To determine the effects of exercise training on sympathetic nerve activity in heart failure patients.
MethodsA systematic review was performed. An electronic search of MEDLINE, ProQuest, SciELO, SPORTDiscus, Rehabilitation and Sport Medicine Source, Cumulative Index to Nursing and Allied Health Literature, Tripdatabase, Science Direct and PEDrO was performed from their inception to February 2017. Clinical trials and quasi-experimental studies were considered for primary article selection. The studies should include patients diagnosed with chronic heart failure that performed exercise training for at least 4 weeks. Sympathetic nerve activity should be measured by microneurography before and after the intervention. The Cochrane Collaboration's Risk of Bias Tool was used to evaluate risk of bias, and the quality of evidence was rated following the GRADE approach. Standardized mean differences (SMD) were calculated for control and experimental groups. Meta-analysis was performed using the random effects model.
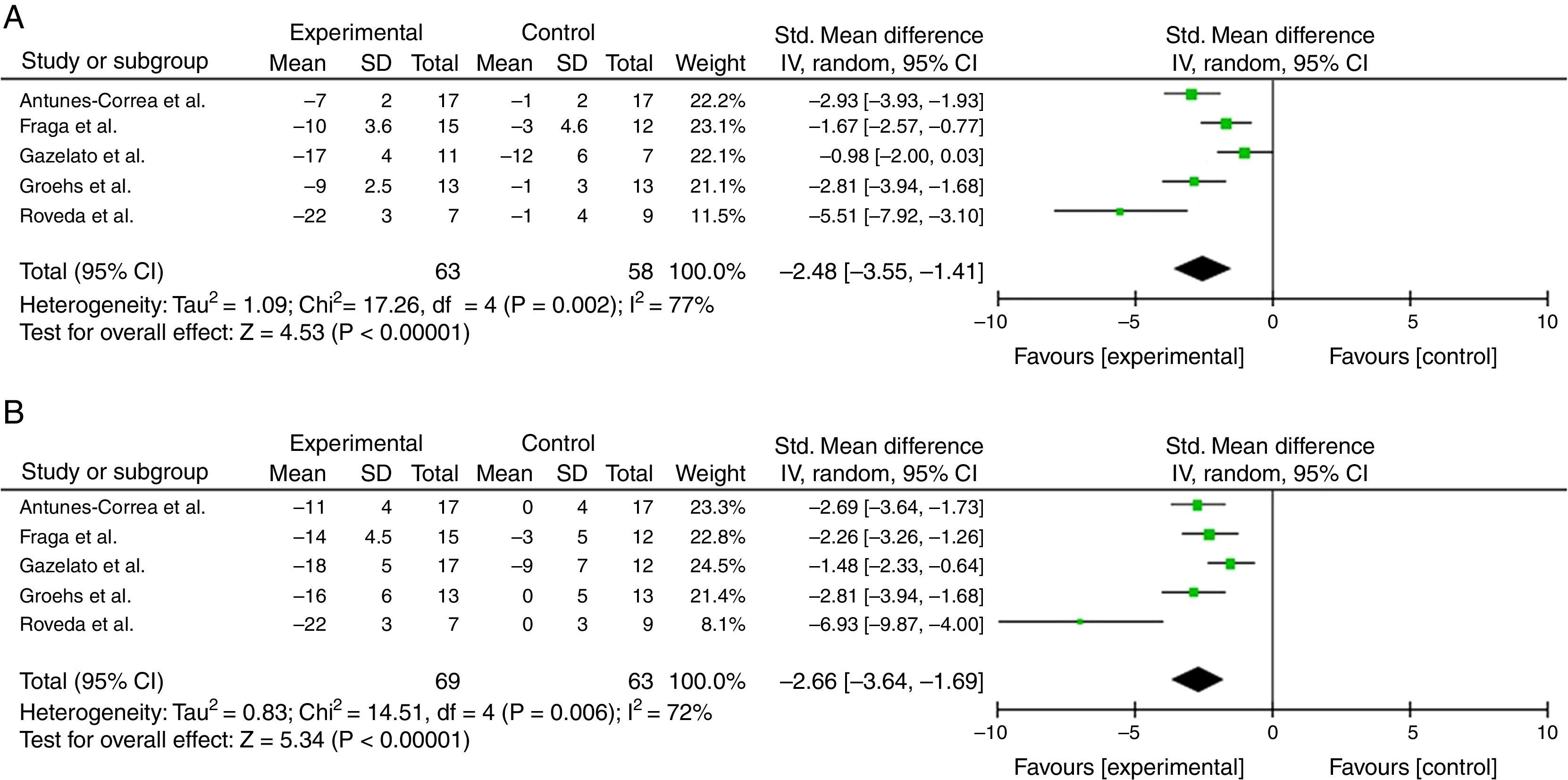
ResultsFive trials were included. Overall, the trials had moderate risk of bias. The experimental group indicated a significant decrease in the number of bursts per minute (SMD −2.48; 95% CI −3.55 to −1.41) when compared to the control group. Meanwhile, a significant decrease was also observed in the prevalence of bursts per 100 beats in the experimental group when compared to the control group (SMD −2.66; 95% CI −3.64 to −1.69).
