Falls in Parkinson Disease (PD) are a complex health problem, with multidimensional causes and consequences.
ObjectivesTo identify the fall predictors in individuals with PD and compare fallers and non-fallers considering their socio-demographic, anthropometric, clinical and functional status.
MethodsA multicenter cross-sectional design was employed. Variables included: age, sex, body mass index, PD progression, levodopa dosage, activities limitation and motor impairments (UPDRS ADL/Motor), level of physical activity (human activity profile – HAP), fear of falls (Falls Efficacy Scale-International-FES-I), freezing of gait (Freezing of Gait Questionnaire – FOG-Q), gait speed (10 meters walk test – 10-MWT), lower limb functional strength (Five Times Sit-to-Stand Test – FTSST), balance (Mini-BESTest), mobility (Timed “Up & Go” – TUG) and dual-task dynamic (TUG-DT). Seventeen potential predictors were identified. Logistic regression and ROC curve were applied.
ResultsThree-hundred and seventy individuals (44.87% fallers and 55.13% non-fallers) completed the study. Fallers presented worse performance in UPDRS motor/ADL/Total, FES-I, FOG-Q, Mini-BESTest, HAP, TUG and TUG-DT and the majority were inactive. The Mini-BESTest Total was the main independent predictor of falls (OR=0.92; p<0.001; 95% CI=0.89, 0.95). For each one-unit increase in the Mini-BESTest, there was an average reduction of 8% in the probability of being a faller. A cut-off point of 21.5/28 (AUC=0.669, sensitivity 70.7% and specificity 55.1%) was determined.
ConclusionBesides characterizing and comparing fallers and non-fallers, this study showed that the Mini-BESTest was the strongest individual predictor of falls in individuals with PD, highlighting the importance of evaluating dynamic balance ability during fall risk assessment.
Falls in Parkinson disease (PD) are a complex health problem, with multidimensional causes and consequences and rates ranging from 35 to 90% for the occurrence of at least one fall,1 and from 18 to 65% for the occurrence of two or more falls.2 The direct consequences of falls such as fractures, head trauma, bruises and other injuries, increase the chances of hospitalization and institutionalization for patients with PD.3 The indirect consequences include limitations in activities of daily living, fear of further falls, adoption of a sedentary lifestyle, and poor quality of life.4
Many studies1,5–10 have already identified a large number of factors associated with increased risk of falls in PD that are specific to the disease or factors that overlap with those already defined for older adults. These factors include disease progression,5 severity of symptoms,6,7 freezing,11 levodopa dosage,9 severe motor impairment,7 motor fluctuations,9,11 loss of oscillation of upper limbs,1 and dyskinesia.6 The non-specific factors included history of falls,1,10 fear of falling,10 cognitive deficit,6 balance deficit,7 reduced mobility,6 lower limb muscle weakness,1 urinary incontinence5 and advanced age.6
In Brazil, the estimated prevalence of PD is 3300 per 100000 individuals over 64 years of age, similar to those observed in American (US), European, and some Eastern community-based surveys.12 In addition, an exponential global increase in PD cases is expected in the coming decades.13 Therefore, the incidence of PD-related falls can be expected to have even greater impact on health systems around the world.
Considering the high incidence of falls in a population that tends to live longer,14 it is important to determine the factors that are associated or could predict falls, especially when prevention may be possible, avoiding morbidity and mortality. Therefore, the objectives of the present study were: (a) to identify the main predictors of falls in individuals with PD; (b) to characterize and compare PD fallers and non-fallers according to their sociodemographic, anthropometric, clinical and functional variables, thus providing a better understanding of the population characteristics. It was hypothesized that variables related to mobility and postural control that incorporates dynamic tasks would demonstrate greater predictive ability.
