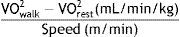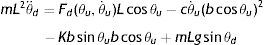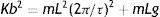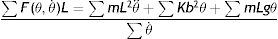Gait speed and metabolic cost are indicators of functional capacity in children with cerebral palsy. Uncovering their mechanisms helps guide therapeutic actions.
ObjectivesTo investigate the contributions of energy-generating and energy-conserving mechanisms to gait speed and metabolic cost of children with unilateral cerebral palsy.
MethodsData on eccentric and concentric muscle work, co-contraction, elastic torque and vertical stiffness of the affected-limb, forcing torque of the non-affected limb, gait speed and metabolic cost were collected from 14 children with unilateral cerebral palsy, aged 6–12 years. Analyses included two groups of multiple regression models. The first group of models tested the association between each dependent variable (i.e., speed and metabolic cost) and the independent variables that met the input criteria. The second group verified the contribution of the non-selected biomechanical variables on the predictors of the first model.
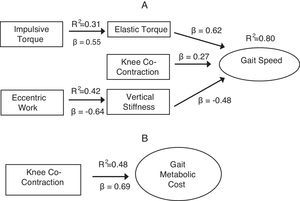
ResultsGait speed (R2=0.80) was predicted by elastic torque (β=0.62; 95%CI: 0.60, 0.63), vertical stiffness (β=−0.477; 95%CI: −0.479, −0.474) and knee co-contraction (β=0.27; 95%CI: −1.96, 2.49). The production of eccentric work by the affected limb proved relevant in adjusting the vertical stiffness (R2=0.42; β=−0.64; 95%CI: 0.86, −0.42); elastic torque of the affected-leg was associated with impulsive torque of the non-affected leg (R2=0.31; β=0.55; 95%CI: 0.46, 0.64). Metabolic cost of gait (R2=0.48) was partially predicted by knee co-contraction (β=0.69; 95%CI: 0.685, 0.694).
ConclusionsThe chain of associations revealed by the two steps models helped uncover the mechanisms involved in the locomotion of children with unilateral cerebral palsy. Intervention that changes specific energy conserving and generating mechanisms may improve gait of these children.
Gait supports the independence of children with cerebral palsy (CP) in different environments.1 Previous studies identified speed and metabolic cost of gait as indicators of the functional capacity of these children.2,3 Such variables are intrinsically related: an increase in gait speed increases the metabolic cost of gait.4,5 Compared to typically developing children, children with CP exhibit higher metabolic cost when walking,6,7 although their gait speed is slower.8
Gait speed is the product of stride frequency and stride length.9 There are two ways in which gait speed can be increased: via an increase in stride length through increased generation of power or via an increase in cadence through increased stiffness of lower limbs’ tissues.9,10 Power generation capacity of lower limbs in children with unilateral CP (UCP) is known to be lower than that of typically developing peers.11 As a result, their ability to increase gait speed by increasing their stride length is reduced.12,13 Increasing cadence, therefore, becomes the most viable strategy for increasing their gait speed.2 To increase cadence, children with UCP must actively increase stiffness, which could theoretically result in higher metabolic cost of gait.14,15
Several factors are associated with gait speed and energetic cost in children with UCP.4,12,16,17 For example, a positive association between lower limb muscle strength and gait speed of children with spastic UCP has been consistently documented.18,19 Leg extensor muscle strength, however, only partially explains the energetic cost of gait in these children.17 Unnithan et al.14 claimed that children with CP's increased metabolic cost of walking could be attributed to higher levels of muscular co-contraction.14 It has been proposed that co-contraction restricts the ability to increase stride length when walking, reducing speed and negatively impacting gait metabolic cost.14 On the other hand, muscle co-contraction could increase joint stiffness, ensuring greater conservation of mechanical energy.12,13,16 As a result, there is no consensus regarding the role of co-contraction on gait speed or energetic cost of children with UCP.12
Despite the interest in identifying factors that influence gait speed and energetic cost in children with UCP, their essential determinants have not yet been uncovered. Theoretical models of gait of children with UCP identify mechanisms related to decreased speed and increased metabolic cost.10,13,20 For example, the biomechanical model of inverted pendulum with springs, proposed by Fonseca et al.,13 describes energy generating and conserving mechanisms that are fundamental to gait. This model captures overall gait adaptations used by children with UCP and may help understand specific determinants of gait speed and metabolic cost.
Child's ability to generate power (e.g., impulsive torque) or to conserve energy stored in elastic tissues (e.g., elastic torque) is essential for walking.13,21 These generating and conserving mechanisms are related, respectively, to concentric work produced by the legs22 and the ability to control joint stiffness via co-contraction or eccentric work.23 Understanding the role of these factors, which are modifiable by means of intervention, may support the development of effective therapeutic strategies. This study aimed to investigate how mechanisms related to the ability to generate power and conserve energy contribute to gait speed and metabolic cost in children with spastic UCP.
MethodsParticipantsParticipants were 17 children with UCP. Inclusion criteria were: medical diagnosis of spastic hemiplegic CP, age 6–12 years, independent ambulation (i.e., Gross Motor Function Classification System – GMFCS levels I or II), not participating in a muscle strengthening program, not having contraindications to exercise, not having had botulinum toxin application or used serial plaster casts in the 6 months prior to evaluation and not having undergone surgery up to twelve months before evaluation. Three children were excluded due to technical problems during data collection, resulting in a group of 14 children (Table 1). The mean age of participants was 7.78 years (standard deviation [SD]=1.31 years). Four participants had undergone Achilles tendon lengthening surgery more than a year previously, 5 received botulinum toxin application to the calf more than 6 months before the study, and 3 had never used bracing. The University Research Ethics Review Committee approved this study's procedures (ETIC: 585/08 – Universidade Federal de Minas Gerais, Belo Horizonte, MG, Brazil). Children's guardians signed informed consent forms authorizing participation.
Participant's characteristics.
| Participant | Age (years) | Sex | Affected side | GMFCSa level |
|---|---|---|---|---|
| 1 | 9 | Girl | Right | II |
| 2 | 6 | Boy | Right | I |
| 3 | 6 | Boy | Left | II |
| 4 | 9 | Boy | Right | II |
| 5 | 6 | Boy | Left | I |
| 6 | 8 | Boy | Right | II |
| 7 | 9 | Girl | Left | II |
| 8 | 9 | Boy | Left | I |
| 9 | 8 | Girl | Right | I |
| 10 | 8 | Girl | Left | I |
| 11 | 6 | Boy | Right | II |
| 12 | 9 | Boy | Right | II |
| 13 | 9 | Boy | Right | II |
| 14 | 7 | Girl | Right | I |
GMFCS: Gross Motor Function Classification, which describes the gross motor function of children and adolescents with cerebral palsy according to self-initiated movement skills. Level I refer to children and youth who are able to perform usual gross motor activities such as running and jumping with decreased speed, balance and coordination. Children classified as level II can climb stairs with a railing, have difficulty with uneven surfaces, inclines or in crowds and have minimal ability to run or jump.
In this cross-sectional study, one trained examiner performed all data collection. In addition to the primary outcomes (gait speed and metabolic cost), the following variables related to energy generation and conservation were measured and/or calculated: concentric and eccentric lower limb muscle work, co-contraction of the affected leg muscles, vertical body stiffness, impulsive torque of the non-affected leg and elastic torque of the affected leg. Children remained barefoot and without orthotic devices in all measurements.
Children's body weight, height, total leg length and length of the thigh, shank and foot were measured. Each child was equipped with a portable gas analyzer (Cosmed K4 b2) to measure oxygen (O2) consumption and calculate the metabolic cost. Data were collected during five minutes at rest and five minutes at self-selected speed. Muscle activity and kinematic parameters were evaluated simultaneously during gait. Active surface electrodes recorded the activity of the gluteus maximus, rectus femoris, vastus lateralis, biceps femoris, tibialis anterior and gastrocnemius muscles. The electromyographic signal was transmitted wirelessly to an ME6000 electromyograph (Mega Eletronics®, Finland) with a sampling frequency of 1000Hz. To normalize the signals obtained during gait, the children performed maximal voluntary contractions (MVCs).