ConclusionExercise training could be effective in reducing sympathetic nerve activity in patients with heart failure. The quality of evidence across the studies was moderate. Future studies are necessary to confirm these results.
Cardiovascular diseases (CVD) are a major cause of mortality and morbidity worldwide.1–3 In Latin America, CVD mortality reaches 30%, of which 23% occurs for people under 60 years of age.4 In addition, these diseases are associated with a reduced quality of life and an increased in disability, which significantly increases medical expenses.4
Heart failure is one of the most prevalent CVDs worldwide. In this regard, epidemiological studies have revealed that this constitutes of 10% of all hospitalizations in the general population and is the leading cause of hospitalization for patients over 65.5,6 CVDs also represents approximately 2% of all medical expenses in developed countries.7
Heart failure can be defined as a structural abnormality of the heart or heart function which inhibits the supply of oxygen at an adequate rate according to tissue needs.8 The pathophysiology is characterized by an imbalance in the autonomic control of the cardiovascular system, with a predominance of sympathetic nervous systems and inhibition of parasympathetic nervous systems.9–33 This imbalance promotes the activation of renin–angiotensin system and mechanisms associated with myocardial remodeling, which worse the disease prognosis and increases patient mortality rates.11–14
One of the most used non-pharmacological interventions for this group of patients is exercise training.34–36 In this context, a recent systematic review revealed that exercise-based interventions are effective in reducing the mortality and hospitalization risk in coronary heart disease patients37; however the underlying mechanisms of this have not been elucidated yet. Therefore, various studies have shown that exercise training promotes sympathetic inhibition, both for humans and experimental models, which could explain the clinical benefits of exercise in the CVD.15,21,31,34–37
Until now, the effects of exercise training on the sympathetic nerve activity (SNA) of patients with heart failure has not been systematically reviewed. Therefore, this systematic review aims to assess the effects of exercise training on the SNA of heart failure patients.
MethodsThe present study corresponds to a systematic literature review conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)38 and on the tutorial for systematic reviews from the Brazilian Journal of Physical Therapy.39
Selection criteria for primary articlesRandomized and quasi-randomized clinical trials aimed at determining the effects of exercise training on the SNA of heart failure patients were considered. Articles were included from their entry into the database until the month of February 2017, in English and Spanish.
Study subjectsAs inclusion criteria were considered men and women older than 18 years, in both hospital-based and community-based settings, diagnosed with chronic heart failure. We excluded studies which included children and/or participants with congenital heart disease, atrial fibrillation, heart transplants or implanted with either cardiac-resynchronization therapy.
InterventionExercise training was defined as aerobic exercise on treadmill or cycle ergometer proportioned at intensities between 50 and 80% maximum oxygen consumption or/and reserve heart rate. Training was set at a minimum duration of 20min for a training period of at least 4 weeks. These exercise parameters are the minimum exercise protocol to promote the main adaptation mechanisms induced by training in patients with CVD.8,40
Variables of interestSNA was considered a variable of interest, measured using the microneurography technique, expressed as burst×min−1, and burst×100beats−1, for peroneal nerves or radial nerves.