MethodsStudy design and participantsThe data used in this study were part of the Rede Parkinson Brasil (REPARK-BR)15 which was a cross-sectional multicenter study composed of a network of researchers dedicated to identify and describe the physical and functional profile of Brazilian individuals with PD. This study was approved by the Ethics and Research Committee of the institutions involved: Universidade Federal de Minas Gerais (CAAE: 15050713.6.1001.5149); Universidade de São Paulo (CAAE: 15050713.6.2004.0065); Universidade Federal do Rio de Janeiro (CAAE: 15050713.6.2001.5257); Universidade de Brasília (CAAE: 15050713.6.2005.0030); Universidade Federal do Rio Grande do Norte (CAAE: 15050713.6.2003.5537); Universidade Federal do Ceará (CAAE: 32195014.5.0000.5054); Universidade Federal do Paraná (CAAE: 15050713.6.2002.0102). Participants were recruited through convenience in public and private outpatient clinics, community and PD support associations between 2014 and 2016.15 Individuals (1) with idiopathic PD, diagnosed by neurologists specialized in movement disorders, according to UK Brain Bank criteria16; (2) classified on the basis ofthe Hoehn and Yahr (HY) disabilities stages 1–517; (3) using antiparkinsonian medication, and (4) medically stable with no significant changes over the last 3 months were included. Those who had other types of Parkinsonism or other associated neurological diseases were excluded. All participants were informed about the research objectives, signed a free and informed consent form and a code was assigned to each of them. All participants were tested by trained examiners, during the “on” phase, within 2h of the last dose of the antiparkinsonian medication. The examiners were researchers and undergraduate or graduate students of the universities involved in the study. All examiners were trained by the main coordinator for three days, and each of them received a manual with the instructions on how to collect the data.
MeasurementsCharacteristics of the participantsInitially, demographic and clinical data such as age, sex, duration of disease, number of drugs used, levodopa dosage, body mass index (BMI), associated diseases, surgeries related to PD, if currently in physical therapy treatment, use of walking aids and presence of a visual deficit were collected.
Fall surveyHistory of falls was investigated by asking the participant to report the number of falls in the last 12 months. A fall definition was provided: “unexpected, unintentional shifting of position, which causes the individual to remain at a lower level, for example, on the furniture or floor, not resulting from a violent blow, loss of consciousness, epilepsy or sudden onset of paralysis as in a stroke”.18 The participants were divided in two groups, one with individuals who had one or no falls (≤1 fall), and the other with recurrent fallers (≥2 falls). Groups were divided based on evidence that supported similarities among single fallers and non-fallers.19
Clinical measures and questionnairesThe Unified Parkinson Disease Rating Scale (UPDRS) was used to measure activities of daily living activities (ADL, Part II) and motor skills (Part III). The total score (sum of the scores of UPDRS – ADL and Motor) was also used to characterize the sample. The UPDRS20 was used instead of the Movement Disorder Society – UPDRS (MDS-UPDRS) with the objective to standardize data collection in all centers, since this version was the one most used by the professionals involved in the current study. The use of the MDS-UPDRS required specific training that would be impracticable for the execution of the study.
The general level of physical activity was measured using the Human Activity Profile (HAP). Two scores were calculated: the maximum activity score (MAS) and the adjusted activity score (AAS). The MAS was the highest item number that the individual was still doing. The AAS was calculated by counting how many activities with lower values than the MAS the individual had stopped doing and subtracting the AAS score from the MAS score. Individuals were classified as inactive (<53), moderately active (53–74) or active (>74).21
Fear of falls was measured using the Falls Efficacy Scale-International (FES-I)22; the subjective perception of the severity and impact of freezing on gait performance with the Freezing of Gait Questionnaire (FOG-Q)23; and the usual gait speed with the 10 meters walk test (10-MWT)24. In addition, the Five Times Sit-to-Stand Test (FTSST)25; the time spent on the Timed “Up & Go” Test (TUG)26 and the Timed “Up & Go” Dual Task (TUG-DT) were also implemented. In the TUG-DT, the participants were asked to complete the motor task while counting backward (continuously subtracting 3) from 100 to 90.
The Mini-BESTest27 was used to measure balance performance. This test has 14 items divided into four domains: (i) anticipatory postural adjustments, (ii) reactive postural control, (iii) sensory orientation, and (iv) dynamic gait. The Mini-BESTest examined performance tasks related to dynamic balance and demonstrated good reliability, construct validity, stability of responses and discrimination capacity between different levels of balance ability in individuals with PD.28 The total score and the score on each domain were analyzed separately.
