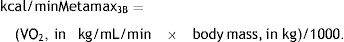To examine the concurrent validity of the GT3X® ActiGraph accelerometer and Google Fit® smartphone application in estimating energy expenditure in people who had suffered a stroke, during fast overground walking.
MethodsThirty community-dwelling stroke individuals walked on a 10-meter hallway over 5min at their fastest speeds, wearing a Cortex Metamax 3B® ergoespirometer, a GT3X® ActiGraph accelerometer, and a smartphone with the Google Fit® application. Pearson correlation coefficients were calculated to verify the associations between measures of energy expenditure, in kilocalories (kcal), estimated by both devices and those obtained with the Cortex Metamax 3B® ergoespirometer (gold-standard measure).
ResultsFair association was found between the energy expenditure values estimated from the combined formula of the ActiGraph GT3X® and those obtained with the gold-standard measure (r=0.37; p=0.04). No significant associations were found between the energy expenditure values estimated by the Google Fit® application and those provided by the gold-standard measure.
ConclusionsThe findings demonstrated that both the GT3X®ActiGraph accelerometer and the Google Fit® smartphone application do not provide valid measures of energy expenditure in chronic stroke individuals during fast overground walking.
Following a stroke, individuals tend to adopt sedentary behaviors, which usually perpetuate during the chronic stages.1,2 However, the paramount importance of active lifestyles for individuals who have had a stroke has already been reported,3,4 since it helps, amongst other aspects, to prevent deconditioning and new cardiovascular events.3 Besides, it is recommended that individuals who have had a stroke should be involved in regular aerobic exercise programs targeted to enhance their aerobic capacity and walking efficiency, in order to improve functional independence.3,5 The reason for these recommendations is based on the fact that individuals who have suffered a stroke have metabolic abnormalities6,7 and cardiovascular adaptations, which are not observed in healthy individuals.8 Therefore, these abnormalities increase the risk of recurrent cardiovascular events.7 Since the prevention of new cardiovascular events is essential for individuals who have suffered a stroke, the objective assessment and monitoring of energy expenditure during physical activity practice becomes of great importance, in order to track the individuals’ physical activity levels.
Devices, such as regular accelerometers, provide objective measures of energy expenditure9,10 and are the most frequently used with stroke individuals, as reported in a previous systematic review.11 One example of a triaxial accelerometer, commonly used with various neurological conditions, is the GT3X® ActiGraph (ActiGraph, Pensacola, FL, USA).12,13 However, its prediction equations used to calculate measures of energy expenditure were developed based upon data of healthy individuals during walking and running on a treadmill.14 Considering the impairment patterns of body functions and structures observed following a stroke, it is reasonable to question whether accelerometers are reliable devices for measuring energy expenditure in this population, since gait patterns of individuals who have suffered a stroke are abruptly different from those of healthy individuals. Also, these devices are mainly used for research purposes, as they are relatively expensive and therefore, it is not always feasible to use them in clinical settings.15
A promising way of assessing and monitoring physical activity of individuals who have suffered a stroke is by using smartphone applications,16 since they provide real-time information and are freely accessible and easy-to-use.15–17 One example of this technology is the Google Fit® (Google Inc., Mountain View, CA, USA), which is an open platform, also available for smartphones.17 Google Fit® smartphone application provides information regarding step counts, distance walked, and calories burnt during physical activity.17 However, since the Google Fit® is a relatively new application,17 its validity has not been evaluated in individuals with neurological conditions, including individuals who have suffered a stroke. Moreover, information regarding the prediction equations for the estimation of energy expenditure is not available for these devices. Thus, it is neither known how they were developed, nor if they would be valid to monitor energy expenditure in stroke individuals.
