The number of researchers and clinicians using movement-evoked pain and sensitivity to movement-evoked pain to assess shoulder pain has increased. However, the intrarater test-retest reliability of movement-evoked pain and sensitivity to movement-evoked pain in people with rotator cuff-related shoulder pain (RCRSP) is still unknown.
ObjectiveWe examined the intrarater test-retest reliability of movement-evoked pain and sensitivity to movement-evoked pain in participants with RCRSP.
MethodsSeventy-four participants with RCRSP performed five trials of active shoulder abduction to elicit pain under two experimental conditions: active shoulder abduction to the onset of pain and maximum range of motion (ROM). The primary outcome measures were pain intensity and ROM. Test-retest reliability of movement-evoked pain and sensitivity to movement-evoked pain was examined using intraclass correlation coefficient (ICC3,1) and minimal detectable change (MDC90).
ResultsThe reliability of movement-evoked pain under both experimental conditions was good to excellent (ICC: 0.81 to 0.95), while the reliability of sensitivity to movement-evoked pain was poor in both conditions (ICC≤0.45). The MDC90 for pain intensity was 1.6 and 1.8 during shoulder abduction to the onset of pain and maximum ROM, respectively. The MDC90 for ROM was 17.5° and 11.2° during shoulder abduction to the onset of pain and maximum ROM condition, respectively.
ConclusionThis study confirms movement-evoked pain testing during active shoulder abduction to the onset of pain or maximum ROM condition is reliable to assess pain associated with movement in patients with RCRSP. The minimal detectable change score of movement-evoked pain can guide clinicians and researchers on how to interpret changes in these outcomes.
Clinicians and researchers often measure shoulder pain intensity and range of motion (ROM) when assessing and monitoring the progress of patients with shoulder pain.1,2 A systematic review exploring core outcome measures for shoulder pain trials reported that shoulder pain intensity and ROM were used as outcome measures in 87% and 67% of studies, respectively.1 Pain intensity is commonly assessed at rest or using self-report recall questionnaires, but both methods have limitations. Pain at rest cannot discern pain related to movement, and self-report recall questionnaires cannot differentiate pain experienced before, during, or after movement.3 In addition, the primary driver of shoulder pain is often associated with movement.4,5 Clinicians and researchers need a reliable way of assessing pain associated with movement.
Movement-evoked pain can be defined as pain experienced during movement in a specific context.4,5 Movement-evoked pain is typically more severe than pain at rest in patients with rotator cuff-related shoulder pain (RCRSP),6 which is the most common diagnosis of shoulder pain,7-9 and seems to be mediated by peripheral and central sensitization mechanisms and psychological factors.3,4,10-12 Repetitive movement tasks are recommended for movement-evoked pain assessment.4,13,14 However, pain intensity may be exacerbated by repeated movement of the shoulder.15 Given the association between movement and pain, movement-evoked pain may contribute to fear of shoulder movement in patients with RCRSP, reducing the amount of movement range at the shoulder joint.16,17 Sensitivity to movement-evoked pain is defined as an increase in pain intensity during movement in response to repeated movements, such as repetitive elevation of the arm.13 Evidence shows sensitivity to movement-evoked pain is correlated with central sensitization, poor physical recovery, and chronic symptoms in patients with chronic pain.18-20
There has been growing interest in exploring movement-evoked pain and sensitivity to movement-evoked pain in patients with shoulder pain.20-22 However, the reliability of movement-evoked pain and sensitivity to movement-evoked pain measures in patients with RCRSP is still unknown. In this study, we aimed to assess the test-retest reliability of (1) movement-evoked pain; and (2) sensitivity to movement-evoked pain in patients with RCRSP.
MethodsStudy designThis test-retest reliability study was conducted within a randomized controlled trial which explored the initial effects of mobilization with movement on shoulder ROM and pain in patients with RCRSP (registration number: ACTRN 12621001723875).23 We conducted the test-retest reliability study on the confirmative screening day of participants’ recruitment and before participants received any intervention from the randomized controlled trial. We reported this test-retest reliability study as per the guidelines for reporting reliability and agreement studies (GRRAS).24 The study was approved by the Ethics Committee of University of Otago (ref. H21/117). All participants signed informed consent forms before participating.
