The DiAbeTes Education Questionnaire (DATE-Q) is a self-administered tool developed to evaluate disease-related knowledge and to assess knowledge of five core components of rehabilitation programs: physical exercise, diet, psychosocial well-being, disease self-management, and complications.
ObjectiveTo translate and cross-culturally adapt into Brazilian Portuguese, and to test the psychometric properties of the DATE-Q for its use in Brazil.
MethodsThe process of translation and cross-cultural adaptation consisted of five steps: translation into Brazilian Portuguese, synthesis of translation, back translation, expert committee, and pilot test of pre-final version. The pre-final version was applied to a sample of 30 patients with diabetes. Psychometric properties (internal consistency, reliability, construct validity, and ceiling and floor effects) of the final version of the Brazilian Portuguese version of the DATE-Q were tested in a sample of 200 adults with diabetes.
ResultsThere was no conceptual divergence between the original and the translated versions. Ten (50%) items of the DATE-Q were culturally adapted. Internal consistency (Cronbach’s alpha coefficient = 0.6), reliability (intraclass correlation coefficient = 0.5), and construct validity (correlation between Diabetes Knowledge Scales and DATE-Q total scores: ρ = 0.7; P < 0.001) were confirmed. Ceiling or floor effects were not identified. The highest scoring item was about healthy eating. The average time for completion of the DATE-Q was 5 min and 51 s, and the completion rate was 100% for all items.
ConclusionThe Brazilian Portuguese version of the DATE-Q showed adequate psychometric properties, and results suggested that the tool can be used to assess disease-related knowledge in adults with diabetes in Brazil.
Diabetes is increasingly prevalent worldwide,1 driven by an aging population and an increase in the prevalence of obesity also driven by an increase in the survival of people with type 1 diabetes due to therapeutic advances.2 Although there is a tendency towards stability or decline of the incidence of diabetes in some high-income countries,2 it is still rising in Brazil3 and its prevalence is higher in individuals who are older and have a lower level of education.4 This is consistent with results from a previous study which identified a high prevalence of diabetes in Brazil and highlighted the need for behavioral change as a strategy to prevent and control this condition and its complications.5
As a chronic disease, diabetes requires comprehensive and continuous medical care6 and development of self-care behavior to achieve therapeutic goals established in each stage of diabetes treatment, which demands disease-related knowledge.7,8 The assessment of diabetes knowledge is essential to understand the knowledge gaps these patients face and design educational strategies. Awareness of how much patients with diabetes know about their condition can also impact the behavior-change process.9–12
Different tools to assess disease-related knowledge in patients with diabetes, which have contrasting content and administration form based on the sociodemographic and clinical characteristics of the target population, have been validated.13–23 Among these, the Spoken Knowledge in Low Literacy in Diabetes Knowledge Assessment Scale (SKILLD)24 and the Diabetes Knowledge Assessment Scale (DKN-A)25 have been translated, cross-culturally adapted, and validated in the Brazilian population. However, the SKILLD19 is a tool designed to assess disease-related knowledge in patients with diabetes and low literacy, and the DKN-A14 does not address critical components of diabetes care (e.g. being active).
Additionally, despite these options, there is a lack of short tools which are self-completed and address the main components of diabetes care. To fill this gap, the research and staff team from the Cardiac Prevention and Rehabilitation Program of Toronto Rehabilitation Institute recently developed a self-administered tool to assess disease-related knowledge in adults with diabetes titled: DiAbeTes Education Questionnaire (DATE-Q).26 This psychometrically validated instrument has 20 items (answer options are: true or false or do not know) equally distributed in five domains (self-management, long-term complications, being active, healthy eating, and psychosocial well-being), and a total score that ranges from 0 to 20 (one point for each correct answer) with higher scores indicating greater diabetes knowledge.26
The objective of this study is to translate, cross-culturally adapt into Brazilian Portuguese, and test the psychometric properties of the DATE-Q for its use in Brazil. Also, secondary objectives include comparing the total DATE-Q scores and the DATE-Q scores in each domain based on age, diabetes type, time elapsed since diagnosis, educational level, and household income.