Search methodPrimary studies were identified through a systematic search through the following electronic databases: MEDLINE, ProQuest, Scientific Electronic Library Online (SciELO), SPORTDiscus, Rehabilitation and Sports Medicine Source (R & SMS), Cumulative Index to Nursing & Allied Health Literature (CINAHL), Tripdatabase, Science Direct and PEDro.
MeSH terms were as follows: Cardiovascular Diseases, Heart Failure, Myocardial Infarction, Exercises, Training, Sympathetic Nervous Systems, Autonomic Nervous Systems, Muscle Sympathetic Nerve Activity, Diabetes, and Obesity. Boolean terms were “AND”, “OR” and “NOT”. The first systematic search was performed during August 2016 and was updated in February 2017.
Identification of articlesAfter the systematic search, titles matching the inclusion criteria were selected. Abstracts from the selected articles were read, followed by an extensive reading of the texts selected. Eligibility criteria were assessed by 2 reviewers independently and disagreements were resolved by consensus. The following variables were recorded in an ad-hoc spreadsheet: author, year of publication, average age, number of male subjects, number of female subjects, total sample size, number of subjects in the control group, number of subjects in the experimental group, exercise training parameters (duration, intensity, exercise mode) and sympathetic activity (mean value and standard deviation) before and after the intervention.
Risk of biasBias risks of individual studies were assessed by investigators (JS, IR) independently, according to recommendations suggested by the Cochrane Handbook for Systematic Reviews of Interventions.41 Thus, bias was determined for selection, performance, detection, attrition and reporting of the included studies.