A motion analysis system (Qualisys ProReflex®, Gothenburg, Sweden) with 6 cameras captured kinematic gait parameters at 120Hz. Passive markers were placed on the head (2 on the temporal bones in front of the ear and 1 on the frontal bone in the glabellar region), trunk (2 on the body of the clavicles and 1 on the body of the sternum), pelvis (cluster with 4 markers), affected thigh (cluster with 3 markers), affected shank (cluster with 3 markers), and feet (calcaneus and the head of the 1st and 5th metatarsals). To create three-dimensional kinematic models, anatomical markers were bilaterally placed in the following body regions: acromion, iliac crest, greater trochanter, lateral epicondyle and medial femur, medial and lateral malleolus, calcaneus and the head of the 1st and 5th metatarsals.17 Each child walked at a self-selected speed on a 10-meter walkway for 20–30 attempts until 3–5 complete gait cycles were captured. A complete gait cycle had to include 2 sequential initial contacts on the affected leg and 2 push-offs of the unaffected leg.
Following the evaluation of gait, an isokinetic dynamometer (Biodex System 3 Pro, Shirley, USA) with a module for evaluation in a closed chain measured the concentric and eccentric extensor muscle work of the affected leg in ranges of motion from 40 to 90° of knee flexion. After familiarization, the child performed 5 maximal repetitions by pushing the dynamometer arm. Children extended the lower limb for the concentric measurement and resisted limb flexion for the eccentric measurement.
Data reductionThe affected leg's initial contact and the toe-off were determined for identification of the beginning and end of the gait cycle.24 For the non-affected leg, the toe-off and push-off phases were defined. Push-off was defined as the time interval from the beginning of the plantar flexion of the non-affected limb until toe off, i.e., the period during which the non-affected limb impelled the center of mass while the affected leg operated as an inverted pendulum.
Electromyographic data of each selected gait cycle were rectified and filtered with a band-pass filter of 500Hz and 10Hz. The signals obtained for each muscle during the gait cycle were normalized by the corresponding activity obtained during MVC. The overlapping area of each agonist/antagonist pair was calculated and represented the magnitude of simultaneous muscle activation, i.e., the co-contraction level.25 Thus, the overlap between the gluteus maximus and rectus femoris characterized the co-contraction index (CCI) of the hip, the vastus lateralis and biceps femoris the CCI of the knee, and the tibialis anterior and lateral portion of the gastrocnemius the CCI of the ankle.25
Information on the volume of oxygen (VO2) during walking were processed as described by Plasschaert et al.26 Data for which the respiratory equivalent ratio represented anaerobic activity or close to it (respiratory exchange ratio [RER]>0.9) were discarded.26 The first 2minutes were removed from the analysis, both at rest and during walking, because they correspond to a stage of adaptation of the respiratory parameters. The metabolic cost of gait was calculated with the following formula26:
Kinematic data were processed with Visual 3D and used to calculate the stance impulsive torque of the non-affected limb, the stance elastic torque of the affected limb, the vertical body stiffness and gait speed. Data were filtered with a fourth-order Butterworth filter with a cut-off frequency of 6Hz.
The impulsive torque, which represents the energy added during walking and elastic torque, which represents the energy conserved by the elastic tissues during walking, were calculated using a biomechanical model of an escapement-driven inverted pendulum.13,20,21 According to this model, the child's behavior while walking is captured by the total torque (mL2θ¨) acting on an inverted pendulum whose mass is located at the child's center of gravity and whose axis of rotation is located at the ankle (i.e., an inverted pendulum oscillating on the child's ankle during the stance phase). This torque is calculated by the following equation.13
where mL2θ¨d represents the total torque on the ankle, Fd(θu,θ˙u)Lcosθu is the impulsive torque produced at push-off, cθ˙u(bcosθu)2 is the dissipative torque, Kbsinθubcosθu is the elastic torque and mLgsinθd is the gravitational torque. The child's mass is represented by m, and the equivalent length of the pendulum is represented by L (assuming the child's center of gravity revolves around the ankle). Due to the nature of the inverted pendulum gait, the impulsive torque of the push-off leg interacts with the elastic and gravitational torques of the stance leg. Details on the calculation of mass and equivalent length are presented by Kugler and Turvey.27Assuming that children walk at their natural frequency and given their body weight, equivalent length of the pendulum and stride frequency (f), the elastic torque can be calculated as:
In this equation, the stance phase period (τ) is represented by 1/f.