Sample sizeThe COnsensus-based Standards for the Selection of health Measurement INstruments (COSMIN) recommends a minimum of 50 participants when conducting a reliability study.25 This study was conducted as part of a randomised controlled trial,23 involving the recruitment of seventy-four participants.
Recruitment and participantsWe recruited participants from the local community through advertisements on social media (i.e., Facebook and Twitter) and through e-mails to the staff and students at the university where this study was conducted. All participants were initially screened by a web-based questionnaire using REDCap software.26 Participants were invited to attend an in-person screening session if they were aged 18–75 years old,27 and reported having shoulder pain. During this initial screening, participants were excluded if they reported a history of shoulder or cervical surgery in the past six months,6,28 corticosteroid injection in the last six weeks,27 history of shoulder subluxation or dislocation, systemic inflammation or disease, neurological disease affecting the shoulder, or tumor.29
All participants who qualified after the web-based screening were invited to attend an in-person confirmative screening. A physical therapist with five years of clinical experience screened participants through physical examination following the British Elbow and Shoulder Society (BESS) guidelines.29 We widened the criteria proposed by the BESS guidelines and added resisted lateral rotation and resisted shoulder abduction tests due to the challenges in diagnosing patients with shoulder pain and the low sensitivity of most clinical tests for the RCRSP.30,31
We included participants who presented with pain during active shoulder abduction, and participants must meet one of the following inclusion criteria: present with painful arc of movement during shoulder abduction, pain on resisted lateral rotation or abduction, or positive Jobe's test.29 We excluded participants who presented with acute rotator cuff tear (history of trauma), massive rotator cuff tears (defined by gross shoulder muscle weakness in the absence of pain),32 other shoulder disorders (i.e., glenohumeral arthritis, acromioclavicular joint pain, adhesive capsulitis), and signs of paresthesia in the upper extremity.29
Experimental procedureDemographic informationWe collected demographic information (sex, age, weight, height, body mass index, ethnicity, education level, employment, dominant hand, painful shoulder, duration of shoulder pain, current medication, or treatment) and number of painful body sites using Michigan Body Map (MBM) from participants. We also collected data on shoulder-related function, shoulder pain and disability, psychological factors, and health-related quality of life using validated patient-reported outcome measures (Table 1). Following the completion of demographic data and patient-reported outcome measures, participants were shown and explained the protocol of testing procedures.
Patient-reported outcome measures.
| Baseline assessment | Questionnaire | Description |
|---|---|---|
| Painful body sites | Michigan Body Map (MBM) | The MBM is a validated graphical tool for patients to report which of 35 specific body sites they have experienced persistent or recurrent pain in for at least three months before enrollment.33 |
| Shoulder-related function | Patient-specific functional scale (PSFS) | The PSFS is a valid and reliable tool for assessing shoulder-related disability, and its minimum clinically important difference (MCID) is 1.3 (small change), 2.3 (medium change), and 2.7 (large change).34 All participants were asked to name up to three important activities that they cannot perform or are having difficulty performing due to shoulder problems. The participant was asked to rate the level of difficulty when performing that activity on an 11-point numeric rating scale (NRS) ranging from 0 (unable to perform the activity) to 10 (able to perform the activity at the same level as before injury or problem). An average PSFS score was calculated by summing the ratings of the nominated activities and dividing by the number of named activities (up to 3). |
| Shoulder pain and disability | Shoulder pain and disability index (SPADI) | The SPADI is a valid and reliable tool for assessing shoulder pain and function, and its minimum clinically important difference is 8 points.35 The SPADI is a patient-reported outcome measure and consists of two subscales: pain intensity and functional disability.36 The pain subscale has five items, and the disability subscale has eight items. Each item ranges from 0 (no pain/no difficulty) to 10 (the worst pain/ so difficult required help). |
| Depression, anxiety and stress | 21-item depression, anxiety, and stress scale (DASS-21) | The DASS-21 is a patient-reported outcome measure and includes three subscales: depression, anxiety, and stress. Each item ranges from 0 to 3, with a total score ranging from 0 to 6. Higher scores indicate higher psychological impairment.37 |
| Dispositional pain catastrophizing | Pain catastrophizing scale (PCS) | The PCS has 13 items, with each item ranging from 0 (not at all) to 4 (all the time). Higher PCS scores indicate higher levels of pain catastrophizing.38 |
| Fear-avoidance beliefs | Fear-avoidance beliefs questionnaire (FABQ) | The shoulder-specific FABQ has two subscales: work and physical activity. The work subscale (FABQ-W) has seven items and the physical activity subscale (FABQ-PA) has four items. Each item ranges from 0 to 6. The total maximum score is 66, and higher scores represent greater levels of fear-avoidance behavior.39 |
| Pain self-efficacy | 2-item short form of pain self-efficacy questionnaire (PSEQ-2) | The items of the PESQ-2 were selected from the original PSEQ version (Items 5 and 9). The maximum PSEQ-2 score is 12 and higher values represent higher confidence levels despite the pain.40 |
| Health-related quality of life | EuroQol five-dimensional Questionnaire (EQ-5D-5 L) | The EQ-5D-5 L can be used to report health-related quality of life in each of the five dimensions and these dimensions can be converted to a health utility score where 1 represents perfect health and 0 indicates health states equal to death.41 The health thermometer visual analogue scale (EQ-VAS) takes values between 0 and 100, where 0 indicates the worst imaginable health and 100 indicates the best imaginable health. |
Given no standardized approach existed for assessing movement-evoked pain in patients with RCRSP, we used active shoulder abduction as the movement to elicit pain during testing. We chose that movement because clinicians commonly use active shoulder abduction when assessing patients with shoulder disorders to identify the presence of painful arc during shoulder abduction in patients with suspected diagnoses of RCRSP.42 We asked participants to actively perform five trials of shoulder abduction in two testing conditions: (1) shoulder abduction to the onset of pain; and (2) shoulder abduction to maximum ROM. A two minutes interval break was implemented between the conditions. During those two testing conditions, pain intensity and ROM were measured. We opted for those two outcome measures due to the complex interaction between pain and ROM.
Participants were assessed twice by the same physical therapist who had pilot testing practice in five participants. The time interval of test-retest measures was set at 10 min.43 The common scenarios in clinical practice and research emphasize the importance of using reliable measurement methods to evaluate the effectiveness of physical therapy interventions immediately after treatment. For example, the interval between the manual therapy is 10 min.44
The order of testing was as follows: (1) shoulder abduction to the onset of pain, (2) shoulder abduction to maximum ROM, (3) pressure pain threshold, and (4) mechanical temporal summation. The description of pressure pain threshold (PPT) and mechanical temporal summation (MTS) testing is provided in the supplementary online material. All participants were seated upright on the hardwood chair with a non-reclining high-back to limit trunk compensation, with their feet remaining flat on the floor.45,46 All participants were instructed to elevate their painful arm with the thumb pointed up toward the ceiling and fingers pointed toward a plastic stadiometer to ensure participants maintained their arms in the frontal plane. If participants presented bilateral shoulder pain, we only assessed the more painful side as reported by the participant.
Shoulder abduction to the onset of painUnder shoulder abduction to the onset of pain, participants were asked to elevate their painful arm until they experienced pain (if participant reported no pain at rest) or until they experienced an increase in their pain (if participant reported pain at rest). Once participants started to experience the onset of pain, they were then asked to maintain that joint position for a few seconds, so that the physical therapist could record their pain intensity and ROM.
Pain intensity was measured with an 11-point NRS ranging from 0 (no pain) to 10 (worst pain imaginable). The NRS is a valid and reliable tool for assessing pain levels.47 Its minimum clinically important difference (MCID) is 1.1 in patients with shoulder pain.48 ROM was measured using a digital inclinometer (Acumar, Model ACU 360, Lafayette Instrument Company). The digital inclinometer can measure a range from 0° to 180° with a precision of 1°. When measuring shoulder ROM, the inclinometer was placed parallel to the humerus, at its distal end, proximal to the elbow, and the measurement was recorded.49 The inclinometer was zeroed using a vertical wall to ensure accurate measurements before performing measurements in each testing condition.
Shoulder abduction to the maximum range of motionUnder shoulder abduction to the maximum ROM, participants were asked to elevate their painful arm as much as possible. Once they reached their maximum ROM during shoulder abduction, they were asked to maintain that joint position for a few seconds, so that the physical therapist measured the ROM. After measuring the ROM of maximum shoulder abduction, all participants were asked to lower their arms to the side of their body. The pain intensity during maximum shoulder abduction was measured. Pain intensity and ROM were measured using NRS and the digital inclinometer as described above.
Outcome measuresWe exposed participants to shoulder abduction to the onset of pain and maximum ROM conditions to assess movement-evoked pain and sensitivity to movement-evoked pain. When assessing movement-evoked pain, we recorded the pain intensity and ROM during those two conditions. When assessing sensitivity to movement-evoked pain, we used pain intensity, as reported by participants, for each condition.
Movement-evoked painRegarding pain intensity for movement-evoked pain, participants provided their pain ratings on the NRS when shoulder abduction to the onset of pain or immediately after shoulder abduction to maximum ROM. In each testing condition, we calculated the mean of five trials of pain intensity for movement-evoked pain. Higher values reflect greater average pain ratings. Regarding ROM for movement-evoked pain, we measured the ROM in two conditions: the ROM of shoulder abduction for when pain increased (i.e., onset of pain) relative to the resting pain and the maximum ROM of shoulder abduction. In each testing condition, we calculated mean of five trials. Higher values reflect greater average shoulder ROM.
Sensitivity to movement-evoked painGiven no guideline for calculating sensitivity to movement-evoked pain, we calculated the sensitivity to movement-evoked pain index by subtracting the mean pain intensity ratings of the first two shoulder elevation from the mean pain intensity ratings of the last two shoulder elevation of the five trials in each condition. Higher values reflect greater increase in pain intensity across repetitive shoulder abduction.
Statistical analysesWe reported the means and standard deviations of the pain intensity, ROM, and sensitivity to movement-evoked pain index at the two experimental conditions. We used intraclass correlation coefficient (ICC3,1), standard error of measurement (SEM), 90% minimal detectable change (MDC90), and Bland-Altman plot to assess the test-retest reliability of the measures. We estimated the ICC with a 95% confidence interval (CI) based on the two-way mixed-effects model, singular measurement, and absolute agreement.50 The ICC was interpreted using the following criteria: poor (ICC<0.5), moderate (0.5≤ICC≤0.75), good (0.75
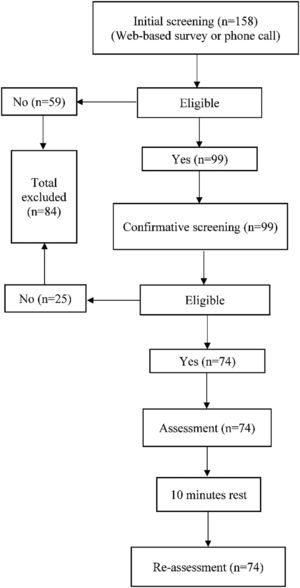
Fig. 1 shows the flow of participants through the study. 158 participants were interested in participating. 59 participants were excluded during the initial screening, and 99 participants were screened in person for confirmative screening. After the confirmative screening, 74 participants met all inclusion criteria and participated in this study. Once included, no participants withdrew from the study. Participants' demographics are presented in Table 2. For descriptive purposes, pressure pain threshold and mechanical temporal summation at the shoulder and leg muscles are also presented in Table 2.
Characteristics of the participants (n = 74). Data are presented as mean (SD) or n (%) unless otherwise indicated.
| Variables | Descriptive statistics |
|---|---|
| Sex, n (%) | |
| Male | 35 (47) |
| Age (years) | 47.4 (16.8) |
| Weight (kg) | 80.5 (17.8) |
| Height (m) | 1.7 (0.1) |
| Body Mass Index (kg/m2) | 27.8 (5.9) |
| Employment, n (%) | |
| Employed full-time | 41 (55.4) |
| Employed part-time | 8 (10.8) |
| Self-employed | 6 (8.1) |
| Unemployed | 1 (1.4) |
| Retired | 8 (10.8) |
| Student | 10 (13.5) |
| Ethnicity*, n (%) | |
| European | 67 (90.5) |
| Māori | 9 (12.2) |
| Pacific | 1 (1.4) |
| Asian | 5 (6.8) |
| Middle Eastern/Latin American/African | 1 (1.4) |
| Other | 1 (1.4) |
| Unknown | 1 (1.4) |
| Education, n (%) | |
| No qualifications | 2 (3) |
| Secondary school | 17 (23) |
| Post-secondary | 23 (31) |
| University degree or above | 32 (43) |
| Dominant hand, n (%) | |
| Right side | 68 (92) |
| Painful shoulder, n (%) | |
| Right | 37 (50) |
| Bilateral symptoms n (%) | 10 (13.5) |
| Duration of shoulder pain, months⁎⁎ | 12 (4 to 42) |
| Current medication/treatment, n (%) | |
| No treatment | 56 (75.7) |
| Physical therapy | 7 (9.5) |
| Analgesics | 5 (6.8) |
| Physical therapy and analgesics | 1 (1.4) |
| Others | 3 (4.1) |
| Analgesics and others | 2 (2.7) |
| Michigan Body Map | 2.7 (1.7) |
| Patient-specific functional scale (PSFS)# | 4.12 (1.85) |
| Shoulder pain and disability index (SPADI)## | |
| Total | 36.2 (19.6) |
| SPADI pain | 47.0 (20.5) |
| SPADI disability | 29.4 (20.7) |
| Depression, anxiety and stress (DASS-21)⁎⁎,## | |
| Overall score (0–63) | 7 (3–13) |
| Depression subscale (0–21) | 1 (0–3) |
| Anxiety subscale (0–21) | 2 (0–3) |
| Stress subscale (0–21) | 3 (2–6) |
| Pain Catastrophizing Scale (PCS)⁎⁎,## | |
| Overall score (0–52) | 8 (3–13) |
| Rumination subscale (0–16) | 3 (0–4) |
| Magnification subscale (0–12) | 2 (0–3) |
| Helplessness subscale (0–24) | 3 (1–6) |
| Fear-Avoidance Beliefs Questionnaire (FABQ)## | |
| Physical activity (0–24) | 14.5 (5.7) |
| Work (0–42) | 9.8 (7.8) |
| Pain self-efficacy (0–12)# | 10.4 (1.9) |
| EQ-5D-5 L (0–1)⁎⁎,# | 0.82 (0.74–0.87) |
| EQ-VAS (0–100)# | 76.15 (14.22) |
| Pressure pain threshold (kPa) | |
| Shoulder | 347.2 (166.5) |
| Leg | 483.7 (177.1) |
| Mechanical temporal summation | |
| Shoulder | 1.9 (1.6) |
| Leg | 1.8 (1.5) |
Self-identified ethnicity categorized according to the Ministry of Health Ethnicity Data Protocols; a participant can be classified as belonging to multiple ethnic groups; therefore, the total percentage does not equate to 100%.
The mean and standard deviation of the test-retest variables and test-retest reliability are presented in Table 3. The test-retest reliability was good for pain intensity (ICC=0.81) and ROM (ICC=0.88) during shoulder abduction to the onset of pain. Similarly, the test-retest reliability of pain intensity (ICC=0.83) and ROM (ICC=0.95) during shoulder abduction to maximum ROM was good and excellent, respectively. In contrast, the reliability of sensitivity to movement-evoked pain was poor during shoulder abduction to the onset of pain (ICC=0.42) and shoulder abduction to maximum ROM (ICC=0.45).
Test-retest reliability of movement-evoked pain and sensitivity to movement-evoked pain.
ROM, range of motion; SD, standard deviation; CI, confidence interval; SMEP, sensitivity to movement-evoked pain; ICC3,1, intraclass correlation coefficients (two-way mixed-effects model, single measurement, absolute agreement); SEM, standard error of measurement; MDC90, minimal detectable change at the 90% confidence interval; LoA, 95% limits of agreement.
The absolute reliability values of the test-retest measures are presented in Table 3. During shoulder abduction to the onset of pain, the SEM for pain intensity was 0.7 and the SEM for ROM was 7.5°. During shoulder abduction to maximum ROM, the SEM for pain intensity was 0.7 and ROM was 4.8°. The SEM of sensitivity to movement-evoked pain was similar during shoulder abduction to the onset of pain (SEM=1.1) and shoulder abduction to maximum ROM (SEM=1.2). During shoulder abduction to the onset of pain, the MDC90 for pain intensity was 1.6 and the MDC90 for ROM was 17.5°. During shoulder abduction to maximum ROM, The MDC90 for pain intensity was 1.6 and MDC90 for ROM was 11.2°. The MDC90 for sensitivity to movement-evoked pain was similar during shoulder abduction to the onset of pain (MDC=2.6) and shoulder abduction to maximum ROM (MDC=2.8).
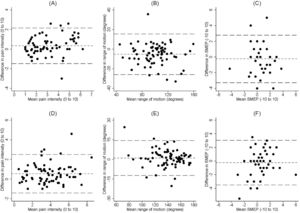
Bland-Altman plotThe mean difference and 95% limits of agreement are presented in Table 3. Little difference were evident in the two repeated measures across all the measurements (Fig. 2), with the most notable difference observed in the range of motion when assessing shoulder adbuction to the onset of pain. The disagreement between the measures increases as the actual values of pain intesity and range of motion increase during the evaluation of shoulder abduction to the onset of pain (Fig. 2A, Fig. 2B). Similarly, an escalated discrepancy has also been observed in pain intensity as the actual pain intensity rise during the assessment of shoulder adbuction to maxium ROM (Fig. 2D).
DiscussionThis study investigated the test-retest reliability of movement-evoked pain and sensitivity to movement-evoked pain in patients with RCRSP. We found that the test-retest reliability of movement-evoked pain ranged from good to excellent when pain was evoked during shoulder abduction to the onset of pain or maximum shoulder abduction. In contrast, we found the reliability of sensitivity to movement-evoked pain was poor when pain was evoked during shoulder abduction to the onset of pain or maximum shoulder abduction. We also determined the MDC90 for movement-evoked pain and sensitivity to movement-evoked pain in patients with RCRSP.
Movement-evoked pain during shoulder abduction to the onset of pain or shoulder maximum abduction was reliable when considering pain intensity and ROM as outcome measures. We did observe a slightly wider CI when analyzing ROM outcomes during shoulder abduction to maximum ROM. Unfortunately, no previous study reported the reliability of pain intensity or ROM during shoulder abduction to the onset of pain for us to compare our results. We found a systematic difference of −5.2° in the ROM during shoulder abduction to the onset of pain between test-retest measurements (i.e., the second measurement was, on average, greater than the first). Given its magnitude, the systematic difference of ROM during shoulder abduction to the onset of pain is likely to be a natural variation between measurements. The reported MDC90 for ROM during shoulder abduction to the onset of pain in our study indicate that a change of ROM during shoulder abduction to the onset of pain greater than 17.5° is the recommended value for clinical interpretation of real change in ROM.
Our findings are similar to previous research in the field,43,56 that reported pain intensity or ROM outcomes were reliable during active shoulder elevation to maximum ROM in patients with shoulder pain. In Riley et al.'s study,56 pain intensity was assessed using active shoulder elevation and their findings suggest pain intensity was reliable (ICC=0.88, 95%CI: 0.77, 0.94). However, it is not clear how participants elevated their arms as they elevated their arm in a self-selected movement pattern and when pain was assessed (i.e., before, during, or after shoulder elevation). In addition, their sample size was small (n=38). Our findings of absolute reliability of maximum ROM are consistent with those reported by Tozzo et al.'s (i.e., SEM=6.6 and MDC90=15.4°) in patients with RCRSP.43 In those studies,43,56 pain intensity or ROM during movement-evoked pain tasks was not assessed in the same participants. The conceptual model of pain evoked by movement highlights that pain and movement can affect each other.57 To address that, our study tested the reliability of movement-evoked pain by measuring pain intensity and ROM in the same group of participants with RCRSP.
Our findings suggest that sensitivity to movement-evoked pain during shoulder abduction to the onset of pain or maximum shoulder abduction was unreliable as assessed using the sensitivity to movement-evoked pain index. The poor reliability of sensitivity to movement-evoked pain may be explained by shoulder abduction during testing performed by participants using different rhythms. Participants in our study performed active shoulder abduction using a self-selected pace rather than controlled pace to allow them to experience the onset of pain. This was aligned with previous studies that self-selected pace of movement was commonly used to test sensitivity to movement-evoked in people with whiplash,13,18,58,59 or people with shoulder pain.20 Unfortunately, those studies did not test the reliability of sensitivity to movement-evoked pain.13,18,20,58,59 The number of repetitions a movement is performed to evoke pain and the weight of the upper limb may also influence sensitivity to movement-evoked pain. Participants in our study only performed five trials of repeated shoulder abduction in each testing condition. A previous study used a canister-lifting task by lifting a series of 18 weighted canisters to measure sensitivity to movement-evoked pain in people with shoulder pain.20 The calculation of sensitivity to movement-evoked pain index may also affect its reliability in patients with RCRSP. Previous studies used different calculations for estimating sensitivity to movement-evoked pain index.20,59 One study recruiting patients with shoulder pain used subtracting mean pain ratings for the first three lifts from the mean pain ratings for the last three lifts of the 18 canisters,20 while another study recruiting patients with knee osteoarthritis used subtracting participants’ first ratings from their peak ratings of knee discomfort during the six-minute walk test.60 In our study, we calculated the sensitivity to movement-evoked pain index by subtracting the mean pain intensity ratings provided for the first two elevations from the mean pain intensity ratings provided for the last two elevations of the five trials in each condition. Our findings indicate the test-retest reliability of sensitivity to movement-evoked pain is poor, suggesting researchers should explore other ways of assessing or calculating sensitivity to movement-evoked pain in this population.
There are some limitations to this study. Firstly, all participants in this study were diagnosed with RCRSP. We cannot generalize our findings to patients with other shoulder disorders, such as frozen shoulder. It should be noted that shoulder pain is multifactorial, and the diagnosis of RCRSP accounts for more than half of shoulder pain in primary care.61 Given these considerations, we provided more information related to participants' clinical characteristics (e.g., PSFS, SPADI, and PCS) in our study. Secondly, the duration of symptoms and bilateral shoulder pain could influence our findings. The median shoulder pain duration of the recruited sample was 12 months (interquartile range: 4 to 42), which suggests most participants had chronic pain. Hence, the reliability of movement-evoked pain may differ in patients with acute RCRSP, pending on the irritability of their condition. Only 10 participants presented with bilateral shoulder pain, and we consider it unlikely this would influence our results. Thirdly, we only used active shoulder elevation in the frontal plane with a self-selected pace to assess movement-evoked pain and sensitivity to movement-evoked pain. We did not use participants' preferred movement patterns or functional tasks with a controlled pace to assess movement-evoked pain and sensitivity to movement-evoked pain, thus influencing the magnitude of observed pain summation responses. Fourthly, we only assessed the intra-rater reliability of movement-evoked pain and sensitivity to movement-evoked pain in patients with RCRSP. To generalize this assessment among clinicians or researchers, an investigation of the interrater reliability of this testing is necessary.
ConclusionsThis study provides evidence that movement-evoked pain testing during active shoulder abduction to the onset of pain or maximum ROM is a reliable way to assess pain associated with movement in patients with RCRSP. The reliability of sensitivity to movement-evoked pain index in patients with RCRSP was found to be poor. The minimal detectable change score can help to interpret changes in movement evoked pain intensity and ROM, thus making informed decisions regarding treatment plans.
We thank the participants for participating in this study. This project was partially supported by the School of Physiotherapy Fund (N/A), the Dunedin School of Medicine Research Student Support Committee of University of Otago (GL.10.NB.M01), and New Zealand Manipulative Physiotherapists Association Educational Trust Fund (N/A). Part of this work was conducted during the Sir Charles Hercus Health Research Fellowship (18/111). SW was supported by the University of Otago Doctoral Scholarship.