Therefore, the aim of this study was to examine the concurrent validity of both the GT3X® ActiGraph accelerometer and Google Fit® smartphone application in estimating energy expenditure in individuals who have suffered a stroke, during their fastest overground walking. The relationships between the estimated energy expenditure measures, in kilocalories (kcal), provided by both devices and those, obtained with the Cortex Metamax 3B® ergoespirometer (the gold-standard measure) were investigated. This information may be useful when recommending these devices for monitoring energy expenditure.
MethodsThis study investigated the concurrent validity of the GT3X® ActiGraph accelerometer and Google Fit® smartphone application in estimating energy expenditure of individuals who have suffered a stroke during fast over ground walking. For this, the data estimated by both the GT3X® ActiGraph accelerometer and Google Fit® smartphone application were compared to those provided by the Cortex Metamax 3B® ergoespirometer (gold-standard measure).18 The COSMIN checklist for evaluating the methodological quality of studies on measurement properties was employed to ascertain the quality of the present study.19
ParticipantsIndividuals, who had a single unilateral stroke, were recruited from the general community, from August to December 2015. To be included, the participants had to be over 20 years of age; had to have a mean time since the onset of the stroke of at least 6 months; had to be able to walk independently with or without assistive devices; had hemiparesis (i.e., residual weakness of the paretic knee extensor and/or ankle plantar flexor/dorsal flexor muscles – strength deficit >10% compared to the non-paretic side),20 assessed by a hand-held dynamometer, and/or increased tonus of the paretic knee extensor muscles determined by scores different from zero on the modified Ashworth scale; and had no cognitive impairments as determined by the following education-adjusted cut-off scores on the Mini Mental State Examination: 13 for the individuals with illiteracy and 18 for those with basic education.21 Participants were excluded if they had any other associated neurological, respiratory, and/or orthopedic conditions. All participants provided written consent, based upon previous approval from the Institutional Ethical Review Board – Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, Minas Gerais, Brazil (CAAE – 47256815.9.0000.5149).
Sample sizeThe sample size of 30 participants was calculated a posteriori by the GPower 3.1 software, resulting in a power of 0.98.
Instruments and proceduresInitially, the participants underwent an interview and physical examination to collect their demographic, anthropometric, and clinical data, which included age, sex, body mass, height, time since the onset of the stroke, habitual and maximal walking speeds (using a 10-meter Walking Test – 10MWT), motor recovery of the paretic lower limb (based on the Fugl-Meyer lower-extremity section scores), strength of knee extensor and ankle plantar flexor/dorsal flexor muscles (as measured with a hand-held dynamometer), and tonus of the knee extensor muscles (based on the Ashworth Modified Scale). They were then asked to walk back and forth on a 10-meter, flat, and straight hallway over 5min, at their maximum speeds, following previously recommended procedures.22 The reason that maximum speed was chosen was because we wanted to know if these devices would provide reliable measures of energy expenditure during physical activity practice, which usually involves increased energy demands. Besides, it has been reported that walking at higher speeds improves cardiovascular health, more than walking at comfortable speeds for chronic stroke individuals,23 which is usually the main goal of a conditioning program.
During the walking test, participants wore the GT3X® ActiGraph accelerometer on their paretic ankle, the smartphone with the Google Fit® application in the front pocket of the pants of their paretic lower limb, and the Cortex Metamax 3B® ergoespirometer (the gold-standard measure),18 following previously recommended procedures.1,24
The GT3X ActiGraph® accelerometerThe GT3X®ActiGraph triaxial accelerometer is a small (3.8cm width×3.7cm length×1.8cm depth; 27g), commercially available device, which captures changes in acceleration ranging in magnitudes from 0.05 to 2.5G's, at a sample rate of 30Hz in three individual axes: anterior–posterior (AP) or X axis, medial-lateral (ML) or Z axis, and vertical (VT or Y axis),25,26 as well as a composite vector magnitude (VM) of the three axes.26 The accelerometer gives its outputs as counts per period of time, called epochs, and in the present study, these were set at 60-s epochs, as previously applied with individuals who have suffered a stroke.1 The GT3X® ActiGraph accelerometer estimates energy expenditure, by converting its counts/min from the VT axis into kcal. To do this, it applies two previously established equations and one combined formula, as follows27:
- (1)
Work-energy theorem (WET) equation: kcal/minWET=0.0000191×counts/min×body mass, in kg.