The impulsive torque Fd(θu,θ˙u)Lcosθu accelerates the child's center of gravity during the gait push-off phase. Thus, after linearizing and rearranging Eq. (2), this variable can be calculated by adding the total torque of the pendulum on the ankle joint, the elastic torque and the gravitational torque during the push-off phase of the non-affected limb.13 The total pendulum torque is calculated as the integral of the angular acceleration of the system (θ¨) from the beginning of the ankle plantar flexion until toe-off multiplied by the pendulum mass (m) and by the square of the equivalent pendulum length (L). To calculate the other elements of Eq. (2), the integral of the system's angular displacement (θ) in the same period was multiplied by gravitational torque and elastic torque. Finally, the impulsive torque was normalized by the integral of the angular speed of the center of gravity on the ankle (θ˙) to remove the effects of the dissipative torque according to the equation below:
Using the same model, vertical body stiffness during the stance phase can be calculated from the ratio between the vertical force acting on the center of gravity and the vertical displacement21:
In this equation, Kvertical represents the vertical stiffness, y¨ and y represent, respectively, the vertical acceleration and displacement of the center of gravity during the stance phase. As the child's mass remains constant, vertical stiffness is obtained by calculating the slope of the regression analysis of acceleration by the vertical displacement of the center of gravity during the stance phase.
To minimize the effect of the mass and length of body segments on the study variables,20 the CCIs for the hip, knee and ankle and the values of elastic torque, vertical stiffness and impulsive torque were normalized by the inverted pendulum gravitational torque (mLg). Additionally, gait speed was normalized by leg length.
Data analysesAnalyses included two groups of multiple linear regression models (hereafter simply referred to as “primary” and “secondary”) that used the stepwise method for selection of independent variables. The primary models tested the association between each dependent variable (i.e., speed and metabolic cost) and the independent variables that met the input criteria (p<0.05). Secondary models verified the contribution of the non-selected biomechanical variables on the predictors of the primary model. Thus, each predictor variable of the primary models was transformed into a dependent variable, and the other predictors remained as independent variables.
ResultsThe first primary model identified elastic torque as the main predictor of gait speed (R2=0.80, β=0.62, 95%CI: 0.60, 0.63, p=0.0001), followed by vertical stiffness (R2=−0.67, β=−0.477, 95%CI: 0.479, −0.474, p=0.001), and knee co-contraction (R2=0.32, β=0.27, 95%CI: −1.96, 2.49, p=0.03). These 3 variables explained 89.4% of the variability of gait speed of children with unilateral CP (Table 2). The second primary model identified knee co-contraction as the sole predictor of metabolic cost (R2=0.48, β=0.69, 95%CI: 0.685, 0.694, p=0.006).
Correlation coefficient (r) matrix showing the bivariate associations of the study's variables.
| Speed | Knee CCI | Ankle CCI | Hip CCI | Elastic torque | Vertical stiffness | Concentric work | Eccentric work | Impulsive torque | Metabolic cost | |
|---|---|---|---|---|---|---|---|---|---|---|
| Knee CCI | 0.32 | |||||||||
| Ankle CCI | 0.24 | 0.47* | ||||||||
| Hip CCI | 0.19 | 0.93 | 0.39 | |||||||
| Elastic torque | 0.80* | 0.11 | 0.02 | 0.02 | ||||||
| Vertical stiffness | −0.67* | 0.03 | 0.07 | 0.07 | −0.32 | |||||
| Concentric work | 0.61* | 0.09 | 0.22 | 0.08 | 0.40 | −0.51* | ||||
| Eccentric work | 0.36 | 0.14 | 0.08 | 0.16 | 0.02 | −0.64* | 0.46 | |||
| Impulsive torque | 0.47* | 0.41 | 0.29 | 0.42 | 0.55* | −0.04 | 0.10 | 0.33 | ||
| Metabolic cost | 0.40 | 0.69* | 0.19 | 0.64* | 0.45 | 0.15 | 0.22 | −0.04 | 0.43 |
CCI, co-contraction index.
