The Charcot-Marie-Tooth disease Pediatric Scale (CMTPedS) has been used to measure aspects of disability in children with all types of Charcot-Marie-Tooth disease (CMT).
ObjectiveTo translate and cross-culturally adapt the CMTPedS into Brazilian–Portuguese and determine its reliability and validity.
MethodsThe translation and cross-cultural adaptation followed international guidelines recommendations. Twenty individuals with CMT were assessed. Two examiners assessed the participants for inter-rater reliability. Face validity was assessed by eight physical therapists that judged the relevance of each test item. The Bland-Altman analysis (bias) and standard error of measurement (SEM) complemented the analysis. Furthermore, intraclass correlation coefficients (ICC), weighted kappa (k), and internal consistency (Cronbach’s alpha) was determined.
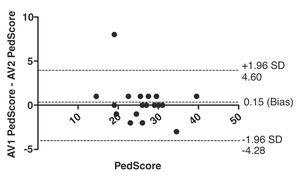
ResultsThe CMTPedS was successfully translated and cross-culturally adapted. Twenty children/youth were enrolled in the study. Of these, the majority (55%) were girls with a mean age of 13.9 (range: from 6 to 18) years. Regarding face validity, the CMTPedS-Br showed relevant items for assessing children and youth with CMT. The ICC for the total score showed excellent reliability (ICC2.1 = 0.93, 95% CI = 0.84, 0.97). The most reliable items were grip, dorsiflexion and plantar flexion strength while the least reliable items were pinprick, vibration, and gait. The internal consistency was excellent (α = 0.96, 95% CI = 0.91, 0.99) and the agreement showed small variability (bias = 0.15, 95% CI= -4.28, 4.60).
ConclusionThe CMTPedS-Br showed adequate reliability and face validity to measure disability in individuals with CMT. This tool will allow Brazil to be part of multicentered studies on such a rare but debilitating condition.
Charcot-Marie-Tooth disease (CMT) is a rare hereditary neuropathy that affects the peripheral nerves. The burden of CMT disease in Brazil is unknown, but the prevalence in the world population is 1/2500.1,2 CMT is divided into two major groups: demyelinating (CMT1) and axonal neuropathy (CMT2), with CMT1 being the most prevalent. The first clinical signs are delayed motor development and toe walking, along with tripping or falling in toddlers.3,4 Older children and youth with CMT show impairments and activity limitations in several areas of functioning such as sensory function and pain (e.g. balance and vibration perception), neuromusculoskeletal and movement-related activities (e.g. foot deformities, muscle weakness, ankle instability, hip luxation, and gait deviation) and mobility (e.g. limited/impaired walking, jumping, etc).1,5–7 These impairments and limitations impact on child/youth’s participation (e.g. sports activities) and quality of life, which requires appropriate assessment to identify specific needs of the population and to determine best management.8
The lack of available instruments for measuring disability among children with neuromuscular disease is a challenge for researchers and rehabilitation professionals.9 The Motor Function Measure (MFM) is the gold standard for assessing gross motor function in children with neuromuscular disease.9 However, the clinical applicability of the MFM for children with CMT is limited as the impairments frequently seen in CMT are different from those seen in children with other neuromuscular conditions. For instance, while the MFM focuses on gross motor abilities, children with CMT typically also present with peripheral symptoms (e.g. sensation and strength deficits)10 which are not assessed by the MFM. This highlights the need of a more specific and relevant instrument for CMT.10
The CMT Neuropathy Score was the first validated standardized instrument for adults with CMT.11,12 From this instrument, consistent with the guidelines of the Inherited Neuropathies Consortium, the Charcot-Marie-Tooth Pediatric Scale (CMTPedS) was developed and validated for assessing children and youth with CMT.2,13 The CMTPedS is an 11-item, norm-referenced tool intended to measure strength, dexterity, sensation, gait, balance, power, and endurance in patients with CMT aged 3–20 years.13 This tool allows specific evaluation of baseline performance and disease severity, as well as assessment of outcomes in longitudinal studies investigating current or novel intervention (e.g. exercise or pharmacological approaches).13
The original English version of the CMTPedS has demonstrated good internal consistency and excellent inter-rater reliability 13 The CMTPedS has been translated into French14 and Italian15 and been tested in 14 and 17 children with CMT, respectively. There is evidence to suggest that assessment with the French and Italian versions was well-tolerated by the children and therefore, the scale may be considered a promising outcome measure for assessing and monitoring children with CMT.14,15 Thus, considering the lack of assessment tool to appropriately assess children and youth with CMT in Brazil, the translation and cross-cultural adaption of the CMTPedS to Brazilian-Portuguese is warranted. Furthermore, the assessment of other measurement properties such as reliability and face-validity will complement the original work on the CMTPedS and help inform the clinical utility of this tool.13 Thus, the objectives of this study were to translate and cross-culturally adapt the CMTPedS for the Brazilian-Portuguese population, and evaluate its measurement properties, including face validity, inter-rater reliability, and internal consistency.