- (2)
Freedson equation: kcal/minFreedson=0.00094×counts/min+0.1346×body mass, in kg – 7.37418.
- (3)
Combined formula: WET equation for counts/min ≤1952 and the Freedson equation for counts/min >1952.
The accelerometer can be positioned on different body regions and, for the present study, it was placed on the paretic ankle, as recommended by the manufacturer and previously used with individuals who had suffered a stroke.1 Energy expenditure estimates, in kcal, provided by all three equations, over the 5-min monitoring period were averaged and used for analyses. The collected data were analyzed by the ActiLife data analysis software (version 4.1.0). This software was provided by the GT3X® ActiGraph accelerometer manufacturer. The GT3X® ActiGraph accelerometer provided reliable measures during overground walking in healthy individuals.28
Google Fit® smartphone applicationThe Google Fit® is an open platform developed by Google Inc., that allows users to control their fitness data. It is also available as a free application for smartphones, which works on versions above 4.0 in Android systems.17 The Google Fit® consists of a set of high level sensors, such as an accelerometer, a gyroscope, and a global positioning system, which detect changes in position and distinguish amongst various types of movements, several types of data, and various bouts of activity.17 The smartphone application used in the present study was the LG Nexus 5, which weighted 130g and had the following dimensions: 69.17mm width×137.84mm length×8.59mm depth.
According to the manufacturer, Google Fit® application provides energy expenditure estimates in calories (cal), but according to the application developers, it provides measures in kcal.17 The data obtained during the 5-min monitoring test were also averaged and used for analyses, because the Google Fit® application provided energy expenditure output as total burnt kcal, and not on a minute-by-minute basis.
Prior to the walking test, the smartphone was positioned in the participants’ front pocket of their paretic lower limb, as previously applied24 and calibrated, based on the following users’ data: sex, body mass (kg), and height (cm). Table 1 shows the technical specifications of both devices.
Technical specifications of the GT3X® ActiGraph accelerometer and the Google Fit® smartphone application.
| Specification | ActiGraph GT3X® | Google Fit® smartphone application |
|---|---|---|
| Sample rate | 30Hz | Not available |
| Data storage | 16MB | 16GB |
| Battery life | 31 days | 17h to 12.5 days |
| Accelerometer sensor | ADXL335 triaxial accelerometer (Analog Devices, USA) | MPU6515 triaxial accelerometer+gyroscope (Invense Inc., USA) |
| Registered range of acceleration | ±3g | Not available |
| Measured outcomes | Acceleration around three axes and vector magnitude | Acceleration around three axes |
| Estimated outcomes | Number of steps taken, energy expenditure (kcal), and duration of physical activity (min) | Number of steps taken, type of activity, traveled distance (miles or km), energy expenditure (cal), and duration of physical activity (min) |
The Cortex Metamax 3B® ergoespirometer, which was used as a gold-standard measure,18 gives real-time corrected measures of VO2, in kg/mL/min.29 The VO2 was measured minute by minute, using an open circuit ergoespirometry, which provided reliable measures during over ground walking in individuals who had suffered a stroke (Intraclass Correlation Coefficient ranging from 0.76 to 0.97).22 The gases were collected during each breathing cycle through a silicone mask adapted to the individual's face.29 For analyses, the VO2 values of the entire 5-min monitoring test were averaged and converted into kcal, by applying the following formula30:
The ergoespirometer was calibrated before each data collection in three steps, following the manufacturer's recommendations: (1) barometric; (2) gas, by using verified gases of known concentration (12% O2, 5% CO2, and balance N2: ±0.02% absolute); and (3) volume, by using a 3L syringe (Hans Rudolph Inc.).8,29
Statistical analysesDescriptive statistics and tests for normality (using the Shapiro–Wilk Test) were carried-out with the SPSS software (version 19.0). The anthropometric data were presented as means and standard deviations. Pearson's correlation coefficients were calculated to examine the associations between the energy expenditure values (in kcal) estimated from both the GT3X® ActiGraph accelerometer equations and the Google Fit® smartphone application, with those provided by the gold-standard measure. The correlation analyses considered the following cut-off values31: 0–0.25: little or none; 0.26–0.50: fair; 0.51–0.75: moderate to good; and >0.75 good to excellent relationships. The significance level was set at 5% for all analyses.
