Executive dysfunction and risk of falling are hallmarks of Parkinson’s disease (PD). However, it is unclear how executive dysfunction predisposes people with PD to falling.
ObjectivesTo: (i) identify sensorimotor, balance, and cardiovascular risk factors for falls that discriminate between those with normal executive function and those with mild and marked executive dysfunction in people with PD and (ii) determine whether mild and marked executive dysfunction are significant risk factors for falls when adjusting for PD duration and severity and freezing of gait (FOG).
MethodsUsing the Frontal Assessment Battery, 243 participants were classified into normal executive function (n = 87), mild executive dysfunction (n = 100), and marked executive dysfunction (n = 56) groups. Participants were asked if they had episodes of FOG in the last month and were assessed with the Movement Disorders Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the Hoehn and Yahr Scale, the physiological profile assessment, and tests of orthostatic hypotension, coordinated stability, and gait and were then followed-up prospectively for falls for 32–52 weeks.
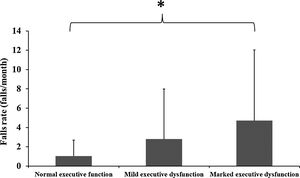
ResultsSeveral PD-specific (elevated Hoehn and Yahr stage, higher MDS-UPDRS scale scores, a history of FOG, Postural Instability and Gait Difficulty subtype, and longer PD duration), sensorimotor (poor vision, knee extension weakness, slow simple reaction time), and balance (greater postural sway and poor controlled leaning balance) factors discriminated among the normal executive function and mild and marked executive dysfunction groups. Fall rates (mean ± SD) differed significantly among the groups (normal executive function: 1.0 ± 1.7; mild executive dysfunction: 2.8 ± 5.2; marked executive dysfunction: 4.7 ± 7.3) with the presence of both mild and marked executive dysfunction identified as significant risk factors for falls when adjusting for three measures of PD severity (Hoehn and Yahr scale scores, disease duration, and FOG).
ConclusionsSeveral PD-specific, sensorimotor, and balance factors differed significantly among the normal, mild, and marked executive dysfunction groups and both mild and marked executive dysfunction were identified as independent risk factors for falls in people with PD.
About two thirds of people with Parkinson’s disease (PD) fall every year,1 with many suffering recurrent falls.1 Falls in people with PD have a high health care cost as many result in serious injuries, including fractures that require hospitalization.2 Many motor and non-motor factors3 have been shown to significantly increase fall risk in prospective studies,4–6 and cognitive deficits such as poor attention and executive function have been increasingly recognised as key fall risk factors in people with PD.4,5,7
People with PD categorised as having cognitive impairment have been found to be at increased fall risk. For example, Wood et al.8 reported that dementia increases fall risk almost seven fold in people with PD, and Camicioli and Majumdar9 found mild cognitive impairment to be the most important clinical risk factor for falls in 52 people with PD. In contrast, Amar et al.10 did not observe significantly increased fall rates in people with PD with either mild or moderate cognitive impairment, however these null findings may have been due to the short (three months) follow-up period. Others have studied gait impairment as a risk factor for progression of cognitive impairment in people with PD, but have not reported on the relationship between impaired gait and cognitive impairment progression in relation to falls.11,12 Only a few studies have contrasted the relative importance of physical and cognitive risk factors for falls in people with PD4,5,13 and these studies have not compared fall rates and fall risk factors between people with PD with normal executive function and those with mild and marked executive dysfunction. Finally, while it is known that cognitive impairments increase in severity with disease progression, few studies have determined whether mild and marked cognitive impairments increase fall risk independently of PD severity.8,9
Accordingly, the aims of this study were to determine: (i) whether mild and marked executive dysfunctions are significant risk factors for falls when adjusting for PD duration, PD severity, and freezing of gait (FOG), and (ii) whether people with PD with mild and marked executive dysfunction have an increased prevalence of sensorimotor, balance, and cardiovascular risk factors for falls.
MethodsParticipantsThis is a secondary analysis of data from two studies: 113 people with PD recruited from a volunteer database, hospital outpatient clinics, PD support groups, and the general community in Sydney, Australia4 (sample 1); and 130 people with PD recruited from community and hospital settings in the UK (primarily South West England) and assessed at North Bristol NHS Trust Hospital, Bristol, UK (sample 2).14
Participants were assessed with the Frontal Assessment Battery (FAB): a validated clinical test for frontal lobe dysfunction and executive dysfunction15 that has been further developed and validated as a screening tool for mild cognitive impairment and dementia in people with PD.16 Using Biundo et al.’s16 classification for normal cognition, mild cognitive impairment, and dementia, participants were classified into three groups: normal executive function (FAB scores >17; n = 87), mild executive dysfunction (FAB scores <16 and ≥13; n = 100), and marked executive dysfunction (FAB scores <12; n = 56). For both studies, individuals were eligible to participate if they had a diagnosis of idiopathic PD17 and could perform a gait assessment unassisted. In both studies, researchers administered the assessments, typically mid-morning, when the participants stated that they felt as comfortable as they usually felt when their antiparkinsonian medications were working (i.e. in their “on” state). All participants provided written informed consent and ethics approval was obtained by the Human Studies Ethics Committee at the University of Sydney, Australia (sample 1) and the South West Research Ethics Committee, UK (sample 2).
PD-related measurementsDuration of disease since date of diagnosis was recorded. Participants in Sample 1 were assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS)18 and participants in Sample 2 were assessed using the Movement Disorder Society - Unified Parkinson’s Disease Rating Scale (MDS-UPDRS).19 Following Goetz et al.20 we transformed UPDRS scores of parts II (activities of daily living score) and III (motor score) into the corresponding scores of the MDS-UPDRS. Motor subtypes for PD were based on clinical features, according to Jankovic et al.21 Using the MDS-UPDRS, if the participants were predominantly affected by Postural Instability and Gait Difficulty (PIGD) they were considered as PIGD subtype, and the participants with tremor dominant or mixed symptoms were allocated into the non-PIGD subtype. More details on how to classify participants into PD subtypes using the MDS-UPDRS are described by Stebbins et al.22 We classified the participants’ stage of the disease using the Hoehn and Yahr scale: extracted from the UPDRS / MDS-UPDRS.23 Daily levodopa intake was dichotomized as ≤750 mg/day or >750 mg/day. Participants were asked whether they experienced freezing of gait (FOG) in the past month. Use of walking aids was also recorded.
Sensorimotor function, balance, and gaitParticipants were assessed using the Physiological Profile Assessment (PPA)24 which comprises tests evaluating key functions of the human balance system: proprioception, visual contrast sensitivity, quadriceps strength, simple reaction time, and postural sway when standing on a compliant surface. Descriptions of the apparatus, procedures, and test-retest reliability for these tests have been previously reported.24,25 The coordinated stability test was used to assess how well participants could adjust their balance in a controlled manner when near their leaning balance range. Higher scores indicate poorer dynamic postural stability.26
Gait analysis was performed using a tri-axial piezo-resistant accelerometer attached to the participant’s pelvis on a belt at the level of the sacrum.4,14 Participants performed one (sample 1) or three (sample 2) walking trials at a self-selected speed along a 20 m-long (sample 1) or a 22 m-long (sample 2) corridor without the use of walking aids. The middle 15 m and 18 m of steady state walking were analysed for participants from sample 1 (n = 113) and sample 2 (n = 130), respectively. For sample 2, the mean of the three trials was computed. The following gait parameters were extracted from the acceleration traces: (i) average step length (walking distance divided by the number of steps with heel strikes defined by vertical acceleration peaks at the pelvis) measured in cm, (ii) cadence (number of steps divided by the duration of the walking trial) measured in steps/minute, and (iii) gait speed (walking distance divided by the total time taken to complete the distance) measured in m/s.
Orthostatic hypotensionOrthostatic hypotension was identified as a fall in systolic blood pressure of 20 mmHg or more and/or in diastolic blood pressure of 10 mmHg or more, recorded with a sphygmomanometer on the arm, during the first 3 min of standing up from sitting.27
FallsFalls were defined as unexpected events which resulted in the participant unintentionally coming to the ground, floor, or other lower level.28 Participants who reported one or more falls in the past 12 months were defined as past fallers. Falls were collected using monthly calendars and telephone calls and participants who reported a fall were subsequently contacted by telephone to verify details. The prospective falls follow-up period was 52 weeks for sample 1 and 32 weeks for sample 2 with fall rates calculated as number of falls/month.
Statistical analysisTo permit parametric analyses of variables with skewed distributions, data with right-skewed distributions were log transformed. Differences in demographic, clinical, and functional characteristics between cognitive status groups were assessed using chi square tests for cross-tabulation tests or one-way analyses of variance with Bonferroni adjusted post-hoc tests to evaluate statistical differences between groups. Incidence rate ratios (IRRs) using negative binomial regression were calculated to assess the association between executive function status and falls incidence. The first model comprised the unadjusted bivariate association (including the groups with mild and moderate executive dysfunction versus normal executive function); the second model was adjusted for PD duration and PD severity (as assessed with the Hoehn and Yahr scale); and the third model was adjusted for FOG in addition to age, PD duration, and PD severity. All models were also adjusted for the cholinesterase inhibitor rivastigmine (as 67 participants from the intervention arm of sample 2 had been prescribed this medication as the treatment therapy in the randomized-controlled trial) and the length of the follow-up for falls. The data were analysed using IBM SPSS v. 24 for Windows (SPSS, Inc., Chicago, IL) and significance levels set at 0.05.
ResultsDifferences between the normal executive function and the mild and marked executive dysfunction groups with respect to demographic, PD-related, cardiovascular, and sensorimotor, balance and gait assessments are displayed in Table 1. Both the mild and marked executive dysfunction groups were significantly older than the normal executive function group and comprised more past fallers, participants with orthostatic hypotension, and more participants who used a walking aid. Both executive dysfunction groups also had greater postural sway (sub-test of PPA), worse controlled leaning balance (higher coordinated stability scores), and greater PD severity/progression (elevated Hoehn and Yahr stages, higher MDS-UPDRS scale scores, FOG, PIGD subtype, and longer PD duration). In addition, compared with participants with normal executive function, more mild executive dysfunction participants were taking high levodopa doses and participants with marked executive dysfunction exhibited worse visual contrast sensitivity, weaker quadriceps strength, and slower simple reaction time.
Parkinson disease (PD)-specific, sensorimotor, balance, and gait measures for all 3 groups. Data are mean ± SD unless otherwise specified.
| Normal executive function (N = 87) | Mild executive dysfunction (N = 100) | Marked executive dysfunction (N = 56) | |
|---|---|---|---|
| Demographics | |||
| Sex, male (%) | 49 (56%) | 63 (63%) | 34 (61%) |
| Age, years | 64.4 ± 9.9a,b | 69.1 ± 7.5 | 72.2 ± 7.8 |
| BMI, kg/cm2 | 25.39 ± 6.18 | 26.45 ± 4.83 | 26.89 ± 4.66 |
| PD-related measurements | |||
| MDS-UPDRS part II, score | 12.9 ± 7.4a,b | 18.2 ± 7.4 | 20.2 ± 7.8 |
| MDS-UPDRS part III, score | 22.0 ± 13.2a,b | 33.7 ± 13.5c | 41.8 ± 15.5 |
| PIGD subtype (%) | 46 (53%)a,b | 76 (76%) | 45 (80%) |
| Hoehn and Yahr, stage | 1.9 ± 0.8a,b | 2.5 ± 0.7 | 2.8 ± 0.6 |
| Levodopa intake (%) | |||
| >750 mg/day | 32 (37%)a | 54 (54%) | 25 (45%) |
| FOG (%) | 30 (35%)a,b | 70 (70%) | 39 (70%) |
| Gait devices use (%) | 18 (21%)a,b | 59 (59%) | 35 (64%) |
| Sensorimotor, balance, and gait | |||
| Visual contrast sensitivity, dB | 19.8 ± 1.9b | 19.8 ± 1.7c | 18.8 ± 3.3 |
| Simple reaction time, ms | 277 ± 66b | 275 ± 71c | 347 ± 143 |
| Quadriceps strength, kg | 24.8 ± 10.5b | 22.0 ± 11.8 | 20.9 ± 13.8 |
| Proprioception, degrees of error | 2.3 ± 1.3 | 2.0 ± 1.4 | 2.4 ± 2.0 |
| Postural sway, mm | 162 ± 143a,b | 283 ± 209c | 370 ± 219 |
| Coordinated stability, score | 14.0 ± 13.7a,b | 21.1 ± 14.0c | 27.6 ± 15.5 |
| Step velocity, m/s | 1.04 ± 0.27 | 1.02 ± 0.31 | 0.95 ± 0.32 |
| Cadence, steps/min | 107.7 ± 12.8 | 109.7 ± 13.7 | 108.0 ± 16.9 |
| Step length, cm | 58.1 ± 14.1 | 56.1 ± 14.2 | 52.6 ± 16.4 |
| Orthostatic hypotension (%) | 14 (16%)a,b | 37 (37%) | 23 (41%) |
| Participants with previous falls (%) | 26 (30%)a,b | 77 (77%) | 40 (71%) |
BMI, body mass index; MDS-UPDRS, Movement Disorders Society-Unified Parkinson’s Disease Rating Scale; PIGD, Postural Instability and Gait Difficulty subtype; FOG, freezing of gait; MDS-UPDRS, Movement Disorders Society-Unified Parkinson’s Disease Rating Scale; PIGD, Postural Instability and Gait Difficulty subtype. High scores in simple reaction time, proprioception, postural sway, coordinated stability, MDS-UPDRS parts II and III, and Hoehn and Yahr tests and low scores in the visual contrast sensitivity and quadriceps strength tests indicated impaired performance.
Post-hoc p values were Bonferroni - corrected.
The mild and marked executive dysfunction groups had similar disease duration and comprised similar proportions of past fallers. However, the marked executive dysfunction group exhibited poorer visual contrast sensitivity, slower simple reaction time, and poorer overall balance (increased postural sway and coordinated stability scores). There were no differences in PD-specific parameters between the two executive dysfunction groups, with one exception: the marked executive dysfunction group had significantly higher MDS-UPDRS part III scores (worse motor function).
Prospective fallsParticipants reported a total of 5156 falls with 170 participants (70%) reporting at least one fall during the follow-up. The mean (standard deviation) fall rates for the groups were: normal executive function: 1.0 ± 1.7, mild executive dysfunction: 2.8 ± 5.2, and marked executive dysfunction: 4.7 ± 7.3 (Fig. 1). The negative binomial regression analysis revealed that compared with the normal executive function group, both the mild and marked executive function groups had markedly elevated prospective falls in the unadjusted analysis (Table 2). In both models 2 and 3, the IRRs for mild and marked executive dysfunction remained statistically significant, such that when adjusting for age, PD severity and duration, and FOG in the final model, the IRRs for mild and marked executive dysfunction were 3.48 (95% CI = 1.90, 6.40) and 5.68 (95% CI = 2.72, 11.88) respectively (Table 2).
Negative Binomial Regression models of relationship between executive dysfunction and prospective falls.
| Incidence Rate Ratio (95% confidence interval) | p | |
|---|---|---|
| Model 1 | ||
| Executive dysfunction | ||
| Mild | 5.45 (3.08, 9.68) | <0.001 |
| Marked | 12.58 (6.16, 25.67) | <0.001 |
| Model 2 | ||
| Executive dysfunction | ||
| Mild | 3.56 (1.99, 6.36) | <0.001 |
| Marked | 5.63 (2.75, 11.52) | <0.001 |
| Disease duration | 1.05 (1.00, 1.09) | 0.040 |
| Hoehn and Yahr stage | 3.90 (2.60, 5.85) | <0.001 |
| Model 3 | ||
| Executive dysfunction | ||
| Mild | 3.48 (1.90, 6.40) | <0.001 |
| Marked | 5.68 (2.72, 11.88) | <0.001 |
| Age | 0.98 (0.95, 1.00) | 0.107 |
| Disease duration | 1.02 (0.97, 1.06) | 0.475 |
| Hoehn and Yahr stage | 3.43 (2.29, 5.11) | <0.001 |
| FOG | 2.11 (1.20, 3.71) | 0.010 |
All models also adjusted for rivastigmine prescription and the length of the follow-up for falls.
We found that compared with people with PD with normal executive function, people with PD with both mild and marked executive dysfunction have significantly elevated fall rates. However, a major objective of this study was to determine whether mild and marked executive dysfunction increased fall risk independently of PD severity. Such adjustment is required as cognitive impairments typically progress as the disease progresses, and it is necessary to eliminate the possibility that increased fall risk in the executive dysfunction groups is due to loss of balance and gait impairment that accompany disease progression.29 We found that when adjusting for PD severity and duration, the IRRs for both mild and marked executive dysfunction remained statistically significant. FOG also occurs more commonly in the later PD stages, and as this syndrome may reflect underlying impaired attentional set-shifting ability and response inhibition it was entered separately in the negative binomial regression modelling.30 Again, while reduced in strength, the IRRs for both mild and marked executive dysfunction and falls remained statistically significant.
Neural factors may also underlie the association between executive dysfunction and falls in people with PD.7 Degeneration of the cholinergic system in PD can result in both motor and non-motor impairments31 via degeneration in the Nucleus Basalis of Meynert (responsible for cholinergic input to the cerebral cortex) and the Pedunculopontine Nucleus (responsible for cholinergic input to the thalamus, with projections to the striatum, cerebellum, and brainstem), respectively. White matter lesions might also contribute to balance impairments in people with PD with mild and marked executive dysfunction. Such white matter lesions are more prevalent in brain areas which contribute primarily to motor impairments (i.e. FOG episodes)32 and to axial motor impairments (i.e. stooped posture). These cortical lesions would impair brain (cortico-striatal and cortico-cortical) connections and basal ganglia and frontal lobe pathways,32,33 contributing to an increased risk of falling.
In addition to the severity of PD-specific measures (elevated Hoehn and Yahr stage, higher MDS-UPDRS scale scores, longer PD duration, and more participants with the PIGD sub-type and FOG), we found several sensorimotor and balance factors discriminated among the normal and mild and marked executive dysfunction groups. These factors included: worse visual contrast sensitivity, reduced quadriceps strength, slower simple reaction time, greater postural sway, poor controlled leaning balance, and presence of orthostatic hypertension. In most cases, these established risk factors for falls were evident in the mild as well as the marked executive dysfunction group and help elucidate at the impairment level why people with PD and executive dysfunction fall frequently.
Researchers have been debating on the relationship between PIGD classification and executive dysfunction,7 and the potential association between PIGD subtype and increased risk of falls.34 The prevalence of the PIGD subtype was higher in the mild and marked executive dysfunction groups, and may reflect, at least in part, the PIGD group having greater PD severity.35 In contrast, quantitative measures of gait (velocity, step length, and cadence) did not differ among the groups, which suggests substantial motor deficits inherent to PD overshadow subtler effects of executive dysfunction on gait stability.
Strengths of the study include the prospective falls follow-up, the inclusion of people with PD with a wide range of executive function abilities, and the large heterogeneous sample drawn from two study sites. We also acknowledge certain limitations, many of which relate to the secondary analysis of previous study datasets. First, executive dysfunction in this analysis was determined from the single neuropsychological test (FAB) common to both studies that assesses only one cognitive domain. Second, we used the validated question regarding incidence of FOG from the MDS-UPDRS. While this question has high face validity, we acknowledge the use of a FOG questionnaire would have provided more precision for this measure. Third, we did not have a record of education in both studies – a factor known to affect cognitive status. In the future, the use of a more in-depth, standardised assessment of the above measures along with additional PD symptoms such as dyskinesia13 may provide greater understanding of elevated fall risk in people with PD. In addition, different versions of the UPDRS were used in the two samples (UPDRS for sample 1 and MDS-UPDRS for sample 2) and may have affected our results. However, by following the agreed methodology for calibrating the results, scoring differences between participants would have been mitigated.20
This study has important clinical implications. It supports the need to develop fall prevention interventions tailored to people with PD with both mild and marked impairments in executive function. Exercise interventions that have shown to improve motor and balance function36 and prevent falls37 have not included patients with established dementia or more marked cognitive impairment. Similar interventions that include targeting the associated factors (postural instability associated with disease severity and FOG) might also reduce falls in people with PD with mild and marked executive dysfunction.
ConclusionsSeveral PD-specific, sensorimotor, and balance factors differed significantly among the normal and the mild and marked executive dysfunction groups and both mild and marked executive dysfunctions were identified as independent risk factors for falls in people with PD. Tailoring pharmacological and exercise interventions to address sensorimotor deficits, postural instability, and FOG may help reduce the occurrence of falls in people with PD with executive dysfunction.
Conflict of interestsStephen Lord acknowledges the PPA (NeuRA FallScreen) is commercially available through Neuroscience Research Australia. All other authors declare no conflicts of interest.
Paulo Pelicioni was a recipient of a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) PhD scholarship [Grant number: BEX 2194/15-5] and Mark Latt was funded by a University of Sydney PhD Scholarship to undertake this study. Stephen Lord is supported by an NHMRC Research Fellowship. Emily Henderson has received research funding from Parkinson’s UK, the British Geriatrics Society, The Gatsby Foundation and the National Institute of Health Research (NIHR).