MethodsDesignThis was a cross-sectional observational study approved by the Research Ethics Committee of the Universidade Federal dos Vales do Jequitinhonha e Mucuri with the consent from the Ribeirão Preto Medical School - University of São Paulo (02817418.2.1001.5108). This study followed the Guidelines for reporting reliability and agreement studies (GRRAS)16 and Consensus-based Standards for the selection of health Measurement Instruments (COSMIN).17
CMTPedS descriptionThe CMTPedS is a norm-referenced tool that has been used to measure baseline disability and disease severity in all types of CMT. This tool is administered by the clinicians and can be used in children/youth with CMT aged from 3 to 20 years. CMTPedS can be divided into two parts and can be fully completed in 25 min. The first part, known as the patient profile, includes questions about general symptoms (e.g. foot pain, hand tremor, daily trips and falls, etc) and objective assessment of ankle dorsiflexion range of motion, measured using the Lunge test, and foot posture, measured using the Foot Posture Index-6. The second part consists of 11 items, including hand dexterity (functional dexterity test and nine-hole peg test), strength (hand grip and ankle plantar flexion and dorsiflexion), sensation (pinprick and vibration), balance (Bruininks Oseretsky Test), and motor function (gait, long jump, and six-minute walking test). These 11-items are converted to z-score based on age/sex-matched normative reference values from the 1000 Norms Project.13,18,19 To improve interpretation and generate a total Ped score or total CMTPed score, the raw and the z-score are converted to a linear score of disability ranging from 0 (not affected) to 44 points (severely affected) using the specialized scoring software developed to automate z-score conversion and categorization process.13 This software is freely available on the internet and can be downloaded from the website (http://cmtpeds.org/). Furthermore, CMTPedS equipment need and detailed testing instructions are provided on the website. For more details see information published elsewhere.13
Translation and cross-cultural adaptationThe processes of translation and cross-cultural adaptation followed the recommendations of international guidelines.20,21 Initially, we contacted the author of the original version of the CMTPedS (Dr. Joshua Burns)13 and sought permission to translate and cross-culturally adapt this tool into Brazilian-Portuguese. Then, the first step was to translate the English version of the CMTPedS into Brazilian-Portuguese. Two independent Brazilian bilingual translators (one technical translator and one health care professional) who were not aware of the objectives of the study produced two translated versions. This procedure allowed us to detect errors and divergent interpretations of ambiguous items by each of the translators. The first Brazilian-Portuguese version was independently back-translated to English by two native English speakers who had no knowledge of the original tool and were not aware of the objectives of the study. The English and the Brazilian-Portuguese versions were reviewed by a multidisciplinary committee, consisting of three pediatric physical therapists with clinical and research experience in the field. This procedure was intended to compare the original and the back-translated versions, using structured techniques (e.g. decentring technique) to resolve discrepancies, modify the format, reject inappropriate terms, and verify the equivalence of the original and back-translated versions.20 The decentring technique considers the original version and the final version equally important and also permits changes during the process to maintain content validity.20 Finally, the multidisciplinary committee reviewed all translations to obtain a final Brazilian-Portuguese version (CMTPedS-Br). The CMTPedS-Br was provided to a group of 30 physical therapists for feedback. The therapists were identified through purposive sampling and had experience in managing children with neuromuscular diseases. The therapists completed a questionnaire (sent via e-mail) to determine the level of understanding of the terms used, and to check possible uncertainties regarding the terms used in the evaluation of CMTPedS-Br. The items not understood by at least 20% or more of the sample indicate the need to be reformulated.