ResultsThirty individuals who had suffered a stroke (21 men, 9 women), who had a mean age of 62 (SD=12) years and a mean time since the onset of the stroke of 98 (SD=96) months, were included. Twenty-one participants reported not being engaged in any kind of physical activity, 24 of the subjects had had ischemic stroke, and the mean distance covered by the participants over the 5-min walking was 258.9 (SD=155.2) meters. Thirteen participants walked at speeds <0.80m/s. Of the nine individuals who were physically active, five reported walking as the most frequently practiced activity, with bouts of activity ranging from 30min to 1h, three times a week. The characteristics of the participants are presented in Table 2.
Participants’ characteristics for a study looking at the validity of accelerometer and smartphone applications in estimating energy expenditure in stroke patients.
| Characteristics | n=30 |
|---|---|
| Age (yrs.), mean±SD (range: min−max) | 62±12 (24–82) |
| Sex (men/women), n | 21/9 |
| Body mass (kg), mean±SD (range: min−max) | 75.0±12.2 (50–99) |
| Height (cm), mean±SD (range: min−max) | 164.7±8.6 (142–184) |
| Time since stroke (months), mean±SD (range: min−max) | 98.5±96.1 (9–412) |
| Side of paresis (left/right), n | 17/13 |
| MMES (scores: 0–30), mean±SD | 26±3 |
| Gait speed (m/s), mea n±SD (range: min−max) | |
| -Comfortable speed | 0.8±0.3 (0.3–1.4) |
| -Fastest speed | 1.3±1.0 (0.5–2.3) |
| Fugl-Meyer lower-limb section (score: 0–34), mean±SD | 19±5 |
| Tonus of the knee extensor muscles (MAS score: 0–4), n | |
| −0 | 18 |
| −1 | 8 |
| −2 | 2 |
| −3 | 1 |
| −4 | 1 |
| Residual weakness (%±SD) | |
| -Knee extensors | 11.3±4.8 |
| -Ankle plantar flexors | 20.0±3.3 |
| -Ankle dorsiflexors | 19.4±3.9 |
SD=standard deviation, min=minimum, max=maximum, yrs.=years.
Of the three GT3X® ActiGraph accelerometer equations used to estimate the energy expenditure, fair association with the gold-standard measure was only found between the values estimated from the combined formula (r=0.37; p=0.04). In addition, there were no other statistically significant associations found between the values estimated from the other GT3X® ActiGraph equations (i.e., WET and Freedson) and those provided by the gold-standard measure (r=0.04; p>0.05).
Google Fit® smartphone applicationSignificant and fair association was found between the energy expenditure values estimated by the Google Fit® smartphone application and those provided by the gold-standard measure (r=0.30; p=0.01) (Tables 3 and 4).
Pearson's correlation coefficients (r and p values) between the energy expenditure estimations (in kcal) obtained from the GT3X® ActiGraph accelerometer and the Google Fit® smartphone application and those obtained by the gold-standard measure (Cortex Metamax 3B).
| Values | Cortex Metamax 3B | GT3X Actigraph accelerometer | Google fit | ||
|---|---|---|---|---|---|
| WET equation | Freedson equation | Combined formula | |||
| Energy expenditure, in kcal (mean±SD) | 3.6±1.2 | 8.6±6.5 | 8.0±4.9 | 8.0±4.8 | 6.4±3.7 |
| Correlation coefficients | – | 0.04 | 0.04 | 0.37a | 0.30a |
| p-Values | – | 0.36 | 0.66 | 0.04 | 0.01 |
WET=work-energy theorem.