Statistical analysisDescriptive statistics, tests for normality (Shapiro–Wilk), and equality of variances (Levene) were calculated for all outcomes. Clinical and demographic characteristics of the participants were described using measures of central tendency and dispersion. For comparisons between groups, the Mann–Whitney U test for independent samples was used. The Pearson chi-square test and proportions tests for the categorical variables were used when appropriate. For the construction of the logistic model, the association of the dependent variable “falls” (0=non-fallers and 1=fallers) with the independent variables: age, sex, BMI, PD progression, levodopa dosage, activities limitation and motor impairments (UPDRS ADL/Motor/Total), level of physical activity (HAP-AAS), fear of falls (FES-I), freezing of gait (FOG-Q), gait speed (10-MWT), lower limb functional strength (FTSST), balance (Mini-BESTest total and the domains), mobility (TUG) and dual-task dynamic (TUG-DT) were analyzed. Correlation analysis was used as the initial step necessary to determine which variables would be included in the regression analysis. Those variables that showed α=10% in the bivariate analysis were included in the model. Multiple logistic regressions based on forward stepwise method (likelihood ratio) were used. After adjustments, the statistically significant variable for predicting falls remained in the model. The fit of the final logistic model was performed using the Hosmer–Lemeshow test and the residual analysis. The strength of association of the independent variable with the dependent variable was expressed in odds ratios (OR) with a confidence interval of 95%. To determine the cut-off point of the Mini-BESTest to differentiate PD “fallers” from “non-fallers”, the ROC curve was applied and the area under the curve (AUC) calculated, as well as the 95% CI. Youden's index was used to establish the best cutoff point, but also to determine the best sensitivity and specificity values. The SPSS 19.0 software was used for data analysis with a significance level α=0.05.
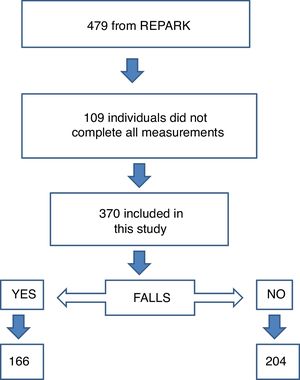
ResultsThree-hundred and seventy individuals from seven Brazilian states completed all measurements, and, therefore, were included in the present analysis. Of these, 166 (44.87%) were classified as fallers and 204 (55.13%) as non-fallers (Fig. 1). The characteristics of the participants are summarized in Table 1.
Demographic and clinical characteristics of the PD fallers and non-fallers.
| Characteristics | All patients (n=370) | Non-fallers (n=204) | Fallers (n=166) | p |
|---|---|---|---|---|
| Age (yr), mean (SD) | 65.8 ± 11 | 65.2 ± 11 | 66.5 ± 11.1 | 0.179 |
| Sex, number of males (%) | 232 (63) | 137 (67) | 95 (57) | 0.050 |
| BMI (kg/m2) | 25.9±3.9 | 26±3.8 | 25.9±4.1 | 0.131 |
| HY=I, n (%) | 45 (12.2) | 29a (14.3) | 16a (9.6) | |