Measurement propertiesFace validity is defined as the extent to which an instrument seems to assess what it proposes to measure.22 For evaluation of this measurement property, 10 Brazilian physical therapists using a purposive sampling strategy were invited to participate. These physical therapists had at least five years of experience in assessing children and youth with neuromuscular diseases. After providing consent to participate, the physical therapists received a questionnaire via email that explored the relevance of each test item to measure physical impairments and activity limitations of children and youth with CMT. The questionnaire included videos of the CMTPedS-Br items assessment available on http://cmtpeds.org. Each question was scored using a three-point Likert scale (not relevant, partially relevant, or extremely relevant). Items with 75% or more satisfactory responses (extremely relevant and partially relevant) were found to be useful and appropriate.23 The face validity questionnaire is available in the Supplemental online material.
Reliability is a measurement property that informs about consistency and variation of a test across repeated trials.22 Two raters using a purposive sampling strategy were invited to participate in this phase. Rater 1 was a master student and Rater 2 had a PhD degree, both were physical therapists with experience in managing children with CMT. The raters participated in an 8-hour workshop facilitated by the researcher in charge of the CMTPedS-Br. The facilitator had previously been trained in the administration of the CMTPedS at The Westmead Children’s Hospital in Sydney, Australia. The inter-rater reliability assessments were performed on the same day with a one-hour interval between testing.
Patients with CMT were invited to participate in the reliability testing of CMTPedS-Br. Among the 80 patients with CMT who underwent clinical assessment at the Rehabilitation Center from the Ribeirão Preto Medical School - University of São Paulo, a convenience sample of 37 patients was recruited. The inclusion criteria were as follows: children and youth aged 3–20 years, diagnosed with hereditary neuropathies classified as CMT1, CMT2, or CMT4 by DNA analysis, and all parents signed the informed consent form. Exclusion criteria included: children diagnosed with acquired neuropathy (drug-related neuropathies), diabetic polyneuropathies, chronic inflammatory demyelinating polyneuropathy, hereditary myopathies, and with severe CMT.
Statistical analysisDescriptive data of the participants were reported as mean ± standard deviation, median (interquartile range; 25th–75th), and frequencies (proportion) when appropriate. Inter-rater reliability was analyzed using intraclass correlation coefficient (ICC2.1) using a two-way random model and a single measurement. ICC values less than 0.69 indicate poor reliability; values between 0.70 and 0.79 are considered acceptable; values between 0.80 and 0.89 indicate good reliability, and from 0.90 to 1.0 excellent reliability.24 Bland-Altman analysis was used to estimate the agreement between the measurements, where a bias close to zero and small confidence intervals of the limits of agreement were interpreted as a good indicator of reliability.24 For item analysis, ICC was used for numerical variables and the weighted kappa index (k) for categorical items. For the k index, values below 0.20 represent poor reliability, values between 0.20 and 0.40 suggest reasonable reliability, between 0.41 and 0.60 moderate reliability, values between 0.61 and 0.80 substantial reliability, and above 0.80 almost perfect reliability 25 The 95% confidence interval (CI) followed the indexes.26 To evaluate internal consistency, Cronbach's alpha coefficient (α) was calculated. An alpha value between 0.7 and 0.9 is considered good and higher than 0.90 excellent.27 The standard error of measurement (SEM) was calculated using this equation: (SD × √[1 − ICC]).28,29 All statistical analyses were conducted on SPSS (version 22.0).
Sample sizeConsidering that CMT is a rare disease (i.e. affects between 1 in 2500 of the population)30 the sample size was obtained from probabilistic models based on half width of a 95% one-sided CI to achieve at least substantial reliability (ICC = 0.61–0.8) with 90% of the empirical assurance probability of achieving the desired precision from pre-specified combination of coefficients. The estimated ICC and the minimum sample size required are published elsewhere.31 Thus, the sample size expected for the study was 19 participants. Furthermore, previous studies investigating other neuromuscular disease and measurement properties also have reported similar sample size.32–34
ResultsTranslation and cross-cultural adaptationFollowing the committee's review, the scale was modified to address some grammatical and translation errors. Some words were reformulated, and some terms were replaced by similar ones, such as “pinprick” translated and adapted to “dor” and “long jump” translated and adapted to “salto”. In the original manual of the CMTPedS, the Citec® hand-held dynamometer is recommended to assess plantar flexion, dorsiflexion and hand grip strength. In the CMTPedS-Br version, we have included an asterisk to highlight that if the researcher or clinician used the Citec® equipment, it is important to multiply the value found by two. In the present study we have used a different hand-held dynamometer (Lafayette® - model 01163), similar to a previous study,13 to assess ankle and dorsiflexion strength and the Hydraulic Hand Dynamometer (SH5001) for hand grip strength.
