High intensity training (HIT) improves disability and physical fitness in persons with chronic nonspecific low back pain (CNSLBP). However, it remains unclear if HIT affects pain processing and psychosocial factors.
ObjectiveTo evaluate 1) the effects of HIT on symptoms of central sensitization and perceived stress and 2) the relationship of symptoms of central sensitization and perceived stress with therapy success, at six-month follow-up, in persons with CNSLBP.
MethodsThis is a secondary analysis of a previously published randomized controlled trial. Persons with CNSLBP (n = 51, age=43.6y) completed the Central Sensitization Inventory (CSI) and Perceived Stress Scale (PSS) at baseline (PRE) and six months after 12-week of HIT consisting of concurrent exercise therapy (FU). Two groups were formed based on CSI scores (low-CSI/high-CSI). First, linear mixed models were fitted for each outcome, with time and groups as covariates. Multiple comparisons were executed to evaluate group (baseline), time (within-group), and interaction (between-group) effects. Second, correlation and regression analyses were performed to evaluate if baseline and changes in CSI/PSS scores were related to therapy success, operationalized as improvements on disability (Modified Oswestry Disability Index), and pain intensity (Numeric Pain Rating Scale).
ResultsTotal sample analyses showed a decrease in both CSI and PSS. Within-group analyses showed a decrease of CSI only in the high-CSI group and a decrease of PSS only in the low-CSI group. Between-group analyses showed a pronounced decrease favouring high-CSI (mean difference: 7.9; 95%CI: 2.1, 12.7) and no differences in PSS (mean difference: 0.1; 95%CI: -3.0, 3.2). CSI, but not PSS, was weakly related to therapy success.
ConclusionHIT improves symptoms of central sensitization in persons with CNSLBP. This effect is the largest in persons with clinically relevant baseline CSI scores. HIT also decreases perceived stress.
Chronic nonspecific low back pain (CNSLBP) is a multi-factorial musculoskeletal disorder affecting up to 20% of the global population.1 It is the leading contributor to years lived with disability, affects mental health and social participation, and imposes significant economic burden on healthcare systems.2
Exercise therapy (ET) is an important component in the therapeutic management of CNSLBP.3 But, effect sizes are modest at best.4 Insufficient information concerning optimal training modes and inconsistencies in recommendations regarding exercise modalities such as volume and intensity4,5 are regularly mentioned as major limitations. Recent studies show that high intensity training (HIT) produces larger effect sizes regarding decreasing disability and improving physical fitness in persons with CNSLBP than training at low or moderate intensity.5,6 Particularly the ability of HIT to decrease disability is an important result, as this is the main burdening factor in this population and is often used as a denominator for overall therapy success.7–9
However, the importance of considering the effect of therapy modalities on pain processing and psychosocial factors in chronic musculoskeletal disorders is currently also emphasized.10,11 Changes in these constructs can have an important underlying effect on perceived disability level in CNSLBP,12,13 and might regulate therapy success of modalities such as HIT. Furthermore, both constructs are interrelated, as they are each regulated by the descending endogenous pain modulation system and can create facilitating effects on nociceptive transmission, thereby increasing pain perception.14 Moreover, variables designed to evaluate these constructs impact disability in other therapeutic interventions. For example, presence of symptoms of central sensitization predicts poor disability outcomes in various musculoskeletal disorders and mediates effects of treatments such as pain neuroscience education and cognitive behavior therapy.10,15,16 Multiple psychosocial factors such as depression, anxiety, and maladaptive cognitions predict development of persistent low back pain17 and are associated with changes in pain and disability after physical therapy.18 Perceived stress specifically influences disability levels and modulates pain perception through inflammatory responses and systematic morphological brain changes.19–21
By performing exercise therapy in chronic pain conditions, an increase in pain tolerance and improvement of psychological function is expected.22 Furthermore, regular exercise affects the ability to inhibit pain.22,23 Hereby, exercising at higher intensities produces larger immediate post-activity hypo-algesic responses.24,25 However, as of yet, it remains unclear to which extent therapeutic exercise performed at a higher intensity can have long-term impact on pain processing and psychosocial factors in chronic musculoskeletal disorders. Recently, a randomized clinical trial compared multiple HIT programs for persons with CNSLBP.26 Herein, HIT improved core set outcomes (i.e., disability and pain intensity), regardless of the HIT modality. While this study focused on short-term effects of HIT on disability and pain intensity, symptoms of central sensitization and perceived stress were also inventoried secondarily as part of a six-month follow-up assessment.