| HY=II, n (%) | 141 (38.2) | 87a (42.9) | 54a (32.5) | |
| HY=III, n (%) | 136 (36.9) | 73a,b(36.0) | 63a,b(38.0) | |
| HY=IV, n (%) | 41 (11.1) | 13c (6.4) | 28c (16.9) | |
| HY=V, n (%) | 6 (1.6) | 1b,c(0.5) | 5b,c (3.0) | |
| UPDRS ADL (0–52) | 15.4 ± 8 | 13.5 ± 6.6 | 17.7 ± 9 | <0.001 |
| UPDRS MOTOR (0–108) | 28.3 ± 16.1 | 24.8 ± 14.7 | 32.5 ± 16.7 | <0.001 |
| UPDRS total | 43.8 ± 22.3 | 38.3 ± 19.3 | 50.6 ± 23.8 | <0.001 |
| LDopa (mg/day) | 568.8 ± 372.9 | 523.9 ± 307.7 | 624.1 ± 434.7 | 0.112 |
| Assistive devices, number (%) | 43 (11.6) | 14 (6.9) | 29 (17.5) | 0.002 |
| Visual impairment, number (%) | 275 (74.3) | 152 (74.5) | 123 (74.1) | 0.928 |
| HAP (AAS) | 57.7 ± 20.2 | 61.7 ± 18.6 | 52.8 ± 21.0 | <0.001 |
| FES-I score (16–64) | 29.7 ± 10.1 | 27.6 ± 9.0 | 32.2 ± 10.8 | <0.001 |
| FOG-Q score (0–24) | 8.1 ± 6.1 | 6.9 ± 5.5 | 9.6 ± 6.5 | <0.001 |
| TUG time, seconds | 13.6 ± 9.2 | 12.3 ± 5.3 | 15.2 ± 12.4 | 0.005 |
| TUG DT time, s | 18.7 ± 14.3 | 16.8 ± 9.1 | 21.3 ± 18.9 | 0.003 |
| FTSST, s | 17.7 ± 8.6 | 16.9 ± 7.4 | 18.8 ± 10.0 | 0.062 |
| 10-MWT time, m/s | 1.10 ± 0.3 | 1.12 ± 0.3 | 1.08 ± 0.3 | 0.607 |
| Mini-BESTest total score (0–28) | 18.8 ± 6.7 | 20.4 ± 6.0 | 16.8 ± 7.1 | <0.001 |
| Anticipatory postural adjustments (0–6) | 4.1 ± 1.6 | 4.5 ± 1.4 | 3.6 ± 1.8 | <0.001 |
| Reactive postural control (0–6) | 3.3 ± 2.1 | 3.6 ± 2.1 | 2.8 ± 2.0 | <0.001 |
| Sensory orientation (0–6) | 4.6 ± 1.6 | 4.9 ± 1.3 | 4.2 ± 1.8 | <0.001 |
| Balance during gait (0–10) | 6.8 ± 2.6 | 7.4 ± 2.2 | 6.1 ± 2.8 | <0.001 |
BMI: Body Mass Index; HY: Hoehn and Yahr disability stages; UPDRS: Unified Parkinson's Disease Rating Scale; ADL: activities of daily living; HAP: Human Activity Profile; AAS: Adjusted Activity Score; FES-I:Falls Efficacy Scale-International; FOGQ: Freezing of Gait Questionnaire; TUG: Timed Up and Go; DT: Dual task; FTSST: Five-Time-Sit-To-Stand Test; 10-MWT: 10 Meter Walk Test; Mini-BESTest: Mini-Balance Evaluation System Test; a,b,c Each subscript denotes a subset of HY categories whose column proportions do not differ significantly from each other at the 0.05 level.
There was no difference between groups regarding the proportion of patients in the five stages of the HY (Table 1). Most participants in both groups were in the mild to moderate stages of the disease. Fallers showed lower motor function and ADL scores (UPDRS ADL/Motor/Total), more fear of falling, lower mean HAP-AAS, worse performance in the FOG-Q, Mini-BESTest, TUG and TUG-DT.
Forty-four percent of the fallers were inactive, 41.6% moderately active, and only 14.5% were active. The non-fallers were mostly moderately active (43.8%), 26.6% were active and 29.4% inactive. The majority of the participants (88.4%) walked without walking aids. Among the fallers, 7.8% used a cane, 4.8% wheelchair, 2.4% walkers, 0.6% crutches and 1.8% other devices. Among the non-fallers, 6.4% used a cane and 0.5% wheelchairs.
