There is limited research on cyclists with a history of exercise associated muscle cramps (hEAMC) defined as muscle cramping (painful, spontaneous, sustained spasm of a muscle) during or after cycling.
ObjectiveTo determine the epidemiology, clinical characteristics, and risk factors associated with hEAMC in cyclists taking part in a mass participation cycling event.
Methods21,460 race entrants from the 2016 Cape Town Cycle Tour completed an online questionnaire, which is based on the guidelines for recreational exercise participation from the European Association of Cardiovascular Prevention and Rehabilitation [EACPR]. The main outcome measures were: the lifetime prevalence hEAMC (%; 95% confidence intervals), independent risk factors (adjusted for age and sex) associated with hEAMC (history of chronic disease, history of allergies, history of chronic medication use, history of medication use before and during race, history of cycling injuries, and cycling training/racing variables). Poisson regression was used to calculate the prevalence (%) of the variables of interest, with 95% confidence intervals.
ResultsThe retrospective lifetime prevalence of hEAMC was 30.51%. EAMC in cyclists affects mainly the quadriceps muscles and occurs in the 4th quarter during a race. Novel independent risk factors associated with an increased risk of hEAMC in cyclists were: increased number of years participating as a recreational cyclist (PR=1.03 per 5 years increase; p<0.0001), a higher chronic disease composite score (PR=1.36 times increased risk for every 2 additional chronic diseases; p<0.0001), a history of any allergies (PR=1.18; p<0.0001), medication use before or during event (PR=1.41; p<0.0001) a history of an acute (PR=1.30; p<0.0001) and gradual onset injury (PR=1.29; p<0.0001).
ConclusionOur study identified novel independent risk factors associated with a hEAMC. These results, in combination with other known risk factors, could assist future targeted prevention programmes and the management of EAMC in recreational cyclists.
Exercise associated muscle cramping (EAMC) is a medical condition defined as a “painful, spasmodic, involuntary contraction of skeletal muscle that occurs during or immediately after physical exercise”.1–3 To prevent injury, such as EAMC, it is important to first understand the extent of the problem through investigating important aspects such as the prevalence of EAMC and common anatomical sites where EAMC occur. Important risk factors that predict EAMC then need to be identified before specific injury prevention strategies can be implemented.4
Most studies report on the period prevalence of EAMC (onset of EAMC over the duration of a specific race) in runners, varying between 18–24 % for marathon runners,5,6 and 14–41 % for ultramarathon runners.7,8 The lifetime prevalence has also been reported as high as 67 % of triathletes.3 Although the lifetime prevalence of EAMC in cyclists has been reported at 60 %3, there are no published data on the annual incidence of EAMC in cyclists. The gap in research is important to address to understand trends of EAMC and having a baseline to evaluate the effectiveness of future preventative interventions.
Studies investigating the muscle groups affected by EAMC in endurance athletes have reported that the calf, hamstrings, and quadriceps muscles are the most commonly affected. However, these studies have focused on runners and triathletes.3,9,10 Currently, there are no data on the muscle groups affected by EAMC in cyclists which hampers the design of targeted prevention strategies in this population.
Risk factors associated with EAMC in runners and triathletes include: history of underlying chronic disease,11 increased exercise intensity,7,12,13 a history of running injury,11,12 increased running experience,11 a history of prerace muscle damage,7 a previous history of muscle cramping,7,12 and possible genetic factors.14 Running involves different biomechanical actions compared to cycling, but both sports expose the lower limb muscles to fatigue through repetitive loading. To date there are no studies reporting risk factors associated with EAMC in cyclists specifically. It has been hypothesized that risk factors for chronic disease, underlying chronic medical conditions, and drugs used to treat these conditions may increase the risk of EAMC.11 The prevalence of chronic disease in recreational cyclists varies between 10 and 16 %,15 but the relationship between chronic diseases and EAMC in cyclists is not known. It is important to determine potential risk factors for EAMC to implement prevention and management strategies.
The aim of this study was to describe the epidemiology and clinical characteristics of a history of EAMC (hEAMC) in cyclists, and to identify selected independent risk factors associated with EAMC in cyclists entering a mass participation one-day cycle event.
MethodsStudy designThis was a retrospective, observational study, with cross-sectional analysis of data. This study forms part of a series of on-going SAFER (Strategies to reduce Adverse medical events For the ExerciseR) studies.16 The Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria, granted ethical approval for the study.
Participants and data collectionParticipants for the study were cyclists who entered the 2016 Cape Town Cycle Tour (CTCT), a mass participation one-day road cycling race of 109 km. No qualification was required to enter the event, and race entrants were defined as any cyclists (amateur to professional) registering for the race. At the time of registration, all race entrants were required to report demographic and race participation data as part of the race entry and registration requirements. Race entrants were also required to complete an online pre-race medical screening questionnaire, hosted on the custom-designed electronic platform. To answer questions related to the questionnaire, the contact details of a research team member was provided to all participants. Participants who completed the screening questionnaire were invited to provide informed consent for their data to be used for research purposes. The questionnaire consisted of a series of questions that were based on the pre-exercise screening guidelines for recreational exercise participation from the European Association of Cardiovascular Prevention and Rehabilitation [EACPR,17] and previous studies in distance runners.11,18 The screening tool for runners was adapted to include questions specifically related to a history of common medical complications encountered during cycling and has been used in previous studies.19
As part of the medical history, race entrants were specifically asked: “Have you ever in your cycling career suffered from muscle cramping (painful, spontaneous, sustained spasm of a muscle) during or immediately after (within 6 h) cycling (in training or competition)?” If the response to the question was “yes”, race entrants reporting a hEAMC were required to answer additional questions related to the muscle cramp. Further questions regarding information related to cycling training/racing history and medical history were asked including: years of recreational cycling, number of years participating in cycling events >2 h, average times per week spent training or racing in the last 12 months, average weekly cycling distance (km) in the last 12 months and average training speed (km/hr). Medical history included any history of cardiovascular disease (CVD), any risk factors for CVD, any symptoms of CVD, any metabolic or hormonal disease, any respiratory disease, any gastrointestinal (GIT) disease, any nervous system/psychiatric disease, any renal or bladder disease, any haematological/immune system disease, and any cancer. Further questions regarding a history of any allergies, and general medication usage for chronic disease, and medication usage before and during racing were asked. A chronic disease composite score out of 10 was calculated using the sum of the individual’s answer to the 10 questions related to a history of chronic disease. Finally, the medical history also included questions related to gradual onset/chronic (“any symptoms of a CHRONIC (no accident) cycling injury (muscles, tendons, bones, ligaments or joints) IN YOUR CYCLING CAREER)” or acute (“any symptoms of an ACUTE ACCIDENTAL cycling injury (muscles, tendons, bones, ligaments or joints) IN YOUR CYCLING CAREER)” injury history.
Study outcomesThe outcomes of this study was to investigate: 1) the epidemiology of hEAMC (lifetime prevalence and retrospective annual incidence), 2) the clinical characteristics of EAMC (n, %), including main muscle groups (quadriceps, hamstrings, calves, etc.) affected and timing of onset during racing / training (first to fourth quarter, or after the race), and 3) the independent risk factors associated with hEAMC (demographics, cycling training / racing history, history of chronic disease, allergies and cycling injuries) amongst race entrants participating in the 2016 CTCT.
Statistical analysisAll consenting cyclists’ self-reported entry data were entered into a database and analysed using SAS statistical software (version 9.4, Cary NC). Cases with missing values were excluded from the analysis. Poisson regression with robust standard errors were used and the various models included the specified independent variables of interest. The dependent variable in the model was the binary-scaled response variable related to the question regarding a lifetime history of EAMC. The demographic data, history of previous injury (gradual onset or acute), and history of any allergy variables were entered into the model as categorical variables. The cycling training variables and the chronic disease composite score were entered into the model as continuous variables and the prevalence of hEAMC ( % and 95 % confidence intervals (CIs)) were reported at the first quartile, median and third quartile for these variables. Univariate unadjusted prevalence ratios (PR) [95 % CIs] were reported for all results for sex and age, cycling experience and cycling training history, history of chronic disease, and history of any allergies. These were also calculated by Poisson regression. The statistical significance level was 5 %, unless otherwise specified. Univariate regression models on all main categories associated with hEAMC were used to obtain the crude prevalence ratios (PRs) of EAMC for each factor separately. Initially, the multiple regression model included all the significant univariate risk factors. The results for the final model only included the retained significant risk factors and were adjusted for sex and age category.
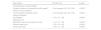
ResultsA total of 35,914 cyclists entered the CTCT in 2016. Of those, 21,460 (60 %) consented and had hEAMC data to be analysed. The profile by sex and age group of all race entrants and race entrants who gave consent to participate in the study is shown in Table 1.
Demographics (sex and age groups) of all race entrants and study participants.
| All race entrants(n = 35,914) | Consenting race entrants in the study (n = 21,460) | p-value | ||||
|---|---|---|---|---|---|---|
| n | % | n | ( %) | |||
| Sex | Males | 28,311 | 78.83 | 16,972 | 79.09 | 0.2114 |
| Females | 7603 | 21.17 | 4488 | 20.91 | ||
| Age group (years) | ≤30 | 6453 | 19.97 | 3253 | 15.16 | <0.0001* |
| 31–40 | 7814 | 21.76 | 4366 | 20.34 | ||
| 41–50 | 10,583 | 29.47 | 6291 | 29.32 | ||
| >50 | 11,064 | 30.81 | 7550 | 35.18 | ||
There was a significant difference between consenting race entrants and all race entrants by age category (p < 0.0001), with a higher percentage of older cyclists in the consenting group.
Epidemiology of hEAMCLifetime prevalence and annual incidenceA total of 6548 cyclists reported a lifetime hEAMC, indicating a lifetime prevalence of 30.5 % (95 % CI: 29.8, 31.3). Of these, 3997 cyclists reported hEAMC in the past 12 months, indicating a retrospective annual incidence of 18.6 % (95 % CI: 18.1, 19.2).
Clinical characteristics of hEAMCMuscle groups affected by hEAMCThe most commonly reported muscle groups affected by EAMC in cyclists with a lifetime hEAMC were the quadriceps muscles (n = 3122, 47.7 %), followed by the calf (n = 1819, 27.8 %), hamstring muscles (n = 1337, 20.4 %), and feet (n = 155, 2.4 %). For 143 (2.2 %) cyclists, the muscle groups affected by EAMC were unspecified.
Timing of onset of hEAMC during racing / trainingThe most common time period for the onset of hEAMC was during the fourth quarter of a race (n = 4152, 63.4 %), followed by after the race (n = 824, 12.6 %), third quarter (n = 819, 12.5 %), second quarter (n = 101, 1.5 %) and first quarter (n = 47, 0.7 %). Among 509 (7.8 %) cyclists, no specific timing of onset was report and 131 (2.0 %) race entrants did not specify the timing of onset related to their cramping.
Risk factors associated with hEAMC (univariate analysis)Demographics (sex and age group)The number (n), frequency ( %; 95 % CI) and prevalence ratio (PR; 95 % CI) of cyclists with hEAMC by sex and age group is shown in Table 2.
Number, prevalence, and prevalence ratio (PR) of cyclists with hEAMC by sex and age group (univariate).
| Characteristics | Consenting race entrants (n = 21,460) | Entrants with hEAMC(n = 6568) | PR (95 % CI) | p-value | ||
|---|---|---|---|---|---|---|
| N | n | Prevalence ( %; 95 % CI) | ||||
| Sex | Males | 16,972 | 5852 | 34.4 (33.5, 35.3) | 2.2 (2.0, 2.4) | <0.0001* |
| Females | 4488 | 716 | 15.8 (14.7, 17.0) | 1.0 | ||
| Age group (years) | ≤30 years | 3253 | 676 | 20.8 (19.3, 22.4) | 1.0 | <0.0001* |
| 31 - 40 years | 4366 | 1172 | 26.8 (25.3, 28.3) | 1.3 (1.2, 1.4) | ||
| 41 - 50 years | 6291 | 1875 | 29.7 (28.4, 31.1) | 1.4 (1.3, 1.6) | ||
| >50 years | 7550 | 2845 | 37.5 (36.2, 38.9) | 1.8 (1.7, 2.0) | ||
PR: Prevalence Ratio.
hEAMC: history of exercise associated muscle cramps.
There was a significantly higher prevalence of male (34.4 %) versus female (15.8 %) cyclists with a hEAMC (PR=2.2, p < 0.0001). There was a significant difference in the prevalence of hEAMC among cyclists in the different age categories, being highest for the oldest age group (37.5 %).
Training/racing variablesThe frequency ( %; 95 % CI) and prevalence ratio (PR) of cyclists with hEAMC by training/racing variables is shown in Table 3.
Prevalence ( %; 95 % CI) and prevalence ratio (PR) (with 95 % CI) of cyclists with hEAMC by training/racing variables (univariate).
| Cycling training / racing variables | Points in the continuous variable# | Entrants with hEAMC (n = 6568)Prevalence ( %; 95 % CI) | PR (95 % CI) | p-value |
|---|---|---|---|---|
| Number of years as a recreational cyclist (years) | 4 | 26.7 (25.9, 27.6) | 5-unit increase 1.08 (1.07, 1.09) | <0.0001* |
| 10 | 29.3 (28.5, 30.0) | |||
| 18 | 33.0 (32.2, 33.9) | |||
| Number of years participating in distance cycling events > 2 h (years) | 3 | 25.6 (24.8, 26.4) | 5-unit increase1.13 (1.11, 1.14) | <0.0001* |
| 6 | 27.5 (26.8, 28.3) | |||
| 15 | 34.1 (33.3, 35.0) | |||
| Average weekly training/ racing frequency in the last 12 months (times per week) | 2 | 30.7 (29.8, 31.5) | 1-unit increase0.99 (0.98, 1.01) | 0.4845 |
| 3 | 30.5 (29.8, 31.2) | |||
| 4 | 30.3 (29.4, 31.3) | |||
| Average weekly cycling distance in the last 12 months (km/week) | 40 | 29.7 (28.9, 30.6) | 40-unit increase1.02 (1.01, 1.03) | 0.0028* |
| 80 | 30.3 (29.5, 31.0) | |||
| 130 | 31.0 (30.2, 31.8) | |||
| Average training speed (km/hr) | 20 | 29.7 (28.8, 30.5) | 2-unit increase1.02 (1.01, 1.03) | 0.0001* |
| 24 | 30.7 (30.0, 31.5) | |||
| 26 | 31.2 (30.4, 32.1) |
PR: Prevalence Ratio.
A number of training/racing variables were associated with an increased PR of hEAMC in cyclists. An increased number of years participating in distance cycling events of >2 h (PR=1.13 times increased risk for every 5-year increase in cycling, p < 0.0001), an increased number of years as a recreational cyclist (PR=1.08 times increased risk for every 5-year increase, p < 0.0001), faster average training speed (PR=1.02 times increased risk for every 2-km/hr increase, p = 0.0001), and increased average cycling distance in the last 12 months (PR=1.02 times increased risk for every 40-km/week increase, p = 0.0028) were associated with an increased risk of hEAMC.
History of chronic disease, allergies, cycling injury, and medication useThe number (n), frequency ( %; 95 % CI) and PR with 95 % CI of cyclists with hEAMC by history of chronic disease, allergies, injury, and medication use is shown in Table 4.
Number, prevalence ( %; 95 % CI) and prevalence ratio (PR) (with 95 % CI) of cyclists with hEAMC by history of chronic disease, allergies, injuries, and medication use (univariate).
| Characteristics | Race entrants with hEAMC (n = 6568) | PR (95 % CI) | p-value | ||
|---|---|---|---|---|---|
| n | Prevalence % (95 % CI) | ||||
| History of chronic disease | |||||
| Chronic Disease Composite Score (0–10)# | 0 | - | 26.6 (25.9, 27.5) | 2-unitIncrease1.5 (1.4, 1.6) | <0.0001* |
| 2 | - | 40.1 (38.6–41.6) | |||
| 4 | - | 60.4 (55.8, 65.4) | |||
| Medication use | |||||
| Chronic prescription medication | Yes | 2041 | 37.4 (35.8, 39.1) | 1.3 (1.3, 1.4) | <0.0001* |
| No | 4509 | 28.3 (27.4, 29.1) | |||
| Missing | 18 | ||||
| Medication use during or before an event | Yes | 861 | 45.8 (42.8, 48.9) | 1.6 (1.5, 1.7) | <0.0001* |
| No | 5649 | 29.1 (28.3, 29.8) | |||
| Missing | 58 | ||||
| History of allergies | |||||
| Any allergies | Yes | 1101 | 37.0 (34.9, 39.3) | 1.3 (1.2, 1.3) | <0.0001* |
| No | 5443 | 29.5 (28.7, 30.3) | |||
| Missing | 24 | ||||
| History of Injury | |||||
| Gradual onset injury | Yes | 456 | 48.5 (44.3, 53.2) | 1.6 (1.5, 1.8) | <0.0001* |
| No | 6060 | 29.7 (29.0, 30.4) | |||
| Missing | 52 | ||||
| Acute injury | Yes | 443 | 45.2 (41.1, 49.6) | 1.5 (1.4, 1.7) | <0.0001* |
| No | 6105 | 29.8 (29.1, 30.6) | |||
| Missing | 20 | ||||
Chronic Disease Composite Score: the composite number of chronic diseases for an individual.
n: number of race entrants with hEAMC.
PR: Prevalence Ratio.
A higher chronic disease composite score was associated with a significantly higher prevalence of hEAMC in cyclists in a “dose-dependent” fashion, meaning the higher number of chronic diseases present, the higher the risk for hEAMC (PR=1.5 times increased risk for every two additional chronic diseases present; p < 0.0001). A history of any allergies was also associated with a significantly higher prevalence of hEAMC in cyclists (PR=1.3; p < 0.0001). Cyclists with a history of a gradual onset injury had a significantly higher prevalence of hEAMC than those without a gradual onset injury (PR=1.6; p < 0.0001), as did those with a history of an acute injury compared to those without (PR=1.5; p < 0.0001). Chronic medication use (PR=1.3; p < 0.0001) and the use of medication before or during an event (PR=1.6; p < 0.0001) were both associated with a significantly higher risk for hEAMC among cyclists.
Independent risk factors associated with a history of hEAMC (multiple regression analysis)The independent risk factors (adjusted for sex and age group) associated with a hEAMC from the multiple regression analysis (adjusted for age and sex) are shown in Table 5.
Independent risk factors for hEAMC (multiple regression analysis) (adjusted for age and sex).
| Risk Factors | PR (95 % CI) | p-value |
|---|---|---|
| Cycling training / racing variables | ||
| Number of years as a recreational cyclist (years)# | 5 unit increase1.03 (1.02, 1.04) | <0.0001* |
| History of chronic disease | ||
| Chronic disease composite score# | 2 unit increase1.36 (1.29, 1.43) | <0.0001* |
| History of allergies | ||
| Any allergies | 1.18 (1.10, 1.26) | <0.0001* |
| Medication use | ||
| Medication use during or before an event | 1.41 (1.31, 1.52) | <0.0001* |
| Gradual onset injury | 1.30 (1.18, 1.43) | <0.0001* |
| Acute onset injury | 1.29 (1.17, 1.43) | <0.0001* |
Adjusted for sex and age.
PR: Prevalence ratio.
Chronic Disease Composite Score: the composite number of chronic diseases for an individual.
Independent risk factors associated with a hEAMC in cyclists were: increased number of years participating as a recreational cyclist (PR=1.03 per 5 years increase; p < 0.0001), a higher chronic disease composite score (PR=1.36 times increased risk for every 2 additional chronic diseases; p < 0.0001), a history of any allergies (PR=1.18; p < 0.0001), a history of medication use before or during the event (PR=1.41; p < 0.0001), a history of an acute (PR=1.29; p < 0.0001) and gradual onset injury (PR=1.30; p < 0.0001).
DiscussionEpidemiology of EAMC in cyclistsLifetime prevalence and annual incidenceOur study is part of only two studies available that reported on the prevalence of hEAMC in cyclists.3 We report a 30.5 % lifetime prevalence of hEAMC in cyclists, which is lower compared to the 60 % reported in one study published over three decades ago.3 The lack of demographical details, training characteristics, and medical history reported in the available literature3 results in challenges when comparing the discrepancies between our findings and the available literature. However, considering the data were collected almost two decades apart, the discrepancy could be attributed to several factors including advancement in training regimes, improved nutritional strategies and potentially better cycling equipment. Studies that focussed on other endurance sports, reported higher EAMC lifetime prevalences with 67 % lifetime in triathletes3 and lower with 14.4 % in ultra marathon running20 and 8.8 % in half marathon running.21 The higher prevalence in our study could be explained by the majority of cyclists being older than 50 years, having chronic diseases and making use of chronic medications, compared to the younger populations in the running studies.20,21 However, it is difficult to make direct comparisons from these studies to the cyclists in our study due to the different nature and physiological demands of these sports. To our knowledge, this is the first study to report on the annual incidence of EAMC in cyclists (18.6 %).
Clinical characteristics of lifetime hEAMC in cyclistsMuscle groups affected by hEAMCThe quadriceps muscle group was the most commonly affected by EAMC (47.7 %), followed by the calf muscles (27.8 %) and the hamstring muscles (20.4 %). These results are similar to those in studies investigating EAMC in distance runners,7,10 and triathletes.7 In the majority of cases, EAMC is confined to muscle groups that are actively used during an athletic event.3 Results from our study correspond with these reports, as the knee extensor group (quadriceps muscle group) is the prime mover used to generate force to the crank in the downstroke phase of cycling,22 and is therefore pivotal to cycling. In addition, EAMC usually occurs in multi-joint muscle groups that span across two joints, and contract in a shortened position, such as the gastrocnemius and quadriceps muscles.2,23 We can theorize that muscle activity in different muscle groups is different when comparing cycling to running and triathlon, which could possibly explain the differences between the distribution of EAMC in commonly affected muscles in different sports.
Timing of onset of EAMC during training/racingThe majority of cyclists experienced EAMC during the final quarter of the race (63.4 %). This finding correlates with studies, which suggested that the development of EAMC is more common in the end stages of a race in marathon runners,6,23 ultra-distance runners,7 and triathletes.7 Exercising for a longer duration results in muscle overload and fatigue, which in turn can lead to the development of EAMC due to a disturbance in neuromuscular control at the level of the spinal cord.24 Our findings are in accordance with the hypothesis that muscle fatigue can lead to EAMC.
Risk factors associated with hEAMC in cyclistsDemographics (sex and age group)We report a significantly higher prevalence of hEAMC in male cyclists (34.4 %) compared to female cyclists (15.8 %) (PR=2.2). This finding correlates with previous studies in male vs. female distance runners.11,25 Females may be less fatigable than males when exercising at similar relative exercise intensities.26 Although speculative, this resistance to fatigue could be attributed to females having a greater proportion of type I muscle fibres that relates to better mitochondrial respiratory capacity and fatigue resistance.9,11 Therefore, evidence of muscle fatigue, resulting in altered neuromuscular control as a causative mechanism in the development of EAMC,24,27 could be a plausible explanation for this finding.
The prevalence of hEAMC was also highest in the oldest age group (PR=1.8). A previous study has shown a significantly higher hEAMC in runners in older age categories compared with runners in the younger (≤30 years) category,11 and that age is a significant indirect predictor of EAMC in distance runners.25 However, in other studies, older age has not been shown to be a risk factor associated with EAMC in runners7 or in endurance athletes with a hEAMC.12,14 The evidence regarding age remains inconclusive, and further research, especially in cyclists is needed.
History of chronic diseaseA novel finding of our study, is that a higher chronic disease composite score was an independent risk factor associated with a hEAMC. For every two additional chronic diseases present, the prevalence of EAMC increased by 36 % in a “dose-dependent” manner. Our findings correlate with results from a previous study that reported an association between a history of EAMC and chronic diseases in some organ systems (cardiovascular, respiratory, GIT, nervous system or psychiatric, haematological or immune and renal) and cancer in distance runners.11 Skeletal muscle cramping is known to be associated with numerous chronic diseases,14,28,29 and associated with chronic disease in a number of organ systems. It is also an unwanted side-effect of certain medications used in the treatment of chronic diseases.11,30 Our univariate analysis’ findings supports this notion, showing a significant increased risk for hEAMC with a history of chronic medication use. However only medication use before or during and event were retained in the multivariate analysis. Muscle cramping can therefore occur due to underlying diseases or as a result of the medication used to treat these conditions.28 The potential mechanism/s to explain this relationship in recreational cyclists requires further study. In future, the chronic disease composite score could be clinically applied to identify cyclists at higher risk for injury during pre-race medical screening. This could aid in EAMC prevention through appropriate medical care, training adjustments and health education.
History of any allergiesA second novel finding of our study was that a history of any allergies was a risk factor associated with hEAMC. This finding was similar to a previous study where it was reported that allergies were an independent risk factor associated with EAMC in distance runners.11 It has been reported that a history of allergies is common in endurance athletes.18,31 Our study shows this association (not a cause-effect) between hEAMC and allergies, and the potential mechanism is unknown. It could be postulated that both the allergy itself and the medication used to treat allergies may be underlying reason for EAMC. This is further supported by the results of our univariate analysis showing that chronic medication use, and medication use before an event were significantly associated with an increase in risk for hEAMC. Anti-histamines are commonly used to treat allergies, but have side-effects such as fatigue and drowsiness.32 We can theorize that if this type of medication is used during training and racing, medication induced fatigue could result in the development of EAMC due to a disturbance in neuromuscular control at the level of the spinal cord. This is speculative but may be a potential reason for the association between allergies and EAMC. Future research should explore the relationship between allergies, the medication used to treat allergies, and EAMC in cyclists.
History of injury (gradual onset or acute)Our third novel finding was that a history of cycling injury, gradual and acute onset, was an independent risk factor associated with a hEAMC. Cyclists with a history of gradual onset injury had a significantly higher prevalence of hEAMC (PR=1.4), as did cyclists with a history of an acute injury (PR=1.34). These findings correlate with studies which have reported that a history of a running injury (past or current running injury) is associated with a history of EAMC in runners and Ironman triathletes respectively.11,12 A history of a running injury is further a significant predictor for EAMC in distance runners.25 In addition, it has been reported that a history of pre-race muscle damage is associated with EAMC in ultra-marathon runners.7 It has been speculated that soft tissue injury could trigger an increase in reflex alpha motor activity,12 or that previously injured areas may be vulnerable to the development of premature fatigue due to weakness of the localized muscles,33 both of which are associated with the development of EAMC.11 However, there are no previous studies investigating the association between previous injury and EAMC in cyclists to directly compare our results to.
Number of years as a recreational cyclistAn increased number of years participating as a recreational cyclist was an independent risk factor associated with an increased risk of hEAMC. A higher risk for EAMC was also noted among road runners with greater number of years of running.6 However, the more experience an athlete is, the older they usually are. Therefore, our finding could be influenced by the fact that older cyclists that likely have chronic diseases and using medication before or during a race, which was also a significant risk factor. Aligned with the neuromuscular control theory, higher training intensities among athletes could lead to increased neuromuscular fatigue and potential muscle cramping.6,33,34
Strengths and limitationsThe main strengths of this study are the large sample size (n = 21,460), and as far as we are aware, it is also the only study reporting on the association between independent risk factors and hEAMC in cyclists. We acknowledge that the study does have has several limitations. Although the study sample was representative of all race entrants by sex, we acknowledge that there was a significant difference between the consenting race entrants and all race entrants by age group. We cannot determine a cause-effect relationship between any of the identified risk factors due to the cross-sectional nature of the study, and all EAMC and training data are self-reported and could have been affected by recall bias. Although some of our study findings could be related to medication use, the lack of information on the exact type of medication, and medication dosage and frequency limits those results. As this is the first study to report on EAMC in cyclists, we could not compare our results with other cycling related studies. Future studies are needed to explore the causal relationship between the risk factors identified and EAMC in cyclists.
ConclusionNovel independent risk factors associated with a hEAMC in cyclists include: a higher chronic disease composite score, history of allergies, history of medication use before or during the event, a history of injury (acute onset or gradual onset), and training/racing variables. Specifically, for every two additional chronic diseases present, the prevalence of EAMC increased 1.36 times in a “dose-dependent” fashion. The results from our study, in combination known risk factors and future research, could assist future prevention programmes and the management of EAMC in recreational cyclists.
The authors report there are no competing interests to declare.
The study was partially funded by a research grant from the International Olympic Committee (IOC) Research Centre (South Africa) at the University of Pretoria.
The South African Medical Research Council (SAMRC) provided partial funding for the statistical analysis.
The authors would like to thank Dr Jannalene Killops for facilitating the data collection, staff at Mediclinic South Africa for storing and then facilitating the export of the raw data, and the event organisers of the Cape Town Cycle Tour, their medical staff, and all the cyclists that participated in this study.