Quality of evidenceThe quality of evidence across studies was evaluated by means of GRADE approach.42 The GRADE comprises five items: presence of risk of bias, inconsistency of results, indirectness of evidence, imprecision of the effect estimates and risk of publication bias. Each non-satisfied item downgraded the overall quality of evidence for each outcome. Thus, the quality of evidence for each outcome was classified into four categories: high, moderate, low and very low.
Data synthesis and statistical analysisMeta-analyses were performed comparing standardized mean differences (SMD) between the experimental and control groups.39 Meanwhile, heterogeneity was determined by calculating the I2 coefficient, which was calculated as I2=100%×(Q×df)/Q; where Q is the Cochran coefficient and df is the degree of freedom. The I2 coefficient ranges from 0 (homogeneity) to 100% (great heterogeneity). If the I2 coefficient was higher than 50% the random effects model was considered for meta-analysis.
Statistical analysis was performed using RevMan 5.3 software and a p <0.05 value was considered significant.
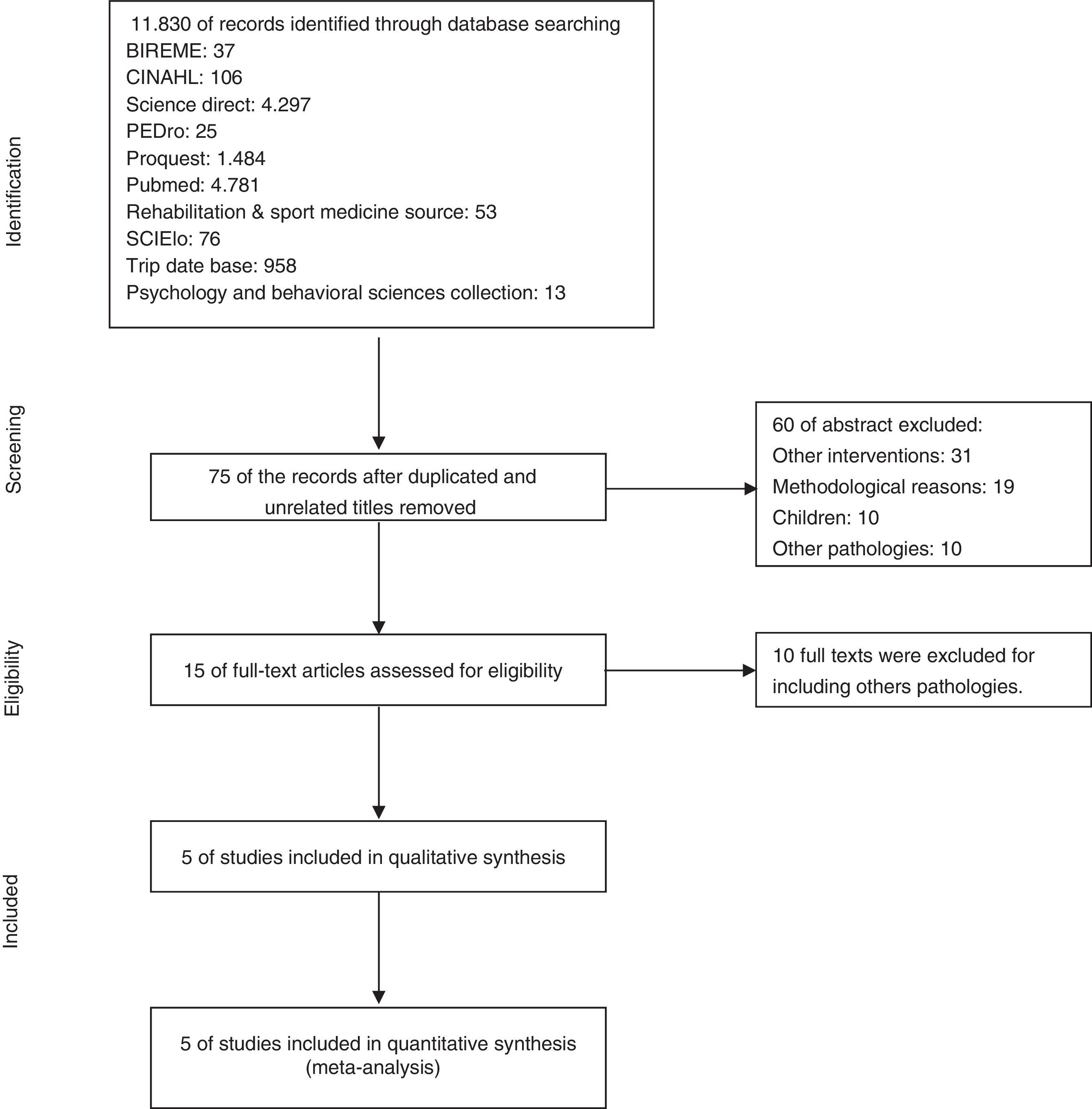
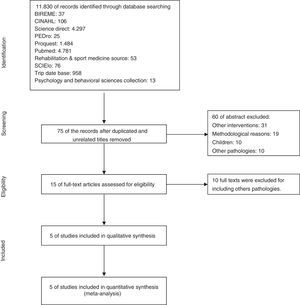
ResultsSearch results11,830 articles were found for the systematic review; 75 abstracts were selected, of which 15 met the inclusion criteria. After extensive readings of the texts, 10 articles were excluded due to the presence of exclusion criteria, leaving 5 primary articles. Fig. 1 presents a flow chart of the selection sequence for the primary articles.
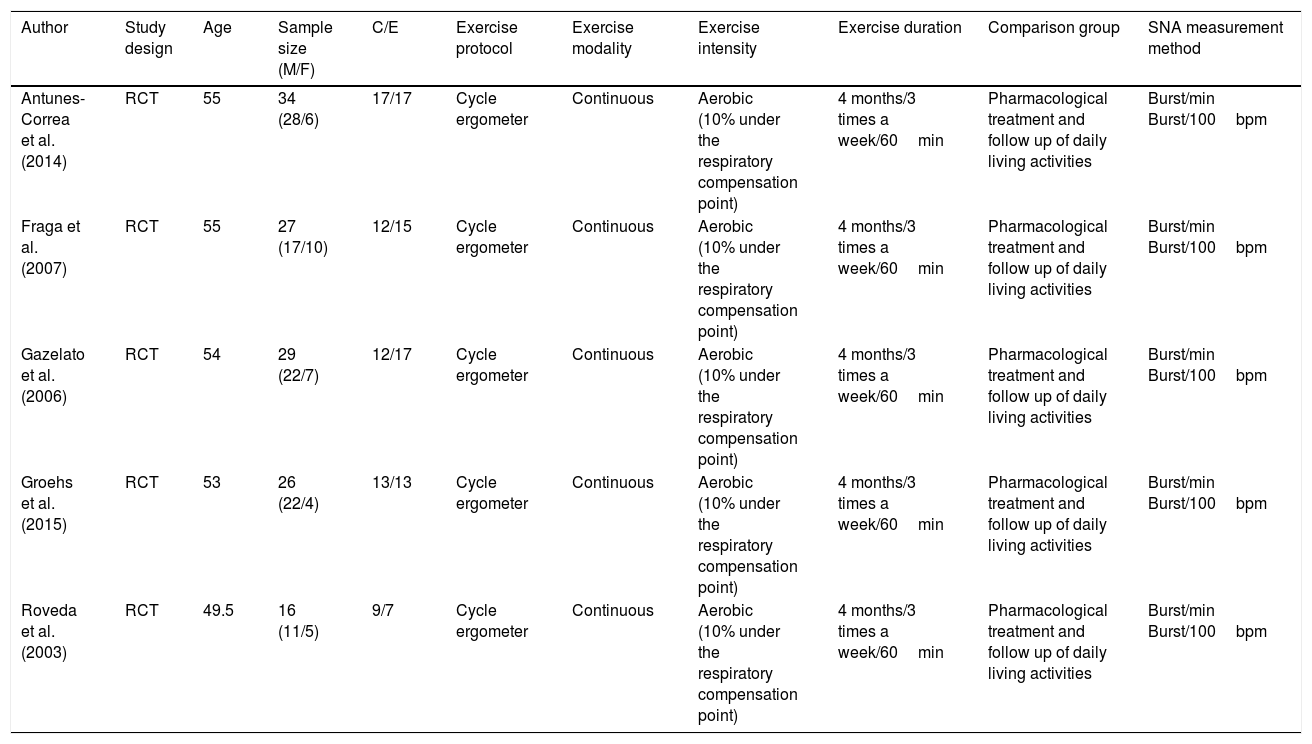
Primary article characteristicsThe 5 articles selected were randomized clinical trials published in English and Table 1 shows their characteristics. The duration of the physical interventions was 4 months for all articles and the type of exercise used was aerobics (on a cycle ergometer) with training intensity ranging between 60 and 72% of the VO2 peak, which is equivalent to 10% lower than the respiratory compensation point in the oxygen consumption test.44–48 The total number of patients was 132, of which 63 were in the control group (no exercise training) and 69 in the experimental group. Subject age in the primary articles ranged between 49 and 55 years. Inclusion criteria used in the articles were as follows: 4 articles included patients with heart failure with an ejection fraction ≤40%,43,45–47 while only 1 used the criterion for inclusion of an ejection fraction ≤35%.44 All articles included patients with a class II to III classification from the New York Heart Association.48 In addition, 3 articles included a <20mL/kg/min oxygen consumption43,44,46 as a criterion.
Characteristics of the included articles.
| Author | Study design | Age | Sample size (M/F) | C/E | Exercise protocol | Exercise modality | Exercise intensity | Exercise duration | Comparison group | SNA measurement method |
|---|---|---|---|---|---|---|---|---|---|---|
| Antunes-Correa et al. (2014) | RCT | 55 | 34 (28/6) | 17/17 | Cycle ergometer | Continuous | Aerobic (10% under the respiratory compensation point) | 4 months/3 times a week/60min | Pharmacological treatment and follow up of daily living activities | Burst/min Burst/100bpm |
| Fraga et al. (2007) | RCT | 55 | 27 (17/10) | 12/15 | Cycle ergometer | Continuous | Aerobic (10% under the respiratory compensation point) | 4 months/3 times a week/60min | Pharmacological treatment and follow up of daily living activities | Burst/min Burst/100bpm |
| Gazelato et al. (2006) | RCT | 54 | 29 (22/7) | 12/17 | Cycle ergometer | Continuous | Aerobic (10% under the respiratory compensation point) | 4 months/3 times a week/60min | Pharmacological treatment and follow up of daily living activities | Burst/min Burst/100bpm |
| Groehs et al. (2015) | RCT | 53 | 26 (22/4) | 13/13 | Cycle ergometer | Continuous | Aerobic (10% under the respiratory compensation point) | 4 months/3 times a week/60min | Pharmacological treatment and follow up of daily living activities | Burst/min Burst/100bpm |
| Roveda et al. (2003) | RCT | 49.5 | 16 (11/5) | 9/7 | Cycle ergometer | Continuous | Aerobic (10% under the respiratory compensation point) | 4 months/3 times a week/60min | Pharmacological treatment and follow up of daily living activities | Burst/min Burst/100bpm |
C, sample size control group; E, sample size experimental group; SNA, measurement method of sympathetic nerve activity.
Sympathetic nerve activity was recorded in all articles for the peroneal nerve, before and after the intervention, and results were reported in burst×min−1 and burst×100beats−1.
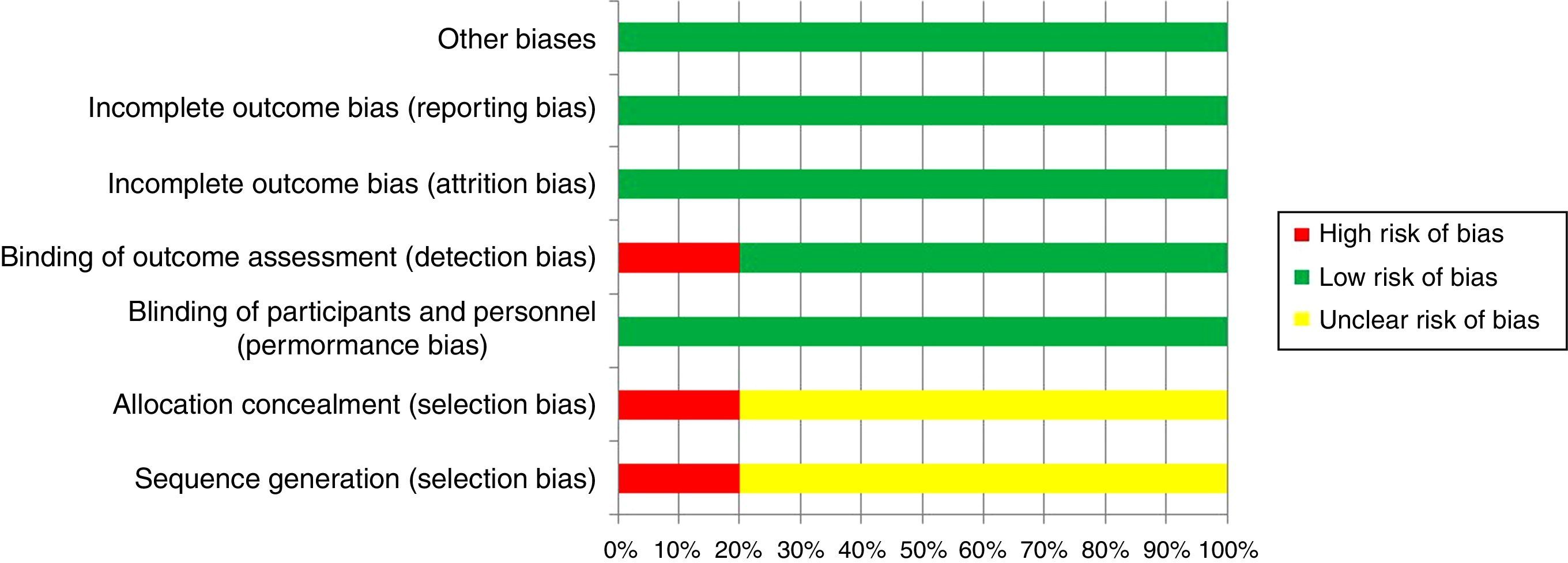
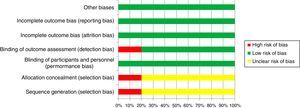
Risk of biasAll studies presented low bias risks for reporting, attrition and performance. Moreover, one study presented a high risk of detection bias and one study had a high risk of selection bias (Fig. 2).
Sympathetic nerve activityA significant decrease in the number of bursts per minute was observed for the experimental group (burst×min−1) (SMD −2.48; 95% CI −3.55 to −1.41) in the meta-analysis of randomized effects, when compared to the control group. Meanwhile, a significant decrease was also observed in the prevalence of bursts per 100 beats in the experimental group when compared to the control group (SMD −2.66; 95% CI −3.64 to −1.69) (Fig. 3A and B). The quality of evidence across the studies was moderate for the sympathetic nerve activity measured by both methods burst×min−1 and burst×100bpm−1. The inconsistency was the main factor that reduced the quality of evidence.
DiscussionThe purpose of this systematic review was to determine the effect of exercise training on SNA in patients with heart failure. Results showed with a moderate quality of evidence that moderate-intensity exercise training is effective in reducing SNA measured through microneurography of the peroneal nerve. Moreover, the overall effect revealed that the intervention restores the SNA near to normal values by reducing the SNA about 2.5 standard deviations (−27.4%), constituting a large effect size.
These findings are consistent with results obtained from other systematic review performed by our group. Segovia et al.49 showed that exercise training increases the heart rate variability and decreases SNA by reducing the LF/HF index in patients with heart failure.49 Additionally, in experimental models it has been demonstrated that 4–8 weeks of exercise training is effective for preventing increases in renal sympathetic activity in rats with experimental heart failure.15,31
Currently, it has been widely reported that sympathetic over-activity is an important part of heart failure pathophysiology. Charkoudian and Rabbitts12 demonstrated that sympathetic activity in the peroneal nerve of those patients is almost twice as much as the sympathetic activity in healthy subjects. In this context, the perpetuation of this physiological response promotes the development of neurohormonal mechanisms such as the renin–angiotensin–aldosterone system, which is closely associated with a poor prognosis of the disease.12
Among the mechanisms underlying reduced sympathetic activity (induced by exercise), it should be noted that evidence is consistent in establishing the existence of a decrease in activity for the rostral ventrolateral medulla (RVLM) and the periventricular nucleus (PVN) of the hypothalamus, as well as an increase in activity for the nucleus of the solitary tract (NST). These adaptive responses, induced by exercise training, promote normalization of sympathetic neurotransmission and restore cardiovascular system reflex control. The previous has been widely demonstrated in experimental studies.15,21,31,34–36
The present review has some limitations that should be identified. Although articles generally presented a low bias risk for reporting, attrition and performance, one article presented high risks of detection and selection bias. Additionally, the studies included small sample sizes, which reduces the statistical power of the results and counterbalance the effect size. Moreover, in the meta-analysis of random effects it was found that there is a high level of heterogeneity between articles, which does not rule out random influences in the magnitude of the observed effect, reducing the quality of evidence across the studies.
This review included only one outcome, constituting a limitation for clinical applicability of the results, since sympathetic nerve activity is not a measuring parameter commonly considered in clinical setting. Moreover, the fact to include just aerobic training impedes to extrapolate the results to others training protocols e.g., strength training or high intensity interval training.
Future studies should be directed to evaluate other protocols and training modes, for example, interval training, high intensity training, or over-training, because there is still no certainty about the real effects of modulating training intensity on autonomic control mechanisms of the cardiovascular system.
From a methodological point of view, future studies should optimize randomization and blinding mechanisms of groups to significantly reduce selection bias in primary articles, increasing the internal validity of future meta-analyses aimed at verifying these hypotheses.
Finally, according to data obtained in this systematic review and meta-analysis, it is possible to conclude that exercise training could be effective in reducing sympathetic nerve activity in patients with heart failure. Thus, the results support a strong recommendation (1B), since the benefits overcome the risks for using moderate exercise training for reducing SNA in heart failure patients.
Financial supportThis work was supported by School of Kinesiology, San Sebastian University, Concepción, Chile.
DisclosureThis work corresponded to Maria Javiera Saavedra's thesis for obtaining the Master of Degree in Cardiopulmonary Kinesiology in San Sebastian University, Concepción, Chile.
Conflicts of interestThe authors declare no conflicts of interest.