MethodsStudy designThe research protocol was approved by the Research Ethics Committee of the Hospital of Universidade Federal de Juiz de Fora, MG, Brazil (CAAE 77831517.0.2002.5133) and all participants gave their informed written consent before being included in the study. Translation, cross-cultural adaptation, and validation of DATE-Q was performed with prior permission from the original authors and consisted of two phases: (1) translation and cross-cultural adaptation and (2) establishing psychometric properties.
First phase: translation and cross-cultural adaptationThis study followed the five steps proposed by Beaton et al:27 (1) Translation into Brazilian Portuguese - two translations from English into Brazilian Portuguese (T1 and T2) were generated independently by two native speakers of Brazilian Portuguese; (2) Synthesis of the translations – the two initial translators and a physical therapist summarized the two translations into one version (T1.2), disagreements were solved by consensus; (3) Back translation – two native English speakers not familiar with the original version of the questionnaire translated the T1.2 version independently back into English (BT1 and BT2); (4) Expert Committee - a committee of experts which included T1, BT1, a physical therapist, a dietitian, an expert physician in diabetes, and an associate professor with expertise in methodology consolidated all previous versions of the questionnaire and developed a pre-final version of the questionnaire in Brazilian Portuguese to be used in a pilot test;27 (5) Pilot testing of the pre-final version – 30 patients with diabetes were selected by convenience from a secondary health service in a Brazilian city according to the following eligibility criteria: diagnosis of type 1 (T1D) or type 2 diabetes (T2D), age 18 years or older, and able to physically exercise. All of them were invited to self-complete the pre-final version of the questionnaire.
The understanding of each item was assessed by individual interviews based on the think-aloud method.28 The participants were interviewed to answer what each item meant to them and to explain the significance of words and expressions.29 To accurately identify possible uncertainties, the interviewer recorded the participants' full answers to each item, and the percentage of uncertainties for each item was computed. The clarity of each item was assessed by a Likert Scale ranging from 1 (I do not understand completely) to 5 (I do understand completely).
After completing the fifth step, the last adaptations were made to obtain the version to be psychometrically tested in the next phase of this study.
Second phase: psychometric propertiesTwo-hundred adults with diabetes were enrolled in this phase. The sample size calculation was based on Hair and Anderson’s recommendation of a minimum sample size of 10 individuals per item, and/or at least 100 participants.30 These individuals were recruited from primary and secondary health services in a Brazilian city and invited to the study according to the same eligibility criteria described previously, in addition of not having participated in the pilot test.
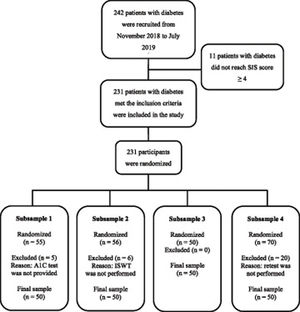
All recruited individuals were asked to answer a six-item test31 to screen for cognitive status, and only potential participants who achieved a score of 4 or greater were invited to sign the consent form and were included in the study. All participants completed a form to report clinical and sociodemographic information. Participants were randomized to one of four subsamples (Fig. 1). The generation of the randomized allocation sequence was performed by the principal investigator (PI) using an online tool, and the allocation sequence was retained in a password-protected file. The randomization information was provided from the PI to the assessor only when the assessments were about to be started to ensure allocation concealment. Participants who did not complete all steps of this study phase, according to the subsample to which they was randomized, were excluded.
The time for completion of the DATE-Q was recorded. Participants completed the questionnaire with neutral monitoring by the assessor.
Subsample 1 — participants randomized to this subsample shared with the research team the glycated hemoglobin (A1C) values from laboratory tests completed as part of their routine clinical care because it is used as a gold standard to estimate the average blood glucose control.6 From the results of previous studies,32,33 it was hypothesized that A1C values and DATE-Q total scores would be negatively associated, and these variables were considered for the assessment of the following psychometric property: construct validity.