Pearson's correlation coefficients (r and p values) between the raw data (in counts) from all of the GT3X® ActiGraph accelerometer axes and those obtained using the Cortex Metamax 3B (VO2, in mL/kg/min).
| Values | Cortex Metamax 3B | GT3X Actigraph accelerometer axes | |||
|---|---|---|---|---|---|
| Vertical | Anterior-posterior | Medial-lateral | Vector magnitude | ||
| Raw data, means (SD) | 9. 5±3.0 | 5975.9 (4317.3) | 3693.9 (2242.5) | 3525.4 (2374.9) | 8412.5 (4568.9) |
| Correlation coefficients | – | 0.28 | 0.12 | 0.53a | <0.71a |
| p-Values | – | 0.14 | 0.51 | <0.001 | <0.001 |
WET=work-energy theorem.
This study was designed to examine the concurrent validity of both the GT3X® ActiGraph accelerometer and Google Fit® smartphone application in estimating energy expenditure in people who had suffered a stroke, during fast over ground walking. To do this, the associations between the energy expenditure data estimated by both devices and those provided by the gold-standard measure (i.e., the Cortex Metamax 3B® ergoespirometer) were compared. Fair associations with the gold-standard measure were found between the data estimated from the combined formula of the GT3X® ActiGraph accelerometer and the Google Fit® smartphone application.
The fair association observed between the energy expenditure data estimated from the GT3X® ActiGraph combined formula and those provided by the gold-standard measure, might be due to the fact that the equations used by the GT3X® ActiGraph accelerometer to estimate energy expenditure (in kcal) were developed for healthy young individuals, during walking/running on a treadmill.14 Previous studies reported that stroke individuals who had higher functional levels (i.e., walked at speeds ˃0.8m/s) had energy expenditure similar to healthy individuals.32–34 However, the results of the present study using a sample, who walked slower (comfortable walking speeds ranging from 0.3 to 1.4m/s) but at their fastest speed, showed that the energy expenditure values estimated from all of the GT3X® ActiGraph equations were about 55% higher than those provided by the gold-standard measure. This overestimation suggests that the equations usually used to predict energy expenditure of healthy individuals during treadmill walking may not be the most appropriate for predicting energy expenditure of community-dwelling stroke individuals during fast over ground walking. The anatomical and physiological differences, usually observed between healthy and stroke individuals, might explain why the equations used to predict energy expenditure of healthy individuals should not be used to predict energy expenditure of stroke individuals.