At the end of this process, the second version of the CMTPedS-Br was obtained, which was sent to the expert committee of 30 physical therapists. Following the cross-cultural adaptation process, there was no item on the scale that presented a misunderstanding index of 20% or higher. Therefore, it was not necessary to make changes to the scale, so it was considered the final version of the CMTPedS-Br. The final CMTPedS-Br form is available for free on the website http://cmtpeds.org/ and as a supplemental online material to this article.
Face validityEight Brazilian physical therapists returned the face validity questionnaire. On average, the physical therapists were 40 ± 7.46 years old and had 14 ± 6.50 years of experience in assessing children and youth with neuromuscular diseases, including CMT. While the majority of the physical therapists (n = 7, 90%) worked in clinical and research settings, only one physical therapist worked full time as a clinician. As described in Table 1, all CMTPedS-Br items were considered relevant to the assessment of physical impairments and activity limitations in children and youth with mild to moderate CMT.
Face validity: relevance of the items of the CMTPedS-Br.
| Items | Extremely relevant | Partially relevant | Not relevant |
|---|---|---|---|
| 1-Functional dexterity test (s) | 80% | 20% | – |
| 2-Nine hole peg test (s) | 70% | 20% | 10% |
| 3-Hand grip strength (N) | 90% | 10% | – |
| 4-Foot plantar flexion (N) | 90% | – | 10% |
| 5-Foot dorsiflexion (N) | 90% | – | 10% |
| 6-Pinprick | 80% | 20% | – |
| 7-Vibration | 90% | 10% | – |
| 8-Balance | 100% | – | – |
| 9-Gait test | 100% | – | – |
| 10-Long Jump (cm) | 90% | 10% | |
| 11-Six minute walk test (m) | 100% | – | – |
CMTPedS-Br: Charcot-Marie-Tooth Pediatric Scale Brazilian Portuguese version.
Among the 37 patients invited to participate in the study, 20 were included in the final sample. The mean age was 13.9 years, ranging from 6 to 18 years old. The others were excluded because they showed severe CMT (n = 2), did not cooperate during the assessments (n = 2), or did not attend on the testing day (n = 12). The characteristics of the participants are shown in the Table 2.
Characteristics of the study participants.
| Variable | (n = 20) |
|---|---|
| Age (years) | 13.9 ± 3.4 |
| Weight (kg) | 56.3 ± 21.4 |
| Height (m) | 1.6 ± 0.2 |
| BMI (kg/m2) | 22.1 ± 6.3 |
| Girls | 11 (55) |
| Brazil region | |
| São Paulo | 13 (65) |
| Minas Gerais | 5 (25) |
| Rio de Janeiro | 1 (5) |
| CMT genetic subtypes | |
| CMT1A | 12 (60) |
| CMT2A | 3 (15) |
| CMT | 5 (25) |
Data are reported as mean ± standard deviation and frequency (proportion). BMI = Body Mass Index, CMT = Charcot-Marie-Tooth disease.
The CMTPedS-Br demonstrated excellent inter-rater reliability (ICC2,1 = 0.93; 95% CI = 0.84, 0.97). Table 3 shows the ICC for each numerical item of CMTPedS-Br considering the raw and z-score; and also, the k index for the categorical items. The most reliable items were grip and ankle dorsiflexion and plantar flexion strength, while the least reliable items were pinprick, vibration, and gait. The limits of agreement between the measurements (−4.28 to 4.60) obtained from the first and the second rater are shown in Fig. 1. A bias close to zero was observed and the agreement variations were between the limits of agreement, except for one patient. The high Cronbach's alpha value of the total CMTPedS-Br score showed good internal consistency (0.96, 95% CI = 0.91, 0.99). The SEM of the inter-rater CMTPedS-Br score was 1.43. To show how comparable our sample is to previous study,13 we have presented in Table 4 the average score from the 2 raters of our data in terms of raw score, z-score, and Ped score (converted from the raw and z-score as described previously).