Subsample 2 — participants randomized to this subsample performed the incremental shuttle walking test (ISWT) to measure functional capacity.34 From the results of previous studies,35,36 it was hypothesized that ISWT distance in meters and DATE-Q total scores would be positively associated, and these variables were considered for the assessment of the following psychometric property: construct validity.
Subsample 3 — participants randomized to this subsample completed the DKN-A to objectively evaluate disease-related knowledge.25 The DKN-A is a 15-item self-administered multiple-choice questionnaire validated in Brazil, and its total score was considered for the assessment of the following psychometric property: construct validity.
Subsample 4 — participants randomized to this subsample completed the instrument twice, with an interval of seven to 21 days between completions. This process was used to assess the following psychometric property: test-retest reliability. Both tests were completed in the same location by the same assessor.
Statistical analysisThe IBM Statistical Package for Social Sciences (SPSS) version 22.0 software was used for storage and data analysis. The normal distribution of data was verified using the Shapiro-Wilk test. Variables with normal distribution were expressed as mean and standard deviation, while those with non-normal distribution were expressed as median and interquartile range. The DATE-Q data were compared as total scores as well as scores in each domain of the original questionnaire (self-management, long-term complications, being active, healthy eating, and psychosocial well-being) according to age, diabetes type, insulin therapy, time elapsed since diagnosis, educational level, and household income using unpaired t-tests. Comparisons for more than two-samples were performed with one-way ANOVA or Kruskal–Wallis test when numerical variables were tested and with Chi-Square Test when categorical variables were tested. The power of the sample was calculated based on the size of Cohen’s effect of these comparisons and values >0.8 were considered significant.37 A significance level of 5% was adopted for all statistical tests. The psychometric properties of the questionnaire were determined based on randomized subsamples.38
The internal consistency was assessed by Cronbach's alpha and considered satisfactory when α > 0.6.37–39 Construct validity was assessed by calculation of Spearman’s correlation coefficient between DATE-Q total scores and A1C values, ISWT distance (meters), and DKN-A total scores. Correlation coefficients <0.19 were considered very weak, from 0.20 to 0.39 weak, from 0.40 to 0.59 moderate, from 0.60 to 0.79 strong, and >0.80 very strong.30,38 Reliability was assessed by intraclass correlation coefficient (ICC), considering the values <0.40 as low reliability, from 0.40 to 0.75 moderate, from 0.75 to 0.90 substantial, and >0.90 excellent.30,37,38 The presence of ceiling and floor effects for the 20-item DATE-Q was determined based on the proportion of occurrence of the highest and lowest scores for the total sample, with values below 15% considered acceptable.38
ResultsTranslation and cross-cultural adaptationAccording to the Expert Committee, there was no divergence between the translated and back-translated versions compared to the original questionnaire. Cross-cultural adaptations were made based on the Expert Committee's recommendations and results from pilot testing, which resulted in 10 items being culturally adapted. All versions of the questionnaire (i.e. from the original to the final version) are available at https://data.mendeley.com/datasets/wfcwb3k79h/1.
Thirty patients with diabetes (median (interquartile range) age of 58 (49–64) years old; 21 females; 87% with T2D; 60% insulin-treated; fasting glucose 115 (92–151) mg/dL; glycated hemoglobin 8.0 (6.5–8.8)%; educational level from illiterate to postgraduate; household income from less than 1 to more than 9–12 times the Brazilian minimum wages received monthly; six-item screening test scores of 5.4 ± 0.8) participated in the pilot test.
The most common value assigned to all DATE-Q items on the Likert scale was 5, and the percentage of uncertainties was lower than 20% in all items, with the exception of items 3 and 6, as many participants did not know what A1C was.