For the secondary models, elastic torque, vertical stiffness and knee co-contraction were the dependent variables. Elastic torque of the affected leg was associated only with impulsive torque of the non-affected leg (R2=0.31, β=0.55, 95%CI: 0.46, 0.64, p=0.04) and vertical stiffness was negatively associated with eccentric leg work (R2=−0.42, β=−0.64, 95%CI: −0.86, −0.42, p=0.013). Knee co-contraction was not related to any variable (Table 3).
Primary and secondary regression models.
| Dependent variable | Predictors | Beta | p-value | Model R2 |
|---|---|---|---|---|
| Primary regression models | ||||
| Speed | Elastic torque | 0.617 | 0.0001 | |
| Vertical stiffness | −0.477 | 0.001 | 0.80 | |
| Knee co-contraction | 0.267 | 0.028 | ||
| Metabolic cost | Knee co-contraction | 0.693 | 0.006 | 0.48 |
| Secondary regression models | ||||
| Elastic torque | Impulsive torque | 0.554 | 0.040 | 0.31 |
| Vertical stiffness | Eccentric work | −0.644 | 0.013 | 0.42 |
| Knee co-contraction | – | – | – | – |
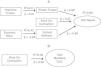
Fig. 1 presents the schematic models developed from the results of the study. They illustrate the contribution of energy generating and conserving mechanisms in both outcomes.
DiscussionThis study identified the contribution of energy generating and conserving mechanisms to gait speed and metabolic cost in children with UCP. Gait speed was largely explained by variables related to energy conserving mechanisms. More specifically, while elastic torque and vertical stiffness are direct measures of biomechanical parameters related to elastic energy return, knee co-contraction is a mechanism that could adjust these variables. In addition, increased metabolic cost was associated with knee co-contraction. Despite its positive impact on gait speed, increasing knee co-contraction to actively adjust knee stiffness seems to be an inefficient mechanism for speed regulation. The multiple roles of co-contraction may explain the lack of consensus on the contribution of this variable to gait speed and energetic cost of children with UCP.
In accordance with theoretical models,13 elastic torque (an energy-conserving element) and impulsive torque of the non-affected limb (a power-generating element) contribute to higher gait speed in children with UCP. The impulsive torque of the non-affected limb provides the energy needed for gait maintenance. This periodic energy input is required because at each cycle, part of the energy is dissipated21 and needs to be replaced. One of the secondary models showed a positive relation between impulsive force and elastic torque. This result suggests that the use of energy conservation mechanisms in the impaired leg (e.g. elastic torque) is dependent on energy producing mechanisms of the opposite leg. In the primary models, there was no direct relation between impulsive torque and gait speed. Thus, interventions that increase muscle force production at push-off of the non-affected limb of children with UCP must be coupled to interventions that favor energy conservation in the affected leg if the goal is to increase gait speed.
The results revealed that variables related to elastic tissue energy return (i.e., elastic torque and co-contraction) contribute positively to the children's gait speed. Elastic torque was the best predictor of gait speed of children with UCP. According to the secondary analyses, this torque is associated with the children's ability to perform eccentric work. Silva et al.23 demonstrated that the eccentric activity of the muscles around a joint contributes to joint stiffness control.23 Thus, children who have the ability to actively resist joint movement when walking (i.e., eccentric contraction) can take advantage of the elastic energy stored in the muscle tissue to increase speed that will likely transpire as increases in cadence.2 This finding suggests that interventions based on eccentric strengthening of children's leg muscles during gait may contribute to provide the energy conservation mechanisms, and therefore, improve functional performance.28 Nevertheless, a recent systematic review with meta-analysis28 demonstrated that strengthening by itself as a type of resistance training, even when properly dosed, does not effectively impact gait speed of children with UCP. The results showed that task-specific gait training performed either on treadmill or overground are more effective than strength training.28 In light of this recent review evidence, we recommend the eccentric strengthening should be implemented within a task-specific gait training.
Vertical stiffness has been related to increased gait speed in children with UCP.13,21 Fonseca et al.21 found that children with UCP increase vertical stiffness at higher gait velocities, suggesting the use of elastic muscle properties at these velocities. However, in the present study, vertical stiffness was negatively related to gait speed. The use of vertical stiffness as an energy conserving mechanism to increase gait speed depends on the child adopting a motion pattern in which the affected limb behaves like a vertical spring. This spring is compressed during stance of the leg, storing elastic energy, which is quickly returned to the body as energy for movement during push-off of this limb.21 However, excessively high or low stiffness values result in a lower energy storage capacity.29 High stiffness values impede elastic tissue deformation and, instead of storing, contribute to transfer energy to other structures. Our results suggest that vertical stiffness in the sample studied appeared not to be related to energy conservation, but rather it was detrimental to gait speed.
The observed association between eccentric work and vertical stiffness in the secondary model may explain the negative contribution of this property to gait speed. In the present study, children with a decreased ability to produce eccentric work had more vertical stiffness than others with greater ability to eccentrically control movement. Instead of using stiffness regulating mechanisms (i.e. muscle eccentric work and co-contraction) to conserve energy, some children with poor eccentric capabilities may have produced greater vertical stiffness by locking their knees during the stance phase. Anatomical joint blockage represents a way to increase joint stiffness, even if it does not contribute to energy conservation. This anatomical blockage is a strategy to increase joint stability, when the child is not able to use the leg as a vertical spring nor has enough eccentric force to actively resist joint perturbations. Thus, during the gait stance phase, most energy would be transferred to the ground and not efficiently used to aid mobility. An individual with adequate vertical stiffness is well-adapted to store elastic energy that may later be returned to the body.21 The absence of appropriate stiffness, associated with muscle weakness, leads to the emergence of alternative means to meet functional demands. Again, eccentric strengthening of leg muscles is suggested as an application of our results.
Contrary to the expectations,5,12 co-contraction of the knee muscles also contributed positively to gait speed. Given its potential for increasing joint stiffness,12,16 knee co-contraction can be seen as a mechanism that actively assists in elastic energy conservation and thus may not represent an inappropriate strategy.13 On the contrary, increased co-contraction may be one of the mechanisms by which the child adjusts stiffness when performing the task.23 As a compensatory strategy to ankle plantar flexors weakness,13,19 knee co-contraction can be used to actively increase leg stiffness, which increases elastic energy storage and return during the push-off phase,20 thus, positively impacting gait speed.
Knee co-contraction, despite being a predictor of gait speed, was not found to be metabolically efficient. This result was partly evidenced by the primary model of gait metabolic cost, which revealed knee co-contraction as the only predictor. In this regard, increased co-contraction may be considered a functional adaptation13,20 that allows increased gait speed at the expense of greater metabolic cost. For example, a child's increase in gait speed to cross a street safely or to play with peers in the playground, will probably be done by a mechanism that increases metabolic cost. Therefore, the optimization parameter used by children with UCP is the accomplishment of a functional demand and not the minimization of metabolic cost, as traditionally argued.3,30
Despite the rigorous implementation of the method of this study, its small sample (n=14) could have reduced the study's power or affected the results’ reproducibility. Despite the identification of significant predictors at both stages of data analyses, the reported chain of associations suggesting direct and indirect courses underlying gait speed and metabolic cost in children with UCP need to be further validated. In addition, to allow some of the study's measurement procedures, children did not wear any orthotic device, which may have modified their usual gait pattern.
By identifying the mechanisms used in the gait of children with UCP, our results help guide evaluation procedures and support interventions that target specific limiting factors to this population's functional locomotion. As such, rehabilitation that focus on changing specific energy conserving and generating mechanisms could be effective in improving the functional performance of children with unilateral CP. For example, our results suggest that therapeutic efforts aimed at enhancing the generation of impulsive torque of the non-affected limb would be valuable. In addition, eccentric muscular strengthening of the affected limb is most promising when implemented on functional programs, under a task-oriented approach.31
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the parents for allowing their children's volunteer participation as well as the Associação Mineira de Reabilitação (AMR), Belo Horizonte, Brazil, for facilitating participants’ recruiting. Financial support for this study was granted by the Brazilian government agencies including the National Council for Scientific and Technological Development (CNPq), the Research Support Foundation from the State of Minas Gerais (FAPEMIG) and by LIM34 – Laboratory from Rehabilitation Sciences, Faculty of Medicine, University of São Paulo (USP), Brazil.