Fallers used on average 4.4 medications per day and non-fallers, 4.1. Both groups presented moderate polypharmacy. Fallers reported on average 1.3 associated diseases and non-fallers 1.2, and the total sample reported presence of: systemic arterial hypertension (35.4%); diabetes mellitus (15.1%); rheumatic (11.1%), cardiac (9.5%), orthopedic (2.7%) and pulmonary (0.8%) diseases. In addition, 3.0% of the fallers and 3.9% of the non-fallers reported they had been submitted to surgery related to PD (Fig. 2).
The regression analyses included seventeen potential predictors. All variables except levodopa dosage, BMI and the 10-MWT were correlated to falls (Table 2) and were included in the regression model. Among these potential predictors, only Mini-BESTest Total score reached significance (p<0.05) and, consequently was kept in the model (Table 3).
Correlations between the dependent variable “falls” and potential predictors.
| Variables | |
|---|---|
| Age | (rho=0.108, p=0.02)a,b |
| Sex | (χ2=3.86, p=0.05)a,c |
| BMI | (rho=−0.051, p=0.28)b |
| PD progression | (rho=0.20, p<0.001)a,b |
| UPDRS ADL | (rho=0, 240, p<0.001)a,b |
| UPDRS MOTOR | (rho=0, 240, p<0.001)a,b |
| UPDRS TOTAL | (rho=0, 264, p<0.001)a,b |
| LDopa dosage (mg/day) | (rho=0.083, p=0.112)b |
| HAP-AAS | (rho=−0, 221, p<0.001)a,b |
| FES-I | (rho=0, 25, p<0.001)a,b |
| FOG-Q | (rho=0, 225, p<0.001)a,b |
| 10-MWT | (rho=− 0.043, p=0.34)b |
| FTSST | (rho=0.13, p=0.009)a,b |
| Mini-BESTest total score | (rho=−0, 280, p<0.001)a,b |
| Anticipatory postural adjustments | (rho=−0, 273, p<0.001)a,b |
| Reactive postural control | (rho=−0, 193, p<0.001)a,b |
| Sensory orientation | (rho=−0, 201, p<0.001)a,b |
| Balance during gait | (rho=−0, 240, p<0.001)a,b |
| TUG | (rho=0.152, p=0.002)a,b |
| TUG-DT | (rho=0.17, p=0.01)a,b |
BMI, Body Mass Index; HY; UPDRS, Unified Parkinson's Disease Rating Scale; ADL, activities of daily living; HAP, Human Activity Profile; AAS, Adjusted Activity Score; FES-I, Falls Efficacy Scale-International; FOGQ, Freezing of Gait Questionnaire; TUG, Timed Up and Go; DT, Dual task; FTSST, Five-Time-Sit-To-Stand Test; 10-MWT, 10 Meter Walk Test; Mini-BESTest, Mini-Balance Evaluation System Test.
Results of multivariate logistic regression for predicting falls in Parkinson Disease participants.
| Independent variables | β (SE) | Wald | df (gl) | p-value | Odds ratio | 95 C.I for OR | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Model 1 | Mini-BESTest-Total | −0.082(0.02) | 23.40 | 1 | <0.0001 | 0.92 | 0.89 | 0.95 |
β, coefficient of the constant; SE, standard error; Wald, Wald chi-square test.
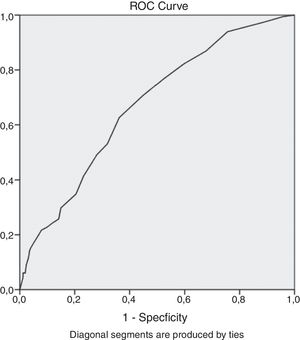
The Mini-BESTest Total score explained alone 59.5% (OR=0.92; p<0.001; 95% CI=0.89, 0.95) of the variance. For each one unit increased in the Mini-BESTest score, there was an average reduction of 8% in the probability of being a faller. Youden's index was used to establish the best cutoff point, but also to determine the best sensitivity and specificity values. The suggested Mini-BESTest cut score was 21.5/28 (AUC=0.669, sensitivity of 70.7% and specificity of 55.1%). Other parameters were calculated for the Mini-BESTest such as positive (57.02%) and negative predictive value (61.03%), likelihood ratio of positive (1.57) and of negative (0.53) test.
