Post-stroke Shoulder Pain (PSSP) is a common stroke-related syndrome that prolongs hospitalization and diminishes quality of life. PSSP studies were unsuccessful in clarifying pathophysiological mechanisms. Therefore, cohort's studies with greater variety of the sample and larger follow-up period could provide additional clinical data and may improve medical care.
ObjectiveTo classify people with PSSP and identify intergroup clinical differences, providing additional data useful for therapeutic care planning.
MethodsOne thousand individuals with stroke were selected from all levels of one health Area and followed up during one year. Demographic data, stroke clinical characteristics, stroke-related symptoms and rehabilitation parameters were collected. The shoulder muscle impairment was used to group participants into three clinical profiles: severe muscular impairment, moderate muscular impairment and low muscular impairment groups.
ResultsA total of 119 individuals were diagnosed with PSSP. The suggested classification criteria showed two groups that differed significantly in relation to the onset and duration of PSSP, presence of sensory and speech impairment, and spasticity. The outcomes did not firmly support the existence of a third suggested PSSP subtype.
ConclusionsPSSP may vary in onset, clinical manifestations, severity and syndrome duration. These results highlight the course of different clinical profiles and require multidisciplinary management approaches.
Post-stroke Shoulder Pain (PSSP) is a common and disabling complication of stroke,1,2 which is described as any subjective complaint of pain in the contralesional, or affected, shoulder following stroke.3,4 This syndrome has been associated mainly with reduced upper limb motor function, shoulder subluxation, spasticity or capsular disorders, rotator cuff injuries, thalamic syndrome and shoulder-hand syndrome.5–9 The incidence of PSSP varies between 22% and 60%, depending on the clinical characteristics of the stroke and study design.2,4,6,7,10–12 This syndrome has been established as one of the most important complications of stroke,1 interfering with rehabilitation care and activities of daily living.13,14
Previous studies proposed several models for accurate physical therapeutic care, but none showed suitable results for all sample cohorts regarding arm functional recovery and pain resolution. The occurrence of a wide variety of symptoms and the relative contributions of various factors to the development of PSSP11,12 suggest that this condition may not manifest itself as a unique entity with its own clinical characteristics or vary considerably during the different stages of stroke.
There is still a great need for studies on the possible etiologic disparity of this stroke-related syndrome, particularly on PSSP with well-defined objective classification criteria. An extended follow-up period and a wide variety in the sample cohort may yield additional data, leading to deeper knowledge of the pathophysiology of PSSP.15 Such a study could result in a better classification of these clinical characteristics into different clinical profiles leading to accurate assessment and individual treatment for individuals with PSSP. The aim of this investigation was to classify people with PSSP and identify intergroup clinical differences, providing additional data that might improve the physical therapeutic management.
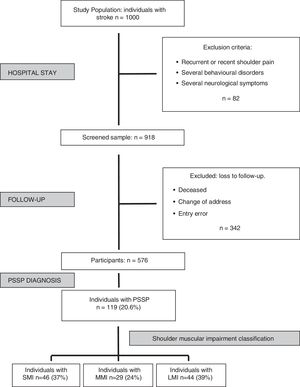
MethodsSubjectsSubjects with stroke admitted to the hospital and attended at any level of our Health Area (e.g., neurology, rehabilitation, internal medicine and primary care departments) were selected. The exclusion criteria was a clinical history of recurrent shoulder pain events (more than one episode over a year), or one referred episode of shoulder pain in the three months prior to stroke. Individuals diagnosed with behavioural disorders or neurological symptoms like anosognosia, hemineglect, Parkinson's disease and Alzheimer's disease, which could complicate evaluation, were also excluded. Participants who did not complete the one-year follow-up period were also excluded.
Study designThis prospective observational study was conducted in a mixed metropolitan and rural region with an estimated population of 500,000 individuals. A total of 1000 subjects diagnosed with stroke and admitted to the hospital in first 24h post-stroke were screened for eligibility by continuous sampling over one year. The appropriate study sample size was calculated based on a maximum loss of screened participants of 49%16 and a minimum incidence of PSSP of 22%7 as reported in previous studies. This means that the minimum sample to be analyzed would be at least 100 cases of PSSP.
The information of interest to the study included clinical data and medical care parameters. All assessments and diagnoses were made by the attending physicians, who recorded the clinical findings in their medical files. The contributing neurologists verified all the documented study-related clinical characteristics and diagnosed the PSSP syndrome during hospital stays or stroke appointments. The follow-up period was established in accordance with previous studies17,18 which considered first year after stroke the PSSP onset period. The participants had three stroke appointments during the study period: at 3, 6 and 12 months. Once patients had been assessed, a trained physical therapist reviewed their medical files to collect the information for the study, entering into a custom-designed database. Clinical data were collected through the IANUS Corporative Information System, which provides information from every healthcare department for each participant.
The Clinical Research Ethics Committee of Galicia (CEIC) approved the free access to medical files (A.R. 2013/440; Santiago de Compostela, Galicia, Spain) according to the principles established in the Declaration of Helsinki and medical research legislation. Patients signed the free informed consent to collect their clinical data necessary for the present study.
ProceduresStroke clinical characteristics (stroke type and classification, affected cerebral hemisphere and contralesional side of the body) and stroke-related symptoms (presence of speech and sensory impairment, impaired shoulder muscle function and involuntary movements) were collected from the initial evaluation of participants’ files. Stroke was classified using the TOAST and Oxfordshire Community Stroke Project – ischaemic stroke – and haemorrhagic stroke classification scale.19–21
Hemineglect was detected using item 11 of National Institute of Health Stroke Scale (NIHSS)22 and guideline drawing, while communication deficits were identified using items 12 and 13 of this scale.22 The sensory exam, including pain, light touch and proprioceptive sensation, was assessed in both arms, testing first on the unaffected side (C5 dermatome). The light touch impairment was determined using a needle and brush over the higher and lower part of the middle deltoid muscle. Proprioception was tested at the thumbs assessing joint position sense.9 All testing was performed 3 times on each side. Participants indicated whether the sensation was the equal, diminished, or non-existent compared to the unaffected side. Based on test values, the sensation deficit was classified as anaesthesia or hypoesthesia.
Upper limb motor function was assessed using Daniels manual muscle testing23–25 (MMT). Shoulder muscle function was assessed on the muscle groups most closely associated with PSSP: the main flexor, external rotator and abductor muscles.26 The final value was obtained by calculating the mean of the MMT scores for the assessed muscle groups.
As the changes in motor control are the most common stroke clinical sign27; muscle function was used as the sort variable to obtain a rating system for PSSP profiles. Indeed, cross-sectional studies showed an association between decreased motor function and PSSP clinical characteristics, suggesting that a new PSSP classification model using upper extremity motor function as the sort variable is possible.28
Rehabilitation parameters collected included proportion of subjects referred, pain response to treatment-recorded using a visual analogue scale (VAS) – and duration of follow-up. The assessments performed during the follow-up period (hospital stay and after discharge) provided information on the presence of stroke symptoms such as spasticity, involuntary movements and neuropathic pain. As several physicians would be responsible for their diagnosis, these assessments could involve evaluation bias. In order to prevent it, spasticity was first assessed by Ashworth scale,24,25,29 and subsequently dichotomized as presence or non-existence of spasticity. Because of the lack of specific diagnostic criteria for neuropathic pain, the diagnosis was based on criteria exposed by a group of experts from the neurologic and pain community30: Neuropathic pain was defined as “pain arising as a direct consequence of a lesion or disease” and its diagnosis required the presence of pain with a distinct plausible distribution, a history suggestive of a relevant lesion, indication of negative or positive sensory signs within the area, and confirmation of the lesion by a diagnostic test. Furthermore, involuntary movement's diagnosis was also based on a previous grading system.31
A consensus assessment was used to diagnose PSSP. Patient – or caregiver – reported the occurrence of pain in the contralesional shoulder and the attending physician confirmed a compatible clinical profile: deep pain on the stroke-affected shoulder that gradually increases and persists at rest, and is aggravated by active or passive movement.32,33 The contributing neurologists verified the diagnosis of PSSP after ruling out other possible causes of pain, such as traumas, central post-stroke pain30 (CPSP) or complex regional pain syndromes.34 The onset of PSSP was recorded as the individual's first report of shoulder pain, even if the PSSP diagnosis was delayed. Data related to PSSP development were also collected: time of onset, detection stage and duration.
Pain-related characteristics (duration of PSSP and pain response to treatment) were assessed according to the individual's ability to communicate (by verbally or physically marking the VAS value).
Statistical analysisData analysis was performed in Statistical Package for Social Science (SPSS) v 20.0 and R software (rcompanion package) v 3.5.1, considering statistically significant the p-values inferior to 0.05.
Stroke clinical characteristics (stroke type, stroke classification, affected cerebral hemisphere and contralesional body side), stroke-related symptoms (presence of speech and sensory impairment, involuntary movements, spasticity, neuropathic pain) and PSSP data (detection stage and response of pain to physical therapy) were codified as qualitative variables and described using frequencies and percentages. Spasticity variable was dichotomized as presence (Ashworth scores>0) or non-existence (Ashworth score=0) of spasticity. PSSP was ranked using muscle function (MMT grades) as a sort variable. The Chi-square test was used to identify association between variables and PSSP groups.
Quantitative variables (PSSP onset and duration, neuropathic pain onset, physical therapy follow-up duration) are described as means and standard deviation (SD), using days as the unit of measurement. One-way ANOVAs were used to compare means among three clinical profiles according to MMT grades after assessing for normal distribution with the Kolmogorov–Smirnov test. The Chi-square pairwise test and Tukey's honestly significant difference (HSD) were used for post hoc analysis of the qualitative and quantitative variables, respectively, when necessary.
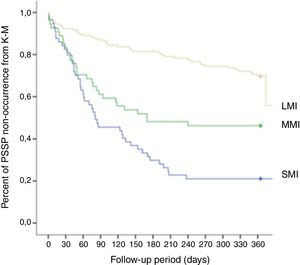
Additionally, a modified Kaplan–Meier function was used to compare the PSSP onset according to the degree of shoulder muscle impairment.
ResultsThe inclusion criteria were met by 576 participants (252 women, 324 men; mean age 70±13 years). The sample includes 434 individuals with ischaemicstroke and 142 with haemorrhagic stroke. A total of 119 participants (21%) were diagnosed with PSSP, which were grouped by the degree of shoulder muscle impairment into three clinical profiles according to MMT grades (Fig. 1):
- •
MMT grade 0–1; severe muscle impairment (SMI): 46 individuals (37%),
- •
MMT grade 2–3; moderate muscle impairment (MMI): 29 individuals (24%) and
- •
MMT grade 4–5; low muscle impairment (LMI): 44 individuals (39%).
Although analyses revealed right cerebral hemisphere and left contralesional body side affected seem to be more frequent in subjects with severe muscle impairment, no clinical characteristics of stroke showed significant differences among the groups of participants with PSSP.
The clinical characteristics of stroke in subjects with PSSP are shown in Table 1.
Clinical characteristics of stroke in participants with PSSP in relation to the degree of muscle impairment.
| SMI Group (n=46) | MMI Group (n=29) | LMI Group (n=44) | p-Value | |
|---|---|---|---|---|
| n (%) [95% CI] | n (%) [95% CI] | n (%) [95% CI] | ||
| Stroke type: ischaemic | 32 (70) [55.18–83.95] | 22 (76) [58.56–93.16] | 37 (84) [72.18–96.03] | 0.182 |
| Affected cerebral hemisphere: right (n=65) | 29 (63) [48.00–78.08] | 16 (55) [35.35–74.99] | 20 (45) [29.61–61.30] | 0.285 |
| Contralesional body side: left (n=56) | 28 (61) [45.68–76.06] | 13 (45) [25.00–64.65] | 15 (34) [18.95–49.23] | 0.489 |
| Individuals with ischaemicstroke (n=91) | ||||
| TOAST scale: | – | |||
| Large-artery atherosclerosis (n=58) | 25 (54) [38.87–69.83] | 14 (48) [28.36–68.19] | 19 (43) [27.41–58.95] | |
| Cardioembolism (n=4) | 1 (2) [0.05–11.53] | 1 (3) [0.09–17.76] | 2 (5) [0.55–15.47] | |
| Small-vessel occlusion (n=29) | 5 (11) [3.62–23.57] | 7 (24) [6.84–41.44] | 17 (39) [23.11–54.16] | |
| Oxfordshire Community Stroke Project scale: | – | |||
| TAC (n=6) | 6 (13) [2.22–23.86] | 0 | 0 | |
| PAC (n=49) | 20 (43) [28.07–58.89] | 14 (48) [28.36–68.19] | 15 (34) [18.95–49.23] | |
| LAC (n=27) | 5 (11) [3.62–23.57] | 6 (21) [7.99–39.72] | 16 (36) [21.01–51.71] | |
| POC (n=9) | 0 | 2 (7) [0.85–22.77] | 7 (16) [3.96–27.85] | |
| Individuals with haemorrhagic stroke (n=32) | ||||
| Lesion area: | ||||
| Deep intracerebral haemorrhage (n=11) | 9 (20) [7.01–32.12] | 2 (7) [0.85–22.77] | 0 | – |
| Lobar intracerebral haemorrhage (n=7) | 4 (9) [2.42–20.79] | 1 (3) [0.09–17.76] | 2 (5) [0.55–15.47] | – |
| Brainstem haemorrhage (n=2) | 0 | 1 (3) [0.09–17.76] | 1 (2) [0.06–12.02] | – |
| Intraventricular haemorrhage (n=2) | 2 (4) [0.53–14.84] | 0 | 0 | – |
| Subarachnoid haemorrhage (n=5) | 1 (2) [0.05–11.53] | 2 (7) [0.85–22.77] | 2 (5) [0.55–15.47] | – |
| Subdural haemorrhage (n=5) | 2 (4) [0.53–14.84] | 1 (3) [0.09–17.76] | 2 (5) [0.55–15.47] | – |
Abbreviations: SMI, severe muscle impairment; MMI, moderate muscle impairment; LMI, low muscle impairment; CI, confidence intervals; TOAST, trial or Org 10172 in acute stroke treatment; TAC, total anterior circulation stroke; PAC, partial anterior circulation stroke; LAC, lacunar stroke; POC, posterior circulation stroke.
The first column of the table shows variables and their total number of cases (n). The values of the stroke characteristics are expressed as frequency (percentage) and CI. Analysis of the stroke classification scales was not possible because the sample size was insufficient in several subcategories for the Chi-square and Fisher exact tests.
Individuals with sensory impairment comprised 59% (n=70). The analysis showed that the proportion of individuals with sensory impairment diminished according with lower impairment of shoulder muscle function, highlighting differences (p<0.001) between SMI (78%) and LMI (36%) groups. Hypoesthesia was the most common sensory impairment among the different groups.
Individuals with speech impairment comprised 56% (n=67) of the total PSSP sample. The post hoc test confirmed significant differences (p=0.003) for incidence of speech impairment between LMI (41%) and SMI (74%) groups.
Analysis showed differences in spasticity between PSSP clinical profiles (p<0.001), and post hoc test specified significant differences of SMI versus LMI (p<0.001) and MMI (p=0.003) groups.
Neuropathic pain was detected in 36% of the participants with PSSP with similar incidences among the groups (28–39%), showing no significant differences between groups (p=0.544). The incidence of involuntary movements showed a trend but not significant association with PSSP groups (p=0.07).
The values for the stroke-related symptoms are summarized in Table 2.
Stroke-related symptoms in participants with PSSP in relation to the degree of muscle impairment.
| SMI group (n=46) | MMI group (n=29) | LMI group (n=44) | p-Value | Intergroup differences p-value | |
|---|---|---|---|---|---|
| n (%) [95% CI] | n (%) [95% CI] | n (%) [95% CI] | |||
| Sensory impairment (n=70) | 36 (78) [65.25–91.27] | 18 (62) [42.68–81.45] | 16 (36) [21.01–51.71] | <0.001 | <0.001a |
| Sensory impairment type: | 0.002 | 0.010a | |||
| Anaesthesia (n=14) | 12 (26) [12.31–39.86] | 2 (7) [0.85–22.77] | 0 | ||
| Hypoesthesia (n=54) | 23 (50) [34.46–65.54] | 16 (55) [35.35–74.99] | 15 (34) [18.95–49.23] | ||
| Speech impairment (n=67) | 34 (74) [60.14–87.69] | 15 (52) [31.81–71.63] | 18 (41) [25.24–56.57] | <0.001 | 0.003a |
| Involuntary mov. (n=20) | 6 (13) [2.22–23.86] | 9 (31) [12.47–49.60] | 5 (11) [3.79–24.56] | 0.070 | |
| Spasticity (n=25) | 21 (46) [30.17–61.13] | 3 (10) [2.19–27.35] | 1 (2) [0.06–12.02] | <0.001 | <0.001a0.003b |
| Neuropathic pain (n=43) | 18 (39) [23.94–54.32] | 8 (28) [9.59–45.57] | 17 (39) [23.11–54.16] | 0.544 | |
| Mean±SD | Mean±SD | Mean±SD | |||
| Onset of neuropathic pain (d) | 73.33±40.82 | 74.44±87.73 | 163.65±184.32 | 0.457 |
Abbreviations: SMI, severe muscle impairment; MMI, moderate muscle impairment; LMI, low muscle impairment; CI, confidence intervals; mov., movements; SD, standard deviation.
The first column of the table shows variables and their total number of cases (n). The values of the stroke-related symptoms are expressed as incidence (percentage) and CI. The time elapsed before the onset of neuropathic pain after stroke is expressed as mean±standard deviation in days (d).
When Chi-square or One way ANOVA analysis showed p<0.05, post hoc tests specified significant differences (right column) between SMI and LMI groups (a) or SMI and MMI groups (b).
Table 3 shows the PSSP characteristics and response to treatment of patients with the syndrome. PSSP onset analysis showed association (p=0.005) with the degree of shoulder muscle impairment (Fig. 2), revealing post hoc test significant differences (p=0.007) between SMI and MMI groups (Table 3). PSSP onset analysis also showed relevant values but inconclusive (p=0.054) between SMI and LMI groups.
PSSP characteristics and response to treatment in relation to the degree of muscle impairment.
| SMI group (n=46) | MMI group (n=29) | LMI group (n=44) | p-Value | Intergroup differences p-value | |
|---|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | |||
| PSSP onset (d) | 90.02±81.25 | 69.00±59.82 | 176.02±150.73 | 0.005 | 0.007b |
| n (%) [95% CI] | n (%) [95% CI] | n (%) [95% CI] | |||
| PSSP stage of detection: | <0.001 | 0.001a0.022c | |||
| Hospital stay (n=53) | 30 (65) [50.37–80.07] | 13 (45) [25.00–64.65] | 10 (23) [9.21–36.25] | ||
| Rehab. follow-up period (n=30) | 13 (28) [14.16–42.36] | 8 (28) [9.59–45.57] | 9 (20) [7.40–33.51] | ||
| Primary care follow-up (n=35) | 3 (7) [1.37–17.90] | 7 (24) [6.84–41.44] | 25 (57) [41.05–72.59] | ||
| Physical therapy (n=69) | 42 (91) [79.21–97.58] | 18 (62) [42.68–81.45] | 9 (20) [7.40–33.51] | <0.001 | 0.001a0.005b<0.001c |
| Response to treatment: | 0.281 | ||||
| Pain resolution (n=35) | 11 (24) [10.50–37.33] | 7 (24) [6.84–41.44] | 17 (39) [23.11–54.16] | ||
| Diminished pain (n=33) | 12 (26) [12.31–39.86] | 9 (31) [12.47–49.60] | 12 (27) [12.98–41.57] | ||
| Persistent pain (n=32) | 17 (37) [21.92–51.99] | 7 (24) [6.84–41.44] | 8 (18) [5.65–30.71] | ||
| PSSP follow-up duration: | 0.074 | ||||
| Less than 3 months (n=35) | 9 (20) [7.01–32.12] | 10 (43) [15.46–53.51] | 16 (47) [21.01–51.71] | ||
| 3–6 months (n=15) | 5 (11) [3.62–23.57] | 4 (17) [3.89–31.66] | 6 (18) [2.36–24.91] | ||
| 6–9 months (n=27) | 11 (24) [10.50–37.33] | 6 (26) [7.99–39.72] | 10 (29) [9.21–36.25] | ||
| More than 9 months (n=18) | 13 (28) [14.16–42.36] | 3 (13) [2.19–27.35] | 2 (6) [0.55–15.47] |
Abbreviations: SMI, severe muscle impairment; MMI, moderate muscle impairment; LMI, low muscle impairment; SD, standard deviation; CI, confidence intervals; PSSP, post-stroke shoulder pain.
The first column of the table shows variables and their total number of cases (n). PSSP onset values are expressed as mean±standard deviation in days (d). The remaining values are expressed as incidence (percentage) and CI.
When Chi-square or One way ANOVA analysis showed p<0.05, post hoc tests (right column) specified significant differences between SMI and LMI groups (a), SMI and MMI groups (b) or MMI and LMI groups (c).
Analysis of the stage of PSSP detection revealed significant differences between groups (p<0.001). Majority (65%) of the SMI group was diagnosed during the hospital stay; whereas the incidence of cases diagnosed during this stage fell to 45% of the MMI group and 23% of the LMI group. This trend reversed with primary care PSSP detection; showing LMI incidences significant higher than SMI (p<0.001) and MMI (p=0.022) groups.
Analysis of subjects with PSSP (n=69, 58%) who received care in the physical therapy department showed significant values (p<0.001), specifying post hoc test significant differences between all PSSP groups (p<0.006). 91% of the SMI group received physical therapy, whereas only 20% of the LMI group received this treatment. The MMI group showed intermediate proportions (62%). Pain resolved during the rehabilitation period in 29% (n=35) of patients with PSSP, showing the highest incidence of pain resolution in LMI group (39%). 37% of the SMI group experienced persistent pain of the same intensity, in contrast with 18% of LMI group. The duration of PSSP required follow-up periods of more than six months in 52% of the individuals with SMI (n=45); 28% of this group was followed up for at least 9 months. MMI and LMI groups ended follow-up period at six months in 60% and 65% of cases respectively. Although the analysis showed no significant values, the follow-up period required tended (p=0.074) to be prolonged in the SMI group, in contrast with the LMI group.
DiscussionThe primary aim of this study was to classify people with PSSP and identify intergroup clinical differences. Thus, this study analyzed the course of the syndrome and evidenced different possible clinical presentations.
PSSP was diagnosed in 119 patients. The suggested method allowed grouping the participants into three different clinical profiles: individuals with severe muscle impairment, moderate muscle impairment and low muscle impairment. Although several models35 can be used to evaluate shoulder muscle impairment, few scales are commonly employed for initial evaluation of stroke patients, mainly highlighting NIHSS and MMT based on Medical Research Council and Daniels scales.24,25 A recent study3 classified the shoulder muscle impairment variable using the item on the NIHSS scale, but it did not include patients with motor function preserved; did not cover all possible muscle function grades and possible PSSP profiles. Thus, we finally decided to use the Daniels scale, which is a simple technique commonly used by physicians and globally tolerated by patients with severe clinical profiles.36 This manual muscle testing version provides reliable results of muscle groups’ assessment of acute stroke patients.24 In addition, this scale may ensure the inclusion of all possible PSSP profiles.
The suggested classification system revealed the existence of two independent PSSP profiles that differed significantly in their clinical characteristics. Although an intermediate profile (MMI) was suggested, our outcomes do not support the existence of a third PSSP subtype; remaining necessary that further studies clarify this point. This PSSP sub-classification may help to explain the conflicting results of previous reports,14,33,37–39 where authors attributed the wide variety of clinical descriptions to sample cohort's differences, health areas included and stroke stage. In this context, we enrolled a mixed group by recruiting subjects followed up at any level of healthcare, extending the data collection period to first post-stroke year.
Regarding stroke main clinical characteristics, our results suggest that stroke type and laterality do not seem to be associated to the development of different PSSP profiles. This information contrasts with findings from previous authors7 who claimed that stroke type might be associated with PSSP. In accordance with some authors,2,9,14,18,26,30,32 we consider that stroke type is not associated with PSSP, but some stroke-related complications might modulate the onset and course of the syndrome. Our results are in line with this assumption since they showed significant differences in stroke-related symptoms between PSSP groups. Thus, the adoption of a management based on the assessment of some stroke symptoms in the early stage of stroke might improve results. Further testing is required to determine if this alternative method can enhance the current approach.
In addition, the high proportion of individuals with right cerebral hemisphere lesion and left contralesional body – significantly more frequent in subjects with hemineglect – suggests a possible association between hemineglect and PSSP.9,37 Hemineglect was considered an exclusion criterion because it could interfere with the evaluation of pain,22 so further studies are needed to complement our data.
