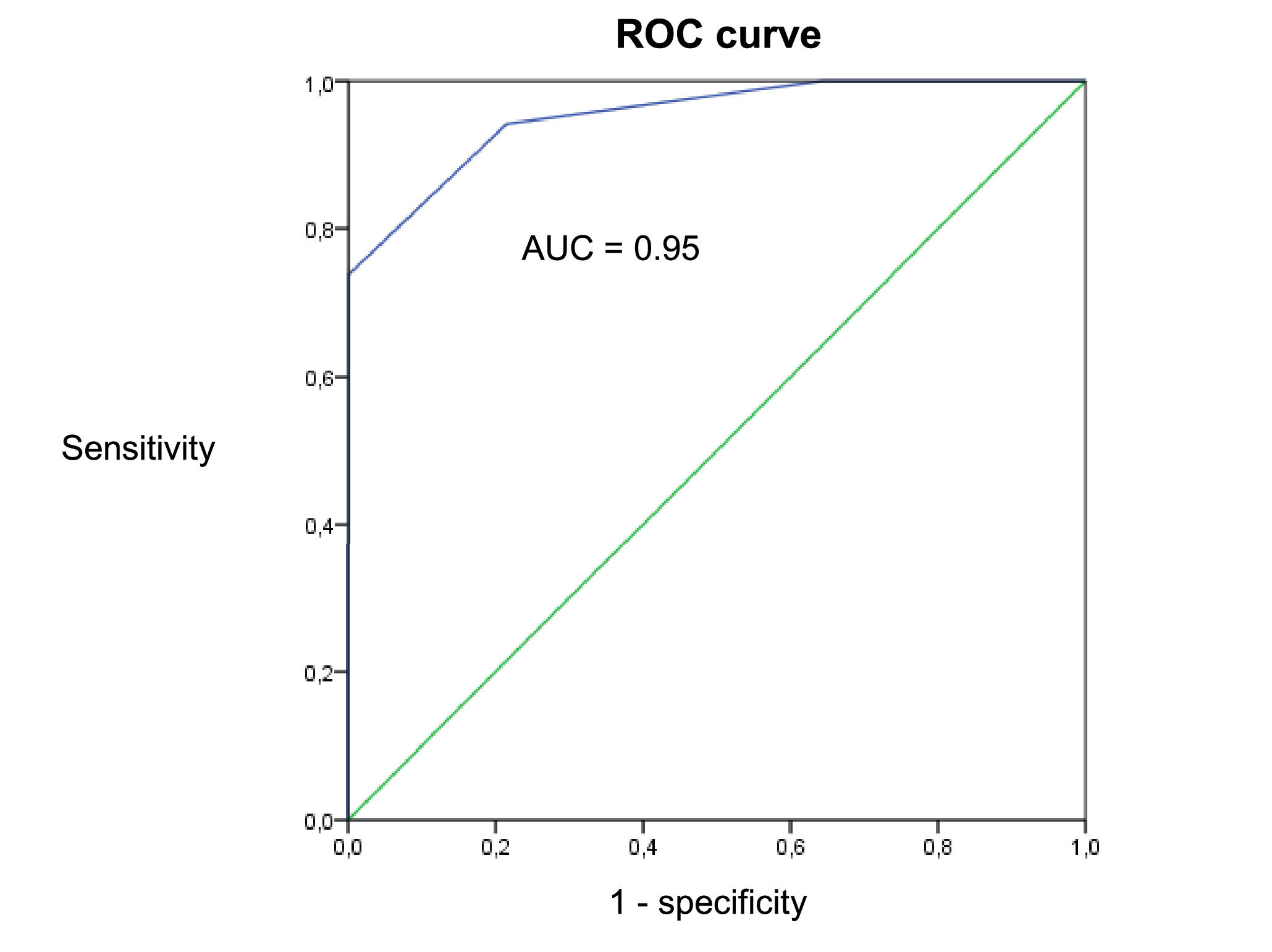
When considering the COSMIN methodology for criterion validity of the short version (with the long version as the ‘gold standard’), we found a sensitivity of 0.94, specificity of 0.64, and an AUC of 0.95 (Fig. 3).
Fifty-three participants who had stable orofacial pain intensity answered the TMD Pain Screener on two different days, and it demonstrated excellent reliability (intrarater) for both the long (ICC = 0.91; 95% CI 0.85, 0.95) and short (ICC = 0.88; 95% CI 0.79, 0.93) versions.