DiscussionThis study demonstrated that the Mini-BESTest was the best independent predictor for falls in individuals with PD. The Mini-BESTest is both a clinical tool and a research outcome measure that examines several postural control systems through the performance of dynamic balance tasks.29 Dynamic balance is associated with movement during transfers and gait, as well as external perturbations and cognitive dual-task performance. As balance impairments increase, balance scores decrease, and there is a progressive increase risk of falling. Considering that balance is a complex construct to measure30 and that individuals with PD have multiple systems affected, multi-item assessment tools such as the Mini-BESTest are very relevant.30
Falls etiology in PD is multifactorial,31 resulting in interactions with age-related processes and various disease specific mechanisms.31 The results of the current study indicated that, even in the face of the complexity of falls, we need to target our interventions mainly to dysfunctions in balance, that seem to be the main cause of falls in patients with PD. Postural instability is commonly placed on the second plan which generally focus on gait impairments by both clinicians and their patients with PD. Accurate identification of those patients at high risk of falls would facilitate appropriate and timely intervention, and could lead to improved quality of care and reduced associated hospital costs, due to reduced admissions and reduced severity of falls. Identifying individuals who are at risk of falling and preventing or minimizing falls is a priority in comprehensive PD patient care.
Fallers in this study had significantly more deficits in reactive postural control and anticipatory postural adjustments, which may have contributed to the occurrence of falls. Although fallers showed poor dynamic balance, only 17.5% used a walking aid. This could result in difficulty in the prescription of walking aids by health care providers or limited access to physical therapy services.15
Compared to similar studies,1,2,5,32 this study had the largest sample size of PD participants. It was found that a Mini-BESTest cutoff point of 21.5/28 (AUC=0.669, sensitivity of 0.707 and specificity of 0.55) that could differentiate fallers from non-fallers. This result is similar to cutoff points established in previous studies30,33–37 that reported cutoff points ranging from 16/32 to 19/28. The sensitivity was also within the reports from those studies (0.62–0.88), although their specificity was higher (0.67–0.78) as was the AUC (0.75–0.87).37 This difference might be explained by the fact that in those studies the authors scored both sides of the items “standing one leg” and “correction with compensatory-lateral step”; thus, reporting a maximum total score of 32 points.37 However, the developers of the Mini-BESTest27suggested that the subtotal and the total score should be calculated using only the side with the lowest score, within a maximum score of 28 points. Among these studies, only one36 considered a maximum total score of 28, identifying a cutoff point of 19 (AUC=0.75, sensitivity of 0.79 and specificity 0.67), which was higher compared to our findings. However, their sample size had 110 participants and reports of sample size calculation and power were unavailable. Therefore, the present study was one of the few that followed all of the instructions recommended by the authors of the Mini-BESTest and the one with the largest sample size to date. Our sample calculation was conducted after data collection. As suggested by Pituch,38 a total of 15 individuals must be recruited for each independent variable. Considering the number of variables included in the present analysis, a total sample of 370 individuals was adequate.
Fallers were in the severe stage of the disease as indicated by the higher scores in UPDRS-ADL, Motor and Total. The UPDRS-ADL was composed of a mixture of PD related physical impairments and ADL items reflecting basic function and mobility. Factors that contributed to a higher score in the UPDRS-ADL may also have caused the falls reported by the PD fallers, as well as falls could affect ADL. The UPDRS-Motor encompassed the clinical spectrum of motor symptoms that gradually compromised general mobility and functional activities and may also have contributed to falls.
Levodopa dosage had no correlation with falls. The effects of dopamine replacement therapy on balance were unclear and may change with disease progression and presence of dyskinesia.39 In addition, there was no correlation between falls and BMI, although both groups had a high mean BMI, indicating overweight. The relationship between falls and BMI had not previously been established in PD.