Our results revealed participants with severe muscle impairment were diagnosed with PSSP in the first 3 months post-stroke, showing a rapid onset and progression, and developing into a long-term syndrome. This PSSP profile was also associated with high rate of sensory deficit, speech impairment and spasticity. This PSSP group seems to include the population with hemiplegic shoulder pain3,14,15,33 (HSP). Additionally, these clinical characteristics coincide with the results showed by Roosink et al.,40 who provided information about persistent Post-stroke Shoulder Pain (pPSSP). This previous study exposed that pPSSP might be caused by central lesions affecting somatosensory pathways as well as ongoing nocioceptive input from the shoulder, leading finally to central nervous system sensitization. Lin et al.41 also reported central sensitization might be caused by input from hemiparetic pain, but other mechanisms such as brain damage from the stroke itself or an abnormality in muscle balance could also contribute. These arguments support that both spasticity as severe motor impairment and altered kinematic movement caused by sensitive deficit and postural imbalance could lead to central sensitization, explaining the long term PSSP subtype. Nevertheless, we did not perform specific analysis to assess somatosensory sensitization. To provide conclusive results on pathogenesis of this PSSP subtype, further studies on lesion cerebral area and affected pathways should be performed.
The present study also reported patients with low muscle impairment (LMI) who had a delayed onset of PSSP and a short-term syndrome with less than three months. LMI cases had a lower incidence and severity of sensory deficit and speech impairment. Only one case of spasticity was detected in this group.
Analysis revealed that most SMI cases were diagnosed by hospital departments, particularly the rehabilitation department; the majority of them receiving physical therapy. Primary care is mainly where PSSP is detected in patients with LMI, and few patients received physical therapy. However, the SMI group had a high rate of persistent pain in contrast with the LMI group. These percentages reinforce the need for modified physical therapy in patients with severe stroke-related symptoms42; including early and targeted management, and long-term daily care.3,11,15,43,44 Individuals with LMI showed appropriate response to standard treatment, but the proportion of patients who received physical therapy could clearly be improved. Their mild severity of the stroke symptoms and delayed onset of PSSP may explain the low number of referrals to physical therapy. Thus, to involve primary care departments by means of targeted protocols and predictive objective measures may improve PSSP identification and referral to rehabilitation services. They may not need an urgent rehabilitation approach, but a longer follow-up including detailed shoulder kinematic tests45,46 and trunk muscle strength assessment.47 Despite the importance reported by previous studies43,48 on the intensity of the rehabilitation, physical therapy interventions could not be analyzed because we cannot guarantee that all participants with PSSP received the same approach and the data related to the procedures may be incomplete. Additionally, we focused pain assessment on pain related to glenohumeral movement – strongly associated with PSSP diagnosis – and response to treatment. Other pain-related measures (e.g. pressure pain threshold, heat pain threshold, conditioned pain modulation, etc.) were not taken account in the current study, so future studies could analyze this issue.
Regarding neuropathic pain, our analysis showed incidence values higher than in the general population.39 Because the onset of neuropathic pain (three to six months after stroke) coincides with the rehabilitation period, previous studies12,13,49 suggested that neuropathic pain is related with physical therapy approach. Our analysis showed similar proportions of neuropathic pain in participants, regardless of whether they underwent physical therapy. This contrast in outcomes could be due to previous studies usually used inclusion criteria like health areas and hospitalization. Therefore, the development of neuropathic pain was independent of the physical therapy approach received by individuals with PSSP in this study.
Since CPSP diagnosis was an exclusion criterion, we focused on identifying the CPSP clinical picture. According to previous reports,9,30 CPSP diagnosis was based mainly in location of the lesion and sensory findings such as burning, painful cold, electric shocks, aching, pressing, stinging, etc. We cannot guarantee that all participants with CPSP were accurately diagnosed, despite intensive efforts. Although we recognize this bias in the diagnosis of PSSP, the incidence of CPSP in the post-stroke population is about 5–11%,50–52 so this confounding factor would be controlled.
In conclusion, PSSP may not develop as a single entity with a unique clinical profile; instead, it varies depending on the characteristics of the subject and stroke phase. Our clinical findings showed that secondary classification of PSSP according to the degree of the muscle impairment of the affected shoulder is possible. These PSSP subtypes showed statistical differences in presence and type of sensory impairment, speech impairment, spasticity and PSSP onset. Contrary to expectations, stroke type and the affected brain area showed no association with the clinical characteristics of PSSP.
Furthermore, individuals with PSSP present different clinical profiles that may need different therapeutic approaches involving different types of health professionals (mainly neurologists, rehabilitation specialists, physical therapists and general practitioners). Further research is required to clarify a practical range of preventive and therapeutic options for PSSP.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank the CHUAC and all the participating healthcare departments for their assistance.