Therefore, the aims of this secondary analysis were to evaluate: effects of HIT on symptoms of central sensitization and perceived stress (aim 1), and the relationship between symptoms of central sensitization or perceived stress with outcomes linked to therapy success (aim 2), at six-month follow-up, in persons with CNSLBP.
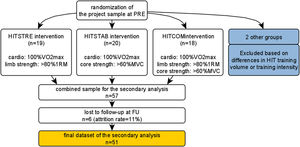
MethodsTrial designThis longitudinal follow-up study consists of a secondary outcome analysis of a larger project evaluating effectiveness of HIT in comparison to moderate intensity training (MIT),6 and effectiveness of various HIT modes, in CNSLBP rehabilitation through a prospectively registered five-arm RCT organized at REVAL (Hasselt University, Diepenbeek, Belgium).26 Participants were measured at baseline (‘PRE’) and at follow-up (‘FU’), i.e., 6 months after finalizing the training program. Because no differences were found on (improvements of) primary outcomes (i.e., disability and pain intensity) between three HIT intervention groups with similar concurrent exercise designs and equal training volume, data were combined into one cohort, with adjustment for group allocation. A comprehensive research design flowchart is displayed in Figure 1. This project was approved by the Medical Ethics Committee of Jessa Hospital (Hasselt, Belgium) and registered at clinicaltrials.gov as NCT02911987.
ParticipantsPersons with CNSLBP living in Limburg (Belgium) were recruited through local advertisements. To be eligible, prospective participants had to speak Dutch, be 25–60 years old, and have a medical diagnosis of CNSLBP.27,28 Persons were excluded if they: had a spinal fusion, had a secondary musculoskeletal disorder, had co-morbidities (e.g., paresis/sensory disturbances by neurological causes), were pregnant, had ongoing compensation claims and work disability >six months, had followed an ET program for CNSLBP in the past three months, or could not attend regular therapy appointments. Interested persons received a patient information letter and were invited for an intake session (consisting of review of information letter, evaluation of study in/exclusion criteria, and signing of informed consent).
InterventionsParticipants enrolled in a 12-week ET program consisting of 24 supervised therapy sessions (2 × 1.5 h/week). They performed high intensity cardiorespiratory interval training at 100%VO2max, coupled with either high intensity general resistance training at 80% of one-repetition maximum (HITSTRE), high intensity core strength training at >40% of maximum voluntary contraction (HITSTAB), or a combined general resistance and core strength program using the same definitions (HITCOM). Training volume for each group was equal. A detailed exercise protocol description has been published previously.26 After finalizing the program, participants were advised to stay active and were not assisted or tracked in any way. They were not aware that they would be invited for a follow-up evaluation.
Testing procedure and outcomesThe following demographic and clinical characteristics were collected at baseline: sex, age, body mass index (BMI), time of onset of CNSLBP, kinesiophobia (Tampa Scale for Kinesiophobia [TSK]) and physical activity (Physical Activity Scale for Individuals with Physical Disabilities [PASIPD]).29,30 For aim 1, primary outcomes were ‘symptoms of central sensitization’ and ‘perceived stress’ measured by the Central Sensitization Inventory (CSI) and Perceived Stress Scale (PSS). For aim 2, outcome variables (i.e., indicators of therapy success31) were improved ‘disability’ and ‘pain intensity’ measured by the Modified Oswestry Disability Index (MODI, minimal clinical important difference [MCID]: 10-point decrease32) and Numeric Pain Rating Scale (NPRS, MCID: 2-point decrease33) and exposure variables were CSI and PSS. All used measures are reliable and valid in persons with chronic musculoskeletal disorders.
Central Sensitization Inventory (CSI) evaluates symptoms of central sensitization and consists of 25 items scored on a five-point scale (0–4 points) with a total score on a 0–100 scale.34 A total score of more than 40 indicates clinically relevant presence of symptoms in an outpatient chronic pain sample.35 CSI was developed as an indirect tool for central sensitization symptomatology evaluation, feasible for clinical practice. Using CSI to evaluate patients is specifically interesting because almost a quarter of persons with chronic low back and neck pain will develop chronic widespread pain, depending on several risk factors assessed with the CSI.36
Perceived Stress Scale (PSS) evaluates the degree to which situations in one's life are appraised as stressful during the previous month and consists of 10 items scored on a five-point scale (0–4 points) with a total score on a 0–40 scale.37 Assessed items are general in nature rather than focusing on specific events or experiences.37 A higher score indicates higher perceived stress.