Diagnosis levelFor inter and intrarater reliability analysis, TMJ diagnoses were treated as independent observations (two observations per participant), resulting in 102 and 106 possible diagnoses related to the joint, respectively. However, some joints diagnosed with disc displacement without reduction (DDWoR) with or without limited opening were excluded from the analysis because the affected side could not be defined in the Symptom Questionnaire (Table 1). There were no cases of disc displacement with reduction (DDWR) with intermittent locking.
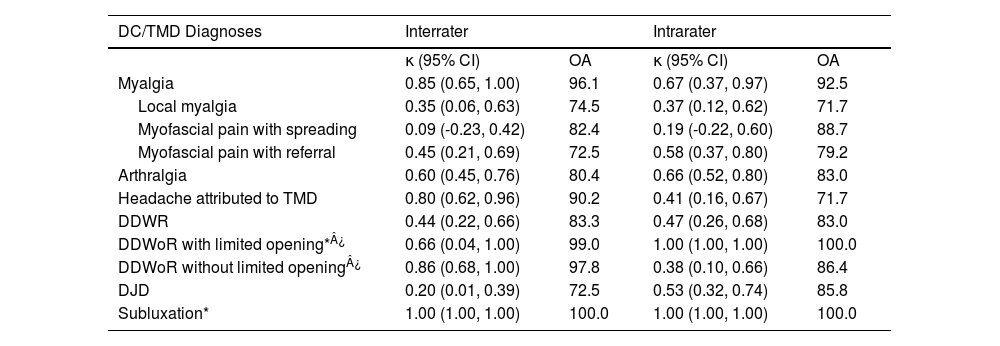
Inter and intrarater reliability of the Brazilian Portuguese version of the DC/TMD regarding diagnosis level.
| DC/TMD Diagnoses | Interrater | Intrarater | ||
|---|---|---|---|---|
| κ (95% CI) | OA | κ (95% CI) | OA | |
| Myalgia | 0.85 (0.65, 1.00) | 96.1 | 0.67 (0.37, 0.97) | 92.5 |
| Local myalgia | 0.35 (0.06, 0.63) | 74.5 | 0.37 (0.12, 0.62) | 71.7 |
| Myofascial pain with spreading | 0.09 (-0.23, 0.42) | 82.4 | 0.19 (-0.22, 0.60) | 88.7 |
| Myofascial pain with referral | 0.45 (0.21, 0.69) | 72.5 | 0.58 (0.37, 0.80) | 79.2 |
| Arthralgia | 0.60 (0.45, 0.76) | 80.4 | 0.66 (0.52, 0.80) | 83.0 |
| Headache attributed to TMD | 0.80 (0.62, 0.96) | 90.2 | 0.41 (0.16, 0.67) | 71.7 |
| DDWR | 0.44 (0.22, 0.66) | 83.3 | 0.47 (0.26, 0.68) | 83.0 |
| DDWoR with limited opening*¿ | 0.66 (0.04, 1.00) | 99.0 | 1.00 (1.00, 1.00) | 100.0 |
| DDWoR without limited opening¿ | 0.86 (0.68, 1.00) | 97.8 | 0.38 (0.10, 0.66) | 86.4 |
| DJD | 0.20 (0.01, 0.39) | 72.5 | 0.53 (0.32, 0.74) | 85.8 |
| Subluxation* | 1.00 (1.00, 1.00) | 100.0 | 1.00 (1.00, 1.00) | 100.0 |
Prevalence < 0.05. ¿One and two joints were excluded from inter and intrarater reliability, respectively. ¿Ten and 18 joints were excluded from inter and intrarater reliability, respectively. Reliability expressed in kappa (κ) and 95% confidence interval (95% CI), and agreement expressed in percentage (%). DDWR, disc displacement with reduction; DDWoR, disc displacement without reduction; DJD, degenerative joint disorder; OA, overall agreement
Table 1 shows inter and intrarater reliability as well as agreement. Myalgia, arthralgia, headache attributed to TMD, and DDWR presented moderate to almost perfect reliability. DDWoR without limited opening demonstrated fair to almost perfect reliability, and degenerative joint disease presented slight to moderate reliability. Regarding subtypes of myalgia, myofascial pain with referral demonstrated moderate reliability, but local myalgia and myofascial pain with spreading showed fair and poor reliability, respectively.