Psychometric propertiesFor the second phase, 242 patients with diabetes were approached, of which 231 (95%) met the inclusion criteria and were enrolled in the study. Of these, 200 (83%) concluded the study (Fig. 1). Sociodemographic and clinical characteristics of these participants and DATE-Q total scores, as well as the comparison between the four subsamples are presented in Table 1. The proportion of participants of subsample 1 on insulin therapy and oral antidiabetics use were significantly higher and lower, respectively, compared to those from other subsamples. Participants’ mean time to complete the DATE-Q was 5 min and 51 s, and the completion rate was 100% for all items.
Participants’ sociodemographic and clinical characteristics and DATE-Q total scores in the second phase of the study.
| Variables | Full sample (n = 200) | Subsample 1 (n = 50) | Subsample 2 (n = 50) | Subsample 3 (n = 50) | Subsample 4 (n = 50) | P | |
|---|---|---|---|---|---|---|---|
| Age (years) | 58 (46–68) | 55 (41–67) | 56 (45–68) | 60 (52–68) | 61 (47–70) | 0.4 | |
| Female (%) | 57 | 49 | 58 | 62 | 60 | 0.5 | |
| Body mass index (kg/m2) | 28.6 (25.8–33.4) | 27.0 (23.7–33.2) | 28.1 (26.1–32.4) | 29.4 (26.3–34.0) | 29.9 (26.7–35.3) | 0.4 | |
| Fasting glucose (mg/dL) | 122 (99.0–161) | 126 (103–163) | 126 (103–169) | 119 (99.3–152) | 113 (92.0–160) | 0.9 | |
| Glycated hemoglobin (%) | 7.3 (6.3–8.4) | 7.7 (6.5–8.4) | 7.3 (6.3–8.1) | 7.4 (6.7–8.5) | 6.9 (6.0–8.9) | 0.7 | |
| Diabetes Type (%) | Type 1 | 19.5 | 30.0 | 20.0 | 14.0 | 14.0 | 0.1 |
| Type 2 | 80.5 | 70.0 | 80.0 | 86.0 | 86.0 | ||
| Oral Antidiabetics (%) | Yes | 80 | 64* | 84 | 86 | 86 | 0.01 |
| Insulin Therapy (%) | Yes | 44 | 62* | 34 | 38 | 40 | 0.02 |
| Regular exercise self-reported (%) | Yes | 51 | 48 | 54 | 46 | 56 | 0.7 |
| Educational Level (%) | Illiterate | 1.5 | 0.0 | 2.0 | 4.0 | 0.0 | |
| Literate non-school | 0.5 | 0.0 | 0.0 | 2.0 | 0.0 | 0.2 | |
| Elementary school not-concluded | 33 | 28 | 28 | 36 | 40 | ||
| Elementary school concluded | 8.0 | 4.0 | 4.0 | 12 | 12 | ||
| High school not-concluded | 4.0 | 2.0 | 6.0 | 6.0 | 2.0 | ||
| High school concluded | 27 | 34 | 38 | 22 | 14 | ||
| Undergraduate not-concluded | 4.5 | 4.0 | 4.0 | 4.0 | 6.0 | ||
| Undergraduate concluded | 12 | 20 | 12 | 6.0 | 10 | ||
| Postgraduate | 9.5 | 8.0 | 6.0 | 8.0 | 16 | ||
| Household incomea | ≤1 | 13 | 10 | 14 | 10 | 18 | >0.9 |
| >1 up to 2 | 23 | 20 | 24 | 22 | 24 | ||
| >2 up to 3 | 25 | 26 | 22 | 26 | 24 | ||
| >3 up to 4 | 13 | 14 | 12 | 14 | 12 | ||
| >4 up to 6 | 14 | 20 | 10 | 12 | 12 | ||
| >6 up to 9 | 4.0 | 4.0 | 6.0 | 6.0 | 0.0 | ||
| >9 up to 12 | 3.0 | 0.0 | 6.0 | 2.0 | 4.0 | ||
| >12 up to 24 | 5.5 | 4.0 | 6.0 | 6.0 | 6.0 | ||
| >24 | 1.0 | 2.0 | 0.0 | 2.0 | 0.0 | ||
| DATE-Q total score | 14 (12–15) | 14 (13–16) | 14 (12–16) | 14 (11–15) | 13 (11–15) | 0.1 | |
P values, comparison between subsamples using a one-way ANOVA (continuous variables) or Chi-Square Test (categorical variables); values are expressed as percentage or median (interquartile range of 25–75%) of the sample and subsample.
The Brazilian Portuguese version of DATE-Q item descriptions and mean score are described in Table 2.
Description of each item of the DATE-Q, answer sheet, and mean score reached by the participants of the second phase of the study in each item (n = 200).
| DATE-Q Items | DATE-Q answer sheet | DATE-Q score per itema |
|---|---|---|
| 1. Quando vivemos com diabetes, é importante controlar a pressão arterial e o colesterol para prevenir complicações. | TRUE | 0.97 ± 0.17 |
| 2. Duas horas depois de comer uma refeição, seu nível de açúcar no sangue deve ser maior do que 160 mg/dL. | FALSE | 0.41 ± 0.49 |
| 3. Os resultados do seu exame de sangue da hemoglobina glicada (HbA1C) mostram seu nível médio de açúcar no sangue no último ano. | FALSE | 0.31 ± 0.46 |
| 4. Treinamento de força (utilizando faixas elásticas ou pesos) pode ajudar a fortalecer seus músculos e diminuir o seu açúcar no sangue. | TRUE | 0.72 ± 0.45 |
| 5. Pular o café da manhã e comer um farto jantar ajuda a prevenir níveis altos e baixos de açúcar no sangue. | FALSE | 0.80 ± 0.40 |
| 6. Manter sua hemoglobina glicada (HbA1C) baixa (menor que 7%) irá ajudar a prevenir complicações do diabetes. | TRUE | 0.70 ± 0.46 |
| 7. Estar consciente dos seus sentimentos e pedir ajuda e apoio pode prevenir que você se torne sobrecarregado por ter diabetes. | TRUE | 0.72 ± 0.45 |
| 8. O exercício é uma boa maneira de ajudar a controlar seu nível de açúcar no sangue. | TRUE | 0.99 ± 0.12 |
| 9. Alimentos industrializados ou processados (como sopa enlatada e comida congelada) são escolhas de alimentos saudáveis para todos os dias. | FALSE | 0.82 ± 0.39 |
| 10. Receber suporte de sua família e amigos é uma boa maneira de te ajudar a lidar com o estresse. | TRUE | 0.95 ± 0.22 |
| 11. Se seu diabetes não for bem controlado, seus vasos sanguíneos e nervos podem ficar danificados. | TRUE | 0.90 ± 0.30 |
| 12. Seu nível de açúcar no sangue pode ser mais alto ou mais baixo que o normal quando você tem um resfriado ou gripe. | TRUE | 0.36 ± 0.48 |
| 13. Você deve verificar seus pés a procura de bolhas, feridas ou úlceras somente antes do exercício. | FALSE | 0.55 ± 0.50 |
| 14. Comer alimentos com fibras (vegetais, cereais integrais, feijão) ajuda a controlar o diabetes porque reduz o nível de açúcar no sangue, o colesterol ruim (LDL) e a pressão arterial. | TRUE | 0.84 ± 0.37 |
| 15. A depressão não afeta o controle do seu diabetes. | FALSE | 0.61 ± 0.49 |
| 16. Se o seu nível de açúcar no sangue está muito baixo, você deve comer chocolate como um carboidrato de ação rápida. | FALSE | 0.20 ± 0.40 |
| 17. Você está se exercitando na intensidade certa quando a sua frequência cardíaca está na faixa desejada e você está com falta de ar. | FALSE | 0.41 ± 0.49 |
| 18. Se você toma insulina ou certas medicações orais para diabetes (comprimidos como por exemplo a glibenclamida), você tem maior chance de baixar o nível de açúcar no sangue. | TRUE | 0.85 ± 0.36 |
| 19. Sono inadequado ou apneia do sono é comum no diabetes tipo 2 e pode piorar sua saúde. | TRUE | 0.49 ± 0.50 |
| 20. Alimentação saudável para o diabetes inclui comer mais alimentos de origem vegetal. Por exemplo: frutas, vegetais, cereais integrais e legumes. | TRUE | 0.99 ± 0.12 |
| Total | 14.0 ± 2.8 | |
Values are expressed as mean ± standard deviation; aDATE-Q scores per item is 1 for a correct answer and 0 for an incorrect answer or if individual answered “I do not know.”