Modified Oswestry Disability Index (MODI) evaluates disability in persons with CNSLBP and consists of 10 items scored on a five-point scale (0–4 points), which is multiplied by 2 for a total score on a 0–100 scale.32 A higher score indicates more disability and indicates a larger degree of functional limitation.
Numeric Pain Rating Scale (NPRS) evaluates average pain intensity in the previous six-week period by choosing a number on a 0–10 scale. A higher score indicates more pain (0=no pain, 10=worst pain imaginable).38
Statistical analysisJMP Pro (12.0, SAS Institute Inc, USA) was used for statistical analysis. Descriptive statistics were used to display baseline group characteristics. Prior to analyses related to answering study aims, interactions between group allocation and CSI/PSS were analyzed to determine if exercise groups could be combined, or if stratified analyses were necessary. No PRE-FU differences between intervention groups were found, thus data were combined into one cohort. This cohort showed equal improvements on disability and pain intensity that surpassed the MCID (Table 1) just as the separate groups evaluated in a previous publication did.26 A split method (high-CSI/low-CSI) with CSI>40/100 as threshold was used to create two relevant groups from the cohort.
Demographic, clinical characteristics and PRE and FU outcome scores for total sample and subgroups.
Categorical variables are expressed as an absolute number, continuous variables are expressed as mean ± SD. Abbreviations: 95%CI, 95% confidence interval with upper and lower limit; FU, long-term measurement 6 months after finalizing the training program; m/f, male/female; PRE, baseline measurement; PASIPD, The Physical Activity Scale for Individuals with Physical Disabilities; TSK, Tampa Scale for Kinesiophobia. *p<0.05 difference in PRE compared with Low-CSI. Ϯp<0.05 difference in FU compared with PRE.
For aim 1, linear mixed models were fitted for each primary outcome (i.e., CSI/PSS), with time (PRE-FU) and group (high-CSI/low-CSI) as covariates and incorporated random intercepts for the participants. Multiple comparisons were executed to evaluate group (baseline differences), time (within-group differences), and interaction effects (between-group differences). For all significance tests, a p-level≤0.05 was used.
For aim 2, Pearson or Spearman (parametric/nonparametric variables) multivariate correlation analyses were executed to determine strength (based on categories proposed by Taylor39) and direction (i.e., negative/positive) of correlations between baseline (PRE) and change (PRE-FU) scores in independent variables (CSI/PSS) and change (PRE-FU) scores in dependent variables (MODI/NPRS). Baseline demographic and clinical characteristics were also included to identify potential confounders. Outcomes showing a correlation with a p-value<0.2 were included in multiple linear regression analyses to evaluate the relationship between those and independent variables.40 A fitted model was built for each dependent variable using a stepwise backward approach. Significant independent variables were put in the model with p-value<0.05 as a value threshold stopping rule. Variables were removed in order of least significance in the model up until only significant variables remained.
ResultsRecruitment and baseline dataIn total, 57 persons were included in this secondary analysis. Six persons (11%) were lost-to-follow-up: three did not respond to research communication, two did not participate due to personal reasons, and one was diagnosed with another chronic musculoskeletal disorder. Baseline characteristics of drop-outs did not differ from the remaining cohort of participants. Finally, data from 51 participants (20 males, mean ± SD age=43.6 ± 10.1y) were used in PRE-FU statistical analyses. They fit the healthy BMI range of >18 and <25 kg/m2,41 displayed symptom duration of 11.7 ± 8.4y, and an activity level of 14.6 ± 9.9 MET h/day, which is similar to previous research in persons of that age with physical disabilities.30 Regarding clinical symptoms, they displayed a pain score of 5.5 ± 1.6, disability level of 21.1 ± 10.1, and a kinesiophobia score of 33.3 ± 5.5. An overview of patient characteristics of the total sample and groups is provided in Table 1.