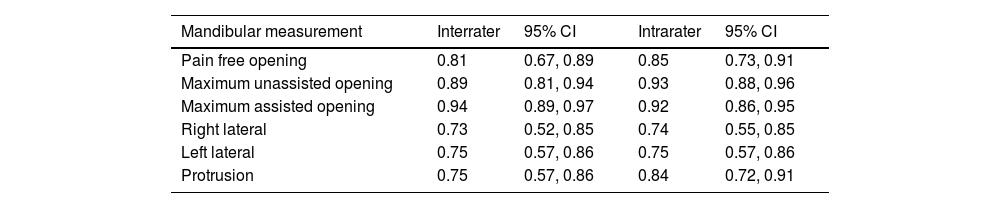
Mandibular range of motionOpening measurements presented excellent intra and interrater reliability, and horizontal movements showed moderate to excellent reliability (Table 2).
Inter and intrarater reliability of the Brazilian Portuguese version of the DC/TMD regarding mandibular measurements.
Data expressed in ICC and 95% confidence interval (95% CI).
Our data support the use of the Brazilian Portuguese version of the TMD Pain Screener as well as the Brazilian version of the DC/TMD Axis I for clinically diagnosing painful TMDs and DDWR.
Both the short and long versions of the Brazilian TMD Pain Screener presented high reliability and accuracy to identify individuals with a DC/TMD pain diagnosis. Nevertheless, the specificity was insufficient for both versions, possibly due to a relatively few nonpainful TMD participants and a lack of participants with conditions other than TMD. The original version of the TMD Pain Screener has demonstrated a sensitivity of 0.99 and specificity of 0.97, with an AUC of 0.99 and ICC test-retest reliability of 0.79.7 Despite similar reliability and sensitivity, the English version showed higher specificity than the current study, probably because it was tested in a larger population including healthy, nonpainful TMD and patients with headache.7 Even so, the short version has never before been compared to the long form. According to COSMIN, a sufficient criterion validity of a short form of a Patient Reported Outcome Measure (PROM) is an AUC ≥ 0.70 when compared to the long version.13 The Brazilian short version of the TMD Pain Screener demonstrated an AUC of 0.95, showing that this version is valid and can be used in clinical and research settings.
To the authors’ knowledge, this is the first DC/TMD reliability and validity study conducted by physical therapists. Our hypothesis was that the interrater reliability would be similar to the original version. Myalgia, DDWR, DDWoR, and DJD presented similar reliability compared to that observed by the authors of the DC/TMD.5 For the diagnoses of arthralgia and headache attributed to TMD, the kappa was similar to that observed by Vilanova et al.22 who assessed reliability in a group of examiners trained and calibrated by self-instruction, as those in the current study. The only diagnosis divergent from other studies was a subgroup of myalgia: myofascial pain with referral, which showed moderate reliability. Some studies showed substantial reliability for this specific subgroup, while others observed almost perfect reliability.5,9,10,22 One of the hypotheses for this difference could be attributed to the number of examiners – only two in our study in contrast to at least three in other studies.9-11,22 On the other hand, a systematic review with meta-analysis with 590 participants found fair reliability (κ = 0.39) for reproducing referred pain in myofascial trigger points. This supports our findings, because referred pain is the clinical measure that differentiates the diagnosis of myofascial pain with referral from the other subtypes of myalgia.5,30
The intrarater reliability was moderate to substantial for most DC/TMD diagnoses, and few diagnoses differed from their respective interrater reliability. The reliability regarding the diagnoses of DDWoR and headache attributed to TMD were lower when assessed by the same examiner on different days, which is likely explained by a learning effect from answering the questionnaires the first time. In contrast, the DJD diagnosis presented higher reliability when assessed by the same examiner. This is most likely due to the fact that assessment of crepitus is difficult to calibrate between operators. Just as the current study, a systematic review that investigated physical examinations for knee disorders found higher intra than interrater reliability on detecting crepitus in the knee joint.31
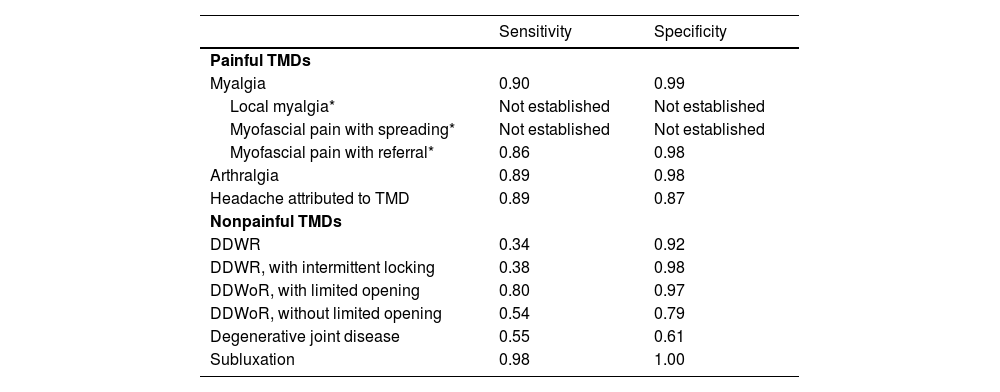