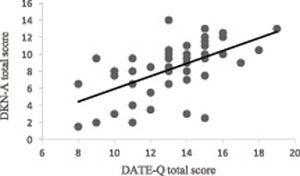
The internal consistency was satisfactory (Cronbach’s alpha = 0.6) and reliability was moderate (ICC = 0.5). The DATE-Q total scores were strongly correlated to DKN-A total scores (ρ = 0.7; P < 0.001; Fig. 2), and weakly correlated to A1C values (ρ = 0.2; P = 0.1) and ISWT distance (ρ = 0.3; P = 0.01). Ceiling and floor effects were not found as only 2 participants scored the maximum total and no participants scored the minimum total.
Table 3 shows the results from the DATE-Q answers compared by age, diabetes type, insulin therapy, time elapsed since diagnosis, educational level, and household income. Participants under 65 years old, who live with T1D, who have a higher educational level or household income, achieved significantly higher DATE-Q “hits” rates and DATE-Q domains’ scores than their counterparts. In addition, insulin-treated participants demonstrated significantly higher DATE-Q “hits” rates and scores in self-management and long-term complications domains than those who were not treated with insulin. Conversely, participants living with diabetes for less than 10 years demonstrated significantly lower scores in self-management and long-term complications domains compared to their counterparts.
Comparison of DATE-Q total scores and scores in each domaina of the original questionnaire for age, diabetes type, time elapsed since diagnosis, educational level, and household income (n = 200).
| Age | < 65 years old (n = 136) | ≥ 65 years old (n = 64) | P | |
|---|---|---|---|---|
| DATE-Q total scores | 13.9 ± 2.7 | 12.4 ± 2.6 | <0.001* | |
| DATE-Q domains’ scores | Self-management | 2.0 ± 1.0 | 1.4 ± 0.8 | 0.5 |
| Long-term complications | 2.9 ± 0.8 | 2.8 ± 0.8 | 0.2 | |
| Being active | 2.7 ± 1.0 | 2.5 ± 0.9 | 0.2 | |
| Healthy eating | 3.5 ± 0.8 | 3.3 ± 0.8 | 0.1 | |
| Psychosocial well-being | 2.9 ± 0.9 | 2.5 ± 1.1 | 0.005* | |
| Diabetes type | Type 1 (n = 39) | Type 2 (n = 161) | ||
| DATE-Q total scores | 15.3 ± 2.8 | 13.0 ± 2.5 | <0.001* | |
| DATE-Q domains’ scores | Self-management | 2.5 ± 0.9 | 1.6 ± 0.9 | <0.001* |
| Long-term complications | 3.4 ± 0.8 | 2.8 ± 0.7 | <0.001* | |
| Being active | 3.1 ± 1.0 | 2.6 ± 0.9 | 0.004* | |
| Healthy eating | 3.6 ± 0.6 | 3.4 ± 0.8 | 0.07 | |
| Psychosocial well-being | 2.9 ± 0.7 | 2.7 ± 1.0 | 0.1 | |
| Insulin therapy | Yes (n = 87) | No (n = 113) | ||
| DATE-Q total scores | 14.2 ± 2.8 | 12.9 ± 2.6 | 0.001* | |
| DATE-Q domains’ scores | Self-management | 2.1 ± 0.9 | 1.6 ± 0.9 | <0.001* |
| Long-term complications | 3.1 ± 0.7 | 2.7 ± 0.8 | <0.001* | |
| Being active | 2.7 ± 1.0 | 2.6 ± 0.9 | 0.5 | |
| Healthy eating | 3.5 ± 0.8 | 3.4 ± 0.8 | 0.8 | |
| Psychosocial well-being | 2.9 ± 0.9 | 2.7 ± 1.0 | 0.2 | |