People with PD became less active as the disease advanced and, after experiencing a fall, may have restricted their physical activities to avoid harm.40 A small proportion of fallers (14.5%) and non-fallers (26.6%) were considered active. Goulart et al.41 found that PD participants decreased their level of physical activity more quickly than asymptomatic subjects at the same age, and that low physical fitness was present in the early and moderate phases of the disease as well as in the advanced stage. This might explain the fact that both groups presented sedentary behavior. Individuals with PD with a history of self-reported falls seemed to avoid activities that they presumably considered as being risky for falls.40 This suggested that fall-related activity avoidance needed to be addressed early to prevent sedentary behavior and falls.
Fear of falls was significantly higher among PD fallers. Balance impairment often induces fear of future falling. This behavior may be protective if it interferes only with hazardous activity and increases caution during performance in all other daily living tasks. However, it can be a problem if it compels patients to restrict their mobility, independence and social participation.10 Fallers with PD spent more time performing the TUG test, confirming earlier findings,5 and were significantly more susceptible to gait changes when performing dual tasks. Mobility in daily life frequently required walking while performing simultaneous cognitive or motor tasks and gait impairments were exacerbated under such dual-task conditions, with increased fall risk. Fallers also had higher scores on FOG-Q. Sudden freezing of gait was one of the most important causes of falls in individuals with PD.11
There was no significant difference between fallers and non-fallers in the performance of the FTSST. This is possibly due to the influence of other unmeasured factors. In the elderly, fall-related factors such as postural sway, reaction time, peripheral sensation, vision, anxiety and pain may have a significant impact on the performance of FTSST.42 There was also no difference between groups and no correlation in the mean walking speed measured by the 10 MWT. Age, disease severity, and balance confidence were significantly related to self-selected walking speeds43 and although they correlated with falls, they might not have influenced the performance in the 10-MWT. The influence of other factors, such as dopaminergic treatment, that has been shown to decrease gait variability parameters in PD should also be considered.
Studying predictors of falls in Brazil is relevant because this country has been undergoing a rapid demographic transition that has occurred unequally across the vast territory, with different regions presenting different patterns of socioeconomic development.44 PD patients require several regular medical and health professional visits to follow up the disease's progression, adjusting medication and managing the complications. Willis et al. investigated the utilization of neurology providers in the treatment of patients with PD in the United States.45 They found that neurologist-treated patients were less likely to be placed in a skilled nursing facility, had lower risk of hip fracture and lower adjusted likelihood of death. The demand for care in the Brazilian public healthcare system (SUS – Sistema Único de Saúde) exceeds supply.44 Another aggravating factor is the difficulty in accessing PD medication. The current policies promoting access to medications are not sufficient and there are failures in the process of drug distribution.44 It is known that 40% of all countries and 80% of low-income countries do not have access to PD medication and this includes the levodopa, which can improve quality of life and reduces mortality.13
This study has some limitations. The inclusion of individuals with cognitive impairments and with comorbidities that could affect balance might have led to misleading results. However, by excluding these participants, we would have ended with a small number of participants, not representative of the PD population. Another limitation was the retrospective assessment of falls. The accuracy of dating past falls might have depended on how subjects were interviewed. We managed to date falls with the combined information of patients, relatives, caregivers, and clinical records. Even in a prospective assessment, some falls were still likely to have been missed. The high incidence of falls quoted was likely to have been an underestimate of the number of falls that actually occurred. Perhaps only highly sophisticated long-term mechanical monitoring methods would be able to give the true incidence, but these were not feasible for the present study.
ConclusionThe present study showed that the Mini-BESTest was the strongest individual predictor of falls in individuals with PD, emphasizing the importance of measuring the dynamic balance capacity during the assessment of the risk of falls. In addition, relevant characteristics of this population were provided. This could facilitate adequate and timely intervention and lead to a better quality of care, thus reducing the severity of falls and hospital costs.
AcknowledgementThis research was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-001), Conselho Nacional de pesquisa (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Pró-reitoria de Pesquisa of Universidade Federal de Minas Gerais (UFMG).
Conflicts of interestThe authors declare no conflicts of interest. We certify that no party has a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, if applicable, we certify that all financial and material support for this research (e.g., NIH or NHS grants) and work are clearly identified in the title page of the manuscript.