A previous study also compared the energy expenditure data estimated from the GT3X® ActiGraph equations with those measured by a metabolic system (Oxycon Pro) using healthy adolescents, young adults, and elderly subjects, walking and running on a treadmill under six different conditions.35 When the data of the elderly were separately analyzed, it was found that out of the three GT3X® ActiGraph equations, the WET one worked the best.35 However, even though the sample of the present study also included primarily elderly individuals (>60years), the data estimated from the WET equation showed no associations with those provided by the gold-standard measure. These differences may be due to the sample characteristics and walking conditions. It is important to point-out that even though a triaxial accelerometer was used, the GT3X® Actigraph only took into account the data from the vertical axis (VT) for the estimation of energy expenditure.35 Compared to movements in the frontal and sagittal planes, joint range of motion in the transverse plane, i.e., internal and external rotations, is more limited even in health individuals. Thus, the vertical axis would probably not be the best to be considered for estimating energy expenditure of individuals who have suffered a stroke, once they have adapted to the movement patterns of their paretic lower limb as a compensatory strategy, due to residual motor impairments.36–38
Moreover, the GT3X® ActiGraph equations were based upon a previous ActiGraph model (GT1M®).14 In this scenario, Sasaki et al.39 compared both devices and observed that the raw data estimated by the two ActiGraph models were not comparable even for healthy young subjects: AP axis mean bias of −515±640 counts; VM axis: mean bias=−231±28 counts. When both ActiGraph models were compared, the main difference was the previous model (i.e., the GT1M) did not take into account the ML axis.39 In the present study, however, the raw data from the ML axis were the only ones that showed some associations with those provided by the gold-standard measure (VO2, in mL/kg/min). These findings could be explained by the abnormal gait patterns of stroke individuals, who showed residual motor impairments and gait asymmetries and that maximal range of motion occurred along the sagittal plane.36 Previous studies reported that the knee extensor and ankle plantar flexor/dorsiflexor muscles were considered the main contributors to the gait performance of stroke individuals36,37 and have been shown to be responsible for the movements of the lower limb along the ML axis.38 The present study observed a relatively high percentage of residual weakness in the above mentioned muscles, which probably confirms the hypothesis that the vertical axis would not be the best axis to use for estimating energy expenditure of stroke individuals. Since the equations used by the GT3X®ActiGraph accelerometer were not considered the most appropriate for measuring energy expenditure in the elderly, Santos-Lozano et al.35 suggested the use of age-specific equations for estimating energy expenditure measures. Thus, it is reasonable to argue that the determination of specific equations for the prediction of energy expenditure, taking into account the data from all three axes, would also be necessary and would be a better alternative for individuals with neurological conditions, including stroke.
The data estimated from the Google Fit® smartphone application were also associated with those provided by the gold-standard measure. The Google Fit® smartphone application estimated energy expenditure values closer to those provided by the gold standard measure. This finding corroborates with the findings of previous studies which have shown that smartphone applications are better physical activity monitors than regular accelerometers.40 Wu et al.,41 for instance, reported that the use of smartphones with a built-in accelerometer and a gyroscope was beneficial for classifying activities of healthy individuals from 19 to 60 years of age. It was also observed that smartphone applications estimated energy expenditure of healthy young individuals during walking and running on a treadmill with better accuracy than the GT3X+® ActiGraph accelerometer (a newer version of the GT3X Actigraph accelerometer).15
Generally, several smartphone applications have been developed for different purposes, such as to administer functional tests, by providing audio and visual instructions42 to help general rehabilitation of stroke individuals, by educating patients and caregivers regarding home-based exercises, posture, and medication control43; and to stimulate the practice of rehabilitation exercises for upper-limb recovery.44 For the goal of post-stroke conditioning, only one smartphone application was found, called Starfish, which has been recently developed to monitor and to increase the number of daily steps of individuals with chronic stroke.16 Even though the Starfish application has demonstrated potential for increasing physical activity levels,16 it does not take into account other forms of physical activity, nor the user's energy expenditure (in kcal) while practicing physical activities. Thus, future studies should focus on the development of smartphone applications with the goal of assessing and monitoring energy expenditure, since these devices have shown to be effective for supporting changes in health behaviors and physical activity levels.45
In summary, the findings of the present study demonstrated that both the GT3X® ActiGraph accelerometer and the Google Fit® smartphone application did not provide valid measures of energy expenditure of chronic stroke individuals during fast over ground walking. Future studies should focus on the development of physical activity monitors, based upon group-specific energy expenditure equations, given that they are free or cheap, easy to use, and provide real-time information on physical activity parameters.
Funding supportBrazilian National Funding Agencies (CNPQ and FAPEMIG).
Conflicts of interestThe authors report no conflicts of interest.
The authors would like to acknowledge the staff members of the “Laboratório de Avaliação e Pesquisa em Desempenho Cardiorrespiratório” (LabCare) for their technical support, and the Brazilian National Funding Agencies (CNPQ and FAPEMIG) for their financial support.