| Time elapsed since diagnosis | < 10 years (n = 87) | ≥ 10 years (n = 112) | ||
| DATE-Q total scores | 13.1 ± 3.0 | 13.7 ± 2.5 | 0.2 | |
| DATE-Q domains’ scores | Self-management | 1.6 ± 0.9 | 1.9 ± 0.9 | 0.03* |
| Long-term complications | 2.7 ± 0.8 | 3.0 ± 0.7 | 0.01* | |
| Being active | 2.7 ± 1.0 | 2.6 ± 0.9 | 0.6 | |
| Healthy eating | 3.4 ± 0.9 | 3.5 ± 0.7 | 0.5 | |
| Psychosocial well-being | 2.7 ± 0.9 | 2.8 ± 1.0 | 0.7 | |
| Educational level | ≤ High school (n = 94) | > High school (n = 106) | ||
| DATE-Q total scores | 12.3 ± 2.4 | 14.4 ± 2.6 | <0.001* | |
| DATE-Q domains’ scores | Self-management | 1.7 ± 0.9 | 2.2 ± 1.0 | 0.002* |
| Long-term complications | 2.7 ± 0.7 | 3.3 ± 0.8 | <0.001* | |
| Being active | 2.5 ± 1.0 | 3.2 ± 0.7 | <0.001* | |
| Healthy eating | 3.3 ± 0.9 | 3.8 ± 0.5 | <0.001* | |
| Psychosocial well-being | 2.7 ± 1.0 | 2.9 ± 0.9 | 0.3 | |
| Household income | ≤ 3 minimum salaries (n = 120) | > 3 minimum salaries (n = 80) | ||
| DATE-Q total scores | 12.7 ± 2.6 | 14.6 ± 2.6 | <0.001* | |
| DATE-Q domains’ scores | Self-management | 1.7 ± 0.9 | 1.9 ± 1.0 | 0.2 |
| Long-term complications | 2.7 ± 0.8 | 3.0 ± 0.8 | 0.008* | |
| Being active | 2.4 ± 1.0 | 2.8 ± 0.9 | 0.001* | |
| Healthy eating | 3.0 ± 0.9 | 3.7 ± 0.6 | <0.001* | |
| Psychosocial well-being | 2.7 ± 0.9 | 2.8 ± 1.0 | 0.7 | |
P values of the comparison between groups with unpaired t-test. aMaximum score per domain = 4; values are expressed as mean ± standard deviation; household income was reported as the number of Brazilian minimum wages received monthly and paid in reais.
This study aimed to translate, cross-culturally adapt, and validate the Brazilian Portuguese version of the DATE-Q to assess disease-related knowledge in individuals with diabetes in Brazil. In the first phase of the study, the pre-final version of DATE-Q showed to be an easily understood self-administered tool, which required few adjustments to 10 of its 20 items, to became more culturally appropriate for Brazilian reality. Although 20 participants did not know what A1C was in items 3 and 6, these items were not revised because this constitutes a knowledge gap reported in the literature40 and not a lack of comprehension skills. In the second phase of the study, the psychometric validation was established, providing preliminary support for its use in Brazil.
Based on data from the Brazilian population census, a majority of the study participants had an education level of uncompleted elementary school and household income between one and three minimum salaries.41,42 The sociodemographic and clinical characteristics of the participants, as well as the finding that DATE-Q takes approximately six minutes to be completed, and the high completion rate of all items, confirm its clinical applicability.
Even though the Cronbach’s alpha was lower than reported in the original questionnaire (α = 0.77)26 and from the other diabetes-knowledge validated questionnaires in Brazil24,25 (i.e. SKILLD, α = 0.75; DKN-A, α = 0.82), the internal consistency of the Brazilian Portuguese version of the DATE-Q was satisfactory. The absence of ceiling or floor effects indicates that the instrument can discriminate different levels of disease-related knowledge in patients with diabetes.43 In addition, similar to the original questionnaire,26 the highest and lowest scoring items were the twentieth and the sixteenth, respectively (Table 2). The result of the analysis of test-retest reliability is predictable, considering that the instrument evaluates knowledge. However, the reliability should be interpreted in context.37
The strong association between the DATE-Q total scores and DKN-A total scores suggest good construct validity. Although DKN-A and DATE-Q contain items related to healthy eating, disease management, and complications, DATE-Q includes additional items related to critical components of diabetes care not included in DKN-A (psychosocial well-being and being active).14,25,26 In addition, DATE-Q has a “don’t know” option to discourage guessing. Despite these differences, the total scores between the 2 questionnaires were strongly correlated. The other diabetes knowledge questionnaire validated for use in Brazil, the SKILLD, is a 10 question tool administered by interview to avoid problems with respect to reading comprehension, which was developed to evaluate diabetes knowledge in elderly patients with low scholastic levels.24 Because of its nature and the non-inclusion of key components of diabetes care such as eating habits, this tool was not used to validate the Brazilian DATE-Q.
Although we hypothesized that individuals with more knowledge of diabetes would have better disease control and functional capacity, the associations found were not significant and weak, respectively. The non-association between A1C and DATE-Q total score was consistent with studies that assessed disease-related knowledge using other tools43,44 as diabetes control is related to disease type and treatment, and psychosocial conditions45 rather than diabetes knowledge. In addition, this result can be related to the date of the exam as 42% of those randomized to the subsample 1 presented blood-test results which had been done more than three months earlier. Despite the recommendation that A1C should be tested twice a year to track disease control,6 this does not meet the reality and capacity of the Brazilian health system. The weakness of the positive association between ISWT distance and knowledge scores can be related to the functional capacity dependence of physical activity level. Both physical activity and diabetes control depend, preferably on active self-care behavior and depend on a positive patient attitude achieved by a supportive social environment than diabetes knowledge.8
Participants under 65 years old showed higher knowledge scores compared to those above this age, this is consistent with studies that identified a negative association between diabetes knowledge and age.14,46 Moreover, knowledge scores were higher in participants with T1D compared to T2D, as has been found in other diabetes knowledge tools validation13,17 and diabetes knowledge assessment studies.42 It is predictable that patients with T1D know more about self-management, long-term complications, and healthy lifestyle than patients with T2D, as the Brazilian healthcare system recommends delivering at least some sort of education to them as part of their primary care45 and T2D usually affects older individuals and is often diagnosed late.1 Likewise, participants treated with insulin had better general, self-management, and long-term complications knowledge than their counterparts, again consistent with the findings from the knowledge of diabetes test validation study.16 This result was expected considering the delivery of specific educational programs about treatment and nutritional management to patients treated with insulin, as recommended by the Brazilian Diabetes Society.6
Furthermore, participants who had been educated beyond high school achieved higher knowledge scores, not only in total scores, but also in all domains of the original questionnaire. These results are comparable to those of other studies.17,45 Those participants with household income higher than average in Brazil42 also had higher knowledge scores compared to participants with household income on the national average or lower. High diabetes knowledge scores were related to higher occupational status in the DKN validation study,14 and it could partially explain the findings of the current study.
We can highlight some points as limitations of the study. First, to enhance the effectiveness of the Brazilian Portuguese version of the DATE-Q in designing potential strategies for educating patients, it would be important to determine if the scale is sensitive to change (i.e., responsiveness). Second, there are other measurement properties of the scale, such as criterion validity, that require assessment. Finally, it is necessary to determine if the DATE-Q is a valuable and valid tool for identifying varying levels of disease related-knowledge in adults with diabetes, and if having received an educational intervention is a better predictor of effective treatment outcomes.
ConclusionThis study suggested that the Brazilian version of the DATE-Q has adequate psychometric properties, providing preliminary evidence for its use to assess the knowledge of adults with diabetes in Brazil.
Conflict of interestsThe authors declare that they have no competing interests.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Finance Code 001.