Inter-rater reliability for each item of the CMTPedS-Br.
| Item | Raw | z-score |
|---|---|---|
| 1-Functional dexterity test (s) | 0.80 (0.56, 0.92) # | 0.82 (0.56, 0.92) # |
| 2-Nine hole peg test (s) | 0.83 (0.58, 0.92) # | 0.83 (0.62, 0.93) # |
| 3-Hand grip strength (N) | 0.97 (0.92, 0.99) # | 0.99 (0.99, 1.00) # |
| 4-Ankle plantar flexion strength (N) | 0.90 (0.78, 0.97) # | 0.99 (0.99, 1.00) # |
| 5-Ankle dorsiflexion strength (N) | 0.92 (0.81, 0.97) # | 1.00 (0.99, 1.00) # |
| 6-Pinprick | 0.54 (0.27, 0.81) * | – |
| 7-Vibration | 0.66 (0.36, 0.92) * | – |
| 8-Balance | 0.84 (0.64, 0.93) # | 0.87 (0.70, 0.95) # |
| 9-Gait | 0.54 (0.15, 0.80) # | – |
| 10-Long Jump (cm) | 0.83 (0.62, 0.93) # | 0.77 (0.51, 0.90) # |
| 11-Six minute walk test (m) | 0.86 (0.68, 0.94) # | 0.91 (0.78, 0.96) # |
Data are # intraclass correlation (ICC) and * kappa index (k) with 95% confidence interval (CI).
CMTPedS-Br: Charcot-Marie-Tooth Pediatric Scale Brazilian Portuguese version.
Mean raw score, median z-score and PedS score obtained from raters 1 and 2.
| Items | Raw-score | Z-score | CMTPedS |
|---|---|---|---|
| 1-Functional dexterity test (s) | 36.84 ± 9.29 | 2.81 [1.57, 5.14] | 2 [1, 4] |
| 2-Nine hole peg test (s) | 27.34 ± 4.91 | 3.17 [1.50, 5.20] | 3 [1, 4] |
| 3-Hand grip strength (N) | 17.58 ± 9.73 | −3.81 [−5.02, −3.06] | 3 [3, 4] |
| 4-Ankle plantar flexion strength (N) | 16.78 ± 6.73 | −4.91 [−5.36, −4.09] | 4 [4, 4] |
| 5-Ankle dorsiflexion strength (N) | 5.17 ± 3.07 | −4.84 [−5.65, −3.59] | 3 [3, 4] |
| 6-Pinprick | 0 [0, 1] | – | 0 [0, 1] |
| 7-Vibration | 3 [0, 3] | – | 3 [0, 3] |
| 8-Balance | 29.07 ± 0.95 | −1.00 [−2.44, 0.71] | 1 [0, 2] |
| 9-Gait test | 6 [5, 7] | – | 2 [2, 3] |
| 10-Long Jump (cm) | 66.67 ± 3 .93 | −3.08 [−3.99, −2.65] | 3 [2, 3] |
| 11-Six minute walk test (m) | 493 ± 12.77 | −2.59 [−3.45, −1.37] | 2 [1, 3] |
| Total CMTPed Score | – | – | 25 [22, 29] |
Data reported are mean ± standard deviation or median [interquartile range; 25th, 75h].
The present study followed the recommendations and methodology of Beaton et al.21 and Guillemin et al.20 and successfully translated the CMTPedS into Brazilian-Portuguese. The CMTPedS-Br tool showed excellent reliability and appropriated face validity to assess the Brazilian pediatric population with CMT.
This research evaluated both equivalence of meaning in both cultures and preserved the meaning of each item in the native language. The small changes that were made, according to the suggestions of the multidisciplinary committee, allowed a better understanding of the CMTPedS-Br. These data are similar to those obtained with the Italian version of the CMTPedS.15
One key change implemented in the CMTPedS-Br was the use of a different dynamometer to assess strength. The original English CMTPedS manual recommends the use of the Citec® hand-held dynamometer.13 However, the literature indicates that other hand-held dynamometers, are also reliable to measure muscle strength.35 Furthermore, results from previous studies of our research group have showed that the Citec®, Nicholas®, and MicroFET2® dynamometers are interchangeable for measuring upper and lower limb muscle strength. In addition, for grip strength, the Citec®, Jamar Plus®, and Baseline Hydraulic dynamometers are interchangeable if the smallest grip setting is selected (data not published). Finally, our own data analysis for strength measurements showed excellent reliability.