Reliability reflects if raters and repeated measures are in concordance, not if the measure is accurate. It is therefore essential to consider the sensitivity and specificity for each DC/TMD diagnosis, as previously described by Schiffman and colleagues.5 Furthermore, reliability values should be evaluated considering clinical purposes, consequences of the results, and not only as statistical thresholds.14 Hence, the Brazilian Portuguese Axis I can be used to classify according to the DC/TMD: painful TMD diagnoses, DDWoR with limited opening, and subluxation because these diagnoses presented good or better reliability (although some had low prevalence) and have presented good sensitivity and specificity in previous research.5 Other subtypes of myalgia, i.e., local myalgia and myofascial pain with spreading, have not been discussed here because their sensitivity and specificity have not been established yet in the original DC/TMD version.5
Regarding mandibular range of motion, the standardized clinical examination used in this study was performed according to DC/TMD instructions. It showed moderate to excellent reliability, consistent with previous studies that found high reliability for opening and horizontal jaw movements.10,32 The German version of the DC/TMD reported an ICC of 0.85 or higher for all mandibular movements assessed.10 The original Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) found that ICC was higher than 0.89 for opening measures, which is very similar to those reported by the current study.27 The horizontal movements showed lower but still moderate reliability, similar to those reported by Schimitter et al.27
Methodological considerationsDespite the large sample size and standardized methods in our study, some limitations should be pointed out. We did not perform the pre-test phase of the Brazilian Portuguese version of the DC/TMD Axis I. Therefore, future studies are required to conduct this phase and verify the instrument's interpretability, adaptation, and understanding by the professionals trained to apply and use the DC/TMD Axis I. Furthermore, the reliability assessment was not performed by an INfORM Reference Examiner or with operators calibrated by a Reference Examiner, as recommended by the INfORM. However, this may not be a factor of importance in this particular study because Vilanova et al.22 showed that self-teaching DC/TMD did not differ regarding TMD pain diagnoses. Also, there are no official Brazilian Reference Examiners recognized by the INfORM at the present time. In addition, subjects with other types of orofacial pain were not included and a dental examination was not conducted to verify the presence of odontogenic pain. Nevertheless, we consider that this did not influence the validity of our results.
ConclusionsThe short version of the Brazilian Portuguese TMD Pain Screener is valid and reliable and is recommended for screening painful TMD in clinical practice and research. The Brazilian Portuguese version of Axis I can be used to assess TMD, especially regarding painful disorders diagnoses and mandibular range of motion.
We would like to thank our research team at Musculoskeletal Research Center (undergraduate, master's, and PhD students) for helping with recruiting participants, data collection, and data tabulation; and José Eduardo Corrente for statistical analyses. This work was partially supported by Universidade Nove de Julho, Malmö University and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES [Coordination for the Advancement of Higher Education Personnel]) (grant number: 88887.612390/2021-00, 88887.818158/2022-00 and 88881.690013/2022-01), finance code 001. The funding sources had no involvement in the research stages or preparation of the article.
TMD diagnoses according to the DC/TMD and their sensitivity and specificity from the original DC/TMD (Schiffman et al., 2014).
DDWR, disc displacement with reduction; DDWoR, disc displacement without reduction. * Myalgia subtype
Excluded participants and specific reasons.
Participants’ diagnoses.
DDWR, disc displacement with reduction; DDWoR, disc displacement without reduction. Of the participants with pain-related TMD, 94.2% presented chronic orofacial pain (1.9% missing).