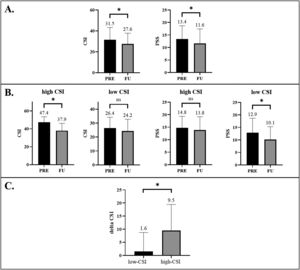
Outcomes of CSI/PSS at follow-upFrom PRE-FU, a decrease in CSI (mean difference: −5.0; 95% confidence interval (CI): −7.4, −2.6), and PSS (mean difference: −2.3; 95%CI: −3.7, −1.0) was found (Figure 2A). An overview of PRE-FU differences of the total sample is provided in Table 1.
Outcomes of CSI/PSS at follow-up in high-CSI/low-CSI groupsThe split method (high-CSI: n = 12 (23%), low-CSI: n = 39 (77%), see Table 1 for baseline characteristics) showed a PRE-FU decrease in the high-CSI group (mean difference: −9.5; 95%CI: −15.8, −3.1) but not in the low-CSI group (mean difference: −1.6; 95%CI: −4.6, 0.9). For PSS, PRE-FU differences were only seen in the low-CSI group (mean difference: −2.8; 95%CI: −4.4, −1.2) but not in the high-CSI group (mean difference: −0.9; 95%CI: −4.0, 2.2)(Figure 2B). Between-group analysis showed a difference in favor of high-CSI (mean difference: 7.9; 95%CI: 2.1, 12.7)(Figure 2C), while no differences were found in PSS (mean difference: 0.1; 95%CI: −3.0, 3.2). An overview of PRE-FU differences in the subgroups is presented in Table 1.
Relationships between CSI/PSS on MODI/NPRSAn overview of multivariate correlation analyses is displayed in Table 2. All datasets had normal distributions.
Results of the correlations between dependent variables and independent variables.
Results are expressed as correlation r (p-value). Abbreviations: CSI, Central Sensitization Inventory; FU, long-term measurement 6 months after finalizing the training program; MODI, Modified Oswestry Disability Index; NPRS, Numeric Pain Rating Scale; PRE, baseline measurement; PSS, Perceived Stress Scale. *variables with p<0.2 that were included in the stepwise linear regression model. +non-normal distributions evaluated with a Spearman p analysis.
Multivariate analysis performed to determine correlations between baseline CSI/PSS and change (PRE-FU) scores of dependent variables, showed weak correlation scores (r ≤ 0.35). However, p-values of correlations between CSI and disability (r = 0.194, p = 0.17), and between CSI and pain intensity (r = 0.194, p = 0.17) were low enough to include in a multilinear regression analysis. Age, PASIPD, and TSK could also be included. However, finally no regression model could be built as both CSI and PSS did not reach ‘the value threshold stopping rule.’
Multivariate analysis performed to analyze correlations between change (PRE-FU) CSI/PSS scores and change (PRE-FU) scores of dependent variables, also showed weak correlation scores (r ≤ 0.35). However, p-values of correlations between CSI and disability (r = 0.273, p = 0.06), and between CSI and pain intensity (r = 0.353, p = 0.02), were low enough to be included in a multilinear regression analysis. Finally, the fitted model for disability included CSI and TSK and explained 16% of the variance (and 9% with CSI as a sole factor). The fitted model for pain intensity included CSI and onset and explained 19% (and 12% with CSI as a sole factor) (Table 3).
Final linear regression models for change (PRE-FU) in disability scores and change (PRE-FU) in pain intensity scores.
Abbreviations: 95%CI, 95% confidence interval with upper and lower limit; FU, long-term measurement 6 months after finalizing the training program; p. est., parameter estimate, PRE, baseline measurement; st. ß, Standardized beta.
This study evaluated effects of HIT on symptoms of central sensitization and perceived stress, and the relationship between symptoms of central sensitization and perceived stress on the one hand and outcomes related to therapy success on the other hand, at six-month follow-up, in persons with CNSLBP. It demonstrated that HIT substantially improves symptoms of central sensitization, especially in persons with clinically relevant scores at baseline. Furthermore, HIT affects perceived stress, regardless of magnitude of baseline or changing symptoms of central sensitization at baseline. Only symptoms of central sensitization were related to decreases in functional disability and in pain intensity. However, impact of (changes in) symptoms of central sensitization on therapy success is low, as only 12% of variance in pain intensity and 9% of variance in functional disability could be explained.
Determining working mechanisms of exercise therapy remain unclear. A recent systematic review by