With respect to face validity, the Brazilian physical therapists indicated that all items of the CMTPedS-Br are relevant and useful to assess impairments and activity limitation in children and youth with CMT. Items that were considered by some physical therapists as partially relevant (e.g. functional dexterity test) and not relevant (e.g. nine-hole peg test) may be influenced by certain factors. Items such as pinprick and functional dexterity test were also indicated as partially relevant by the physical therapists (20%). Some physical therapists reported on the comments section of the questionnaire that they have more experience with young children or less severe cases, for which the items may be less relevant. The physical therapists also reported the same concern for pain assessment, that this item is very difficult to assess in young children, due to comprehension limitation. Indeed, the k index for the pain item showed moderate reliability. Among the 11-items, three showed as extremely relevant (100%): balance, gait, and six-minute walk test.
The inter-rater reliability for the total score of the CMTPedS-Br was excellent (ICC = 0.93), consistent with the original English and Italian versions of the scale, with inter-rater reliability of ICC = 0.95 (95% CI: 0.84, 0.99)13 and 0.99 (95% CI: 0.96, 0.99),15 respectively. Furthermore, in the Bland-Altman analysis the bias was close to zero and the limits of agreement (−4.28 to 4.60) showed minimal variability considering the total CMTPedS-Br score range (0–44). The ICC for the individual items showed excellent reliability for hand grip strength and ankle plantar flexion and dorsiflexion strength measurements. Four items associated with fine and gross motor skills showed good reliability, with the observation that some patients improved their scores by performing the test quickly or with more accuracy for the second rater, suggesting a learning effect. Finally, items such as gait, pinprick, and vibration showed the lowest reliability. Regarding internal consistency, our study showed good reliability (0.96) which is consistent with the original study of validation and reliability of the CMTPedS (Cronbach’s alpha = 0.82).13
Based on the results of the present study, the CMTPedS-Br showed adequate reliability and face validity among children and youth with CMT. Since its inception, the CMTPedS scale has been used to evaluate children diagnosed with CMT to measure impairments and activity limitations caused by the condition.36–38 CMTPedS is a valid and sensitive instrument for different subtypes of CMT, being able to measure the natural history of the condition.13,37 Furthermore, CMTPedS embraces the International Classification of Functioning, Disability and Health (ICF), including domains of body structure and function (e.g. dexterity, sensation, pain, strength, balance) and activity and participation (e.g. six-minute walk test).12,13,2,39 Therefore, the excellent reliability and face validity may give support to the clinical utility of this tool but other measurement properties such as responsiveness are still needed.
This study has strengths, such as adequate sample size considering a rare health condition, as well as the diversity of measurement properties presented. Nevertheless, weaknesses of the study are: (1) a purposive sampling strategy was used to select the practitioners, considering that CMT is a rare disease and not a lot of practitioners has experience in managing this population; (2) it was not possible to present the intra-rater reliability due to data collection logistics and non-attendance of the participants who many resided in distant locations; (3) data collection was obtained from a single national referral center; and (4) the use of a cross-sectional design. Despite these limitations, it is important to mention that in the original validation study13 the authors also only provided data for inter-rater reliability based on the same difficulties as ours. Moreover, the difficulty in obtaining intra-rater reliability or the small sample size does not negatively impact the value of this study, since the inter-rater reliability was excellent. Further studies including those using longitudinal multicenter designs should increase the sample size and explore other measurement properties, such as test-retest and construct validity. Additionally, it is important to highlight that the Inherited Neuropathies Consortium is running a longitudinal study including about 1000 cases of CMT, which will provide other measurement properties, such as minimal detectable change, minimal clinically important difference, and ceiling and floor effects as well. Finally, our study will permit to join in the Inherited Neuropathies Consortium. This center is an integrated group of academic medical centers dedicated to conduct research and improve the care of individuals with CMT.
ConclusionThe CMTPedS-Br showed adequate reliability and face validity. The Brazilian-Portuguese version of the CMTPedS will allow Brazil to be part of multicentered studies on this rare but debilitating condition.
Conflicts of interestThe authors declare no conflicts of interest.
The following is Supplementary data to this article: