Bedrest is toxic for inpatients and consumer grade physical activity monitors offer an economical solution to monitor patient ambulation. But these devices may not be accurate in debilitated hospitalized patients who frequently ambulate very slowly.
ObjectiveTo determine whether measures of physical capacity can help identify inpatients for whom wearable physical activity monitors may accurately measure step count.
MethodsProspective observational study of 54 adult inpatients with acute neurological diagnoses. Patients were assessed using 2 physical capacity assessments (Activity Measure for Post-Acute Care Inpatient Mobility Short Form [AM-PAC IMSF] and Katz Activities of Daily Living [ADL] scale). They also completed a 2-minute walk test (2MWT) wearing a consumer grade physical activity monitor.
ResultsThe wearable activity monitor recorded steps (initiated) in 33 (61%) of the inpatients, and for 94% of inpatients with gait speeds >0.43 m/s. Physical capacity assessments correlated well with gait speed, AM-PAC IMSF r = 0.7, and Katz ADL r = 0.6, p < 0.05. When the physical activity monitor initiated, the mean absolute percent error (SD) comparing device calculated steps to observed steps, was 10% (13). AM-PAC IMSF (T-score >45) and Katz ADL (>5) cutoff scores identified inpatients for whom physical activity monitors initiated with a sensitivity of 94 and 91%, respectively.
ConclusionsPhysical capacity assessments, such as AM-PAC, and Katz ADL, may be a useful and feasible screening strategy to help identify inpatients where wearable physical activity monitors can measure their mobility.
Mobility is emerging as a vital sign for adult inpatients, with increasing evidence that patients who are more mobile have better outcomes.1–3 While the importance of inpatient mobility has gained acceptance, a consensus has not been met on the best methods for measuring mobility. Currently, most hospitals record inpatient mobility using periodic observations by clinicians, which are often descriptive and also do not provide the most complete or accurate depiction of a patients’ mobility levels throughout the course of the day.4,5 For example, a nurse may record that the patient transferred to a chair during an observed interaction with the patient; however, during unobserved periods the patient may transfer to the chair multiple times or walk around in the room, which may not get recorded. Advancements in wearable activity monitors provide an opportunity to capture a more accurate and complete measurement of daily mobility for hospitalized patients.
Several consumer-grade activity monitors have been validated against research-grade activity monitors, which provides an economic approach for hospitals interested in deploying this technology.6,7 Early studies in this area have found physical activity measured by these devices to be associated with clinical outcomes, such as length of stay or discharge readiness.8 Uncertainty regarding patient's mobility level is a frequent barrier to discharge, thus being able to more accurately and thoroughly measure a patient's mobility may facilitate early discharge planning and reduce hospital length of stay. However, studies have also found that the data provided by wearable activity monitors is less accurate at lower gait speeds which means that this technology is likely not appropriate for all hospitalized patients as these patients often have impairments in gait speed.9 Furthermore, it is likely not feasible or economical to utilize wearable activity monitors on all hospitalized patients. A screening method is needed to identify patients for whom wearable physical activity monitors are appropriate and will provide accurate data.
While gait speed testing is likely the most direct way to identify patients suitable for wearable activity monitors,9 this approach would probably add undue burden onto clinical staff in the hospital setting who already work under significant time constraints. An alternative to gait speed testing is the use of physical capacity assessments (e.g., Activity Measure for Post-Acute Care Basic Mobility Inpatient Mobility Short Form [AM-PAC IMSF), Katz Activities of Daily Living [ADL] scale), which are already utilized during routine clinical care and assess patient function based on clinician judgement or asking patients or caregiver questions, rather than observing patients perform specific tasks.4 Because these assessment tools are meant to provide objective measurements of patient mobility, they may be a viable alternative to physical performance tests such as gait speed. The purpose of this study was to compare the use of two physical capacity assessments to gait speed testing in their ability to identify patients for whom activity monitors would provide accurate data. The results of this study will help clinicians identify patients appropriate for wearable activity monitors through routinely collected data.
MethodsStudy population and settingWe enrolled a convenience sample of 54 adult patients with a wide range of functional impairments from the neurology and neurosurgical units at a large academic hospital in 2016. The study was approved by the Johns Hopkins Institutional Review Board (IRB) in Baltimore, Maryland, U.S.A. and all patients provided oral consent, which was approved by the IRB (IRB00116176). Patient characteristics and demographics were obtained from the electronic medical record (EMR).
All in-patients at the study units were eligible unless they met one of the following exclusion criteria: (1) physician order for bedrest, (2) required a monitor or medical device that precluded ambulation, (3) hemodynamically unstable, (4) uncooperative, combative, or resistive, or (5) nurses could not ambulate the patient safely, even with assistance, from the edge of bed to a chair. Patients were allowed to use their assistive devices.
Physical activity monitorWe utilized a consumer-grade, tri-axial physical activity monitor (Fitbug Orb Activity Tracker; Fitbug orb©) to count steps taken by patients during a standardized 2-minute walk test.10–12 This physical activity monitor has been used in previous studies and has been found to be accurate among healthy individuals.10–12 The physical activity monitors used were secured to an elastic gait belt placed around the hips without limiting patient's movement. This location was chosen based on manufacturer information and being a recommended location for device attachment during gait assessment.13–15 The physical activity monitors were wirelessly paired to a tablet computer with the required step count software.
AssessmentsPhysical capacity measurementsTwo physical capacity assessments were completed by nursing for each patient: the AM-PAC Basic Mobility IMSF (also called “6 clicks”) and the Katz ADL scale. Both measures are used widely in the inpatient setting and have been shown to be accurate and reliable when assessed by nurses.4 Additionally, the AM-PAC Basic Mobility IMSF has been translated and cross-culturally adapted into Brazilian-Portuguese.16 The AM-PAC IMSF asks how much assistance (from another person) is needed for a patient to complete 6 distinct functional activities and can be scored via direct observation of patient performance or clinician judgment. Scores range from 6 to 24 with lower scores indicating higher levels of impairment.17 Similarly, the Katz ADL scores patients on a scale of 0 to 6, with higher scores indicating greater independence in physical functioning.18 Nurses completing the physical capacity assessments received training on how to score the AM-PAC IMSF and Katz ADL prior to the study.
Two-minute walk testEach patient completed a two-minute walk test (2MWT), conducted by a nurse and physical therapist. This test has documented reliability, validity, and feasibility in the hospital setting.19,20 The 2MWT was conducted in the unit corridor, which had been measured in advance so that patients’ ambulation distance could be recorded. Patients were instructed to walk as quickly as they could safely ambulate for a full two minutes. At the end of two minutes, patient ambulation distance was calculated based on pre-determined corridor measurements. If patients could not complete the entire 2 min, their distance and amount of time (in seconds) they walked were recorded.
Gait speed was calculated based on the distance walked by the patient and the length of time they walked (2 min). During the 2MWT, the physical therapist followed the patient and counted their steps using a handheld counter.
AnalysesPatient demographics, physical capacity measurements, and physical performance measurements were summarized using descriptive statistics, including mean and standard deviation for continuous variables, and frequency and proportions for categorical variables.
To measure the accuracy of wearable activity monitors, we compared the number of steps recorded by the activity monitors to those observed by the physical therapist (reference standard). An absolute percentage error was calculated for each patient (difference between observed steps and steps recorded by activity monitor divided by observed steps). A mean absolute percentage error was calculated by averaging the absolute percentage error for all patients that the physical activity monitor recorded at least 1 step. Visual displays were created to see the relationship between the APE and gait speed and each physical capacity assessment.
Next, we aimed to identify if AM-PAC and/or Katz ADL cutoff scores could be used to identify patients for whom activity monitors would provide accurate measurements. Because the majority of inaccurate step counts occur when the device detects 0 steps, we sought to identify which patients the activity monitor would detect ≥1 step. To do this, we incrementally divided each functional measure into two categories based on all possible values (i.e. thresholds) of the functional measure. For example, gait speed was evaluated for each 0.01 m/sec, with one of the possible thresholds then being ≤0.43 m/sec versus >0.43 m/sec. We then created a 2 × 2 contingency table comparing the dichotomized functional measure with whether the activity monitor detected ≥1 step. Next, we defined the classification accuracy for device initiation at each threshold using this formula: (True positive + True Negative) / (True positive + True Negative + False Positive + False Negative).21 We identified the threshold for each functional measure that had the highest classification accuracy for device initiation, which balances sensitivity and specificity. For each physical capacity assessment threshold, we also calculated the sensitivity and specificity of the device detecting any steps.
Lastly, we fitted a logistic model using AM-PAC IMSF and Katz ADL as predictors for whether the physical activity monitor detected steps or not. Collinearity between AM-PAC IMSF and Katz ADL was assessed using the variance inflation factor. We computed the area under the curve (AUC) by performing a receiver operating characteristic (ROC) analysis to evaluate the overall predictive performance of the model. Furthermore, we performed a K-fold cross-validation with 10 random splits drawn from original data with a seed set to 1200 to generate a more realistic estimate of the predictive performance given the small sample size in the current study.22
ResultsA total of 54 patients, with a mean ± SD age of 52 ± 16, participated in the study. Of these, 46% were female, 63% Caucasian, and 46% were post-operative (Table 1). Patients ambulated with gait speeds ranging from 0.04 to 1.68 m/s. Pearson correlations between the physical capacity assessments and gait speed were calculated: AM-PAC IMSF r = 0.7 and Katz ADL r = 0.6.
Patient characteristics (n = 54).
| Characteristic | Valuesa | Rangeb |
|---|---|---|
| Age (years) | 52 +/- 16 | 22–83 |
| Female, number (percentage) | 25 (46) | – |
| Caucasian, number (percentage) | 34 (63) | – |
| Diagnosis, number (percentage) | ||
| Spinal surgery | 16 (29) | – |
| Craniotomy | 9 (17) | – |
| Stroke | 8 (15) | – |
| Degenerative disease | 2 (4) | – |
| Other neurological | 19 (35) | – |
| Functional Assessment Measures | ||
| Gait Speed (meters/sec) | 0.7 +/- 0.4 | 0.04–1.68 |
| Katz ADL scalec | 5 +/- 2 | 1–6 |
| AM-PACd | 51 +/- 9 | 35–61 |
Katz ADL, Katz Activities of Daily Living scale; AM-PAC, Activity Measure for Post-Acute Care (inpatient mobility short form.
The physical activity monitor did not initiate (recorded zero steps) in 39% (n = 21) of the inpatients. When the physical activity monitors initiated, the mean average percent error ± SD of the device calculated steps compared to observed steps was 10% ± 13. We determined the following thresholds for accurately classifying whether the physical activity monitor initiated: AM-PAC IMSF: 45, and Katz ADL: 5 (Table 2). The accuracy of classification for AM-PAC and Katz ADL at the aforementioned threshold are 83% and 87%, with a sensitivity of 94 and 91%, respectively.
Association of functional measurement thresholds and physical activity monitor initiationa.
| Functional Measurement | Thresholdb | Accuracy of classificationsc (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Gait speed (meters/sec) | 0.43 | 96 | 100 | 90 |
| Katz ADL scale (range: 0 – 6) | 5 | 87 | 91 | 76 |
| AM-PAC (T-score) | 45.44 | 83 | 94 | 67 |
AMPAC, Activity Measure for Post-Acute Care (inpatient mobility short form); Katz ADL, Katz Activities of Daily Living scale.
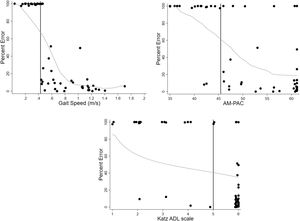
The proportion of patients where the physical activity monitor initiated above the thresholds are outlined in Table 3. The proportion was the highest, 94%, in those with a gait speed >0.43 m/sec during the 2MWT. Among those with AM-PAC IMSF score of >45.44 or Katz ADL >5 the proportion of patients where the physical activity monitor initiated was 85% and 91%, respectively. The relationship between the range of AM-PAC IMSF and Katz ADL scores with physical activity monitor percent error, demonstrated that the physical activity monitors had lower mean absolute percentage error for patients with less functional impairment (Fig. 1).
Physical activity monitor performance in patient subgroups (by functional measure score).
| Functional Measure | Thresholdsa | N | Physical Activity Monitor Initiated, N (%) |
|---|---|---|---|
| All Patients | – | 54 | 33 (61) |
| Subgroup of Patients | |||
| 2 MWT gait speed (meters/sec) | >0.43 | 35 | 33 (94) |
| AM-PAC (T-score) | >45.44 | 34 | 29 (85) |
| Katz ADL scale (range: 0–6) | >5 | 32 | 29 (91) |
MWT, Minute Walk Test; AMPAC, Activity Measure for Post-Acute Care (inpatient mobility short form); Katz ADL, Katz Activities of Daily Living scale.
Threshold was the point at which accuracy of classifications was the highest when the activity monitor initiated (see Table 2).
Scatter plots with jittered dots representing Percent Error of the physical activity monitor across the range of scores for gait speed, AM-PAC IMSF, and Katz ADL. The vertical axis represents the percent error with 100% indicating that the physical activity monitor did not initiate and 0% representing no difference between physical activity monitor and observed steps. The horizontal axis represents the scoring scale for each physical function measure, with higher scores indicating better performance. The bolded black vertical line represents the threshold for each functional measure (Table 3). The gray line represents a fitted locally weighted regression Loess curve.
Abbreviations: Percent Error, (observed steps – physical activity monitor steps)/observed steps; AM-PAC, Activity Measure for Post-Acute Care (inpatient mobility short form, also called “6 Clicks”); Katz ADL, Katz Activities of Daily Living scale
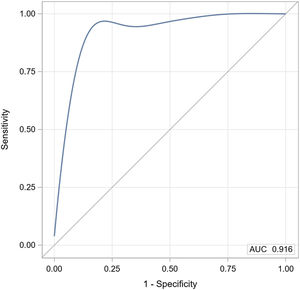
Lastly, AM-PAC and Katz ADL demonstrated a strong discriminatory ability for determining whether the physical activity monitor detected steps or not with an area under the curve (AUC) of 0.92 (Fig. 2). The cross-validated mean AUC was 0.94 and bootstrap bias corrected 95% confidence interval was 0.67 to 0.95.
Receiver operating characteristic (ROC) analysis for a logistic model with AM-PAC IMSF and Katz ADL ability to predict whether the physical activity monitor detects steps.
Abbreviations: AM-PAC, Activity Measure for Post-Acute Care (inpatient mobility short form, also called “6 Clicks”); AUC, Area under the curve; Katz ADL, Katz Activities of Daily Living scale
In this study we sought to examine whether measures of physical capacity may help to discriminate between inpatients for whom consumer-grade wearable physical activity monitors can measure step count accurately or not. Consistent with other studies, our findings highlight that these devices may not detect any steps for a large number (39%) of hospitalized patients, primarily those with slow gait speeds.10,23 We also found cutoff scores for the AM-PAC IMSF and Katz ADL physical capacity assessments where the wearable physical activity monitors recorded step counts with similar accuracy compared to observed steps. Specifically, physical activity monitors may record steps more accurately with patients who have an AM-PAC IMSF score of >45.44 (raw score >19) or Katz ADL score of 6. Towards the goal of capturing mobility as a vital sign for adult inpatients, results of the study may assist clinicians in helping to screen patients where these devices can more accurately measure their mobility.
The physical activity monitor used in this study did not initiate step counting in patients who ambulated with a slow gait speed (≤0.43 m/s or ≤1.0 mph), which represented more than one-third of our study population. These results are consistent with other studies that have used a variety of different devices and also showed that gait speed is an important determinant for step count accuracy. For example, among 34 community dwelling older adults it was found that the Actigraph and the Yamax DigiWalker pedometer underestimated steps when gait speeds were <0.8 m/s (1.8 mph).9 In a different study examining a variety of gait speeds using the activPAL, researchers found a higher mean absolute percent error (23%) when 21 participants (65–87 years old) ambulated at slow speed (0.67 m/s or 1.5 mph) on a treadmill.24 Another study among 36 older adults with physical impairments found that the activPal physical activity monitor significantly underestimated step counts in people with gait speed ≤0.47 m/s (1.1 mph).25 Notably, this study included hospitalized patients and their gait speed cutoff is comparable to our results. Hence, although differences in ranges of gait speed cutoffs have been reported our findings corroborate existing evidence that gait speed is likely the most important factor in determining whether a wearable physical activity monitor will be able to accurately measure step counts. However, assessing gait speed to screen patients for use of physical activity monitors may not be feasible during routine care.26
Given staff time constraints and dynamic environment of the hospital setting, other measures of physical functioning may be a more feasible screening strategy during routine care. We considered reliable and valid physical capacity assessments performed by multiple disciplines such as nurses, nursing assistants, and case managers, and incorporated into routine clinical care without extensive training.4 Specifically, the Katz ADL and AM-PAC IMSF assessments can be scored based on patient or clinician report, without requiring patient performance. The cut-points that we found in our study, AM-PAC 45.44 (raw score, 19) are consistent with other studies describing levels of mobility impairment where patients are likely to be more mobile. For example, a prior study showed that physical therapy consultation for patients with AM-PAC IMSF score >43.63 (raw score, 18) may indicate the patient has no need for therapy consultation.27 Other studies have suggested that admission AM-PAC IMSF scores of 16 to 17 or higher in the hospitalization are associated with eventual home discharge.28,29 While the authors are unaware of previously established threshold scores for Katz ADL a score of 6 is the highest score, which means the patient is independent with bathing, dressing, toileting, transferring, continence, and feeding.18 Thus, the Katz ADL independently or along with the AM-PAC IMSF score, may be used to determine whether to use a physical activity monitor for a patient. Compared to gait speed, these physical capacity assessments offer the possibility to screen large groups of patients for wearable physical activity monitors.
Measuring inpatient mobility over the course of hospitalization, the clinical care team can use these data to determine the needs of the patients. Low levels of mobility or a decline in mobility may put a patient at risk for worse outcomes or may indicate that the patient has additional needs. In the hospital setting, a recent study used physical activity monitors to evaluate 777 patients and found that 1000 steps during the last 24 h of admission may indicate discharge readiness.8 Another study among 154 medical inpatients suggests that step-count is independently associated with hospital length of stay.1 Furthermore, among 177 hospitalized patients 65 years old or older, fewer than 900 steps per day was strongly associated with functional decline.30 Importantly a recent study evaluated ambulation among post-surgical hospital patients and found that 1000 steps the day after surgery was associated with a lower probability of increased hospital length of stay.8 There is an opportunity to incorporate wearable physical activity monitors into routine care for certain patient populations to improve hospital care.
LimitationsThere are limitations to this study. It was conducted at a single academic hospital among patients with primarily neurological disease or post-neurosurgical procedures; hence, generalizability to others is limited and should be further studied. However, our patients had a diverse range of functional impairments with a wide range of gait speeds that are likely to be generalizable to other patient populations. Additionally, although we selected a tri-axial consumer grade physical activity monitor that has been used in prior clinical studies, we only tested one type of device that was available to us at the time of the study. Our gait speed cutoffs were also comparable to other studies, which suggests that our findings are likely generalizable to other devices but need to be verified. Lastly, our results are based on a single episode of ambulation for each patient's hospitalization. It is likely that patient gait speed and functional capacity vary over the course of their disease recovery and hospitalization, which may impact the accuracy of the wearable physical activity monitor.
ConclusionPhysical activity monitors may be a precise means of monitoring patient activity in the subgroup of patients with faster gait speeds, but gait speed may not be feasible to measure among all patients in the hospital during routine care. Identifying patients where physical activity monitors are likely to provide more accurate data to clinicians using less resource intensive clinical measures, such as AM-PAC IMSF and Katz ADL is a useful strategy. Because these clinical instruments can be administered as part of routine clinical care, they may help enable screening hospitalized patients with a wide range of functional impairments for activity monitor use on a larger scale.
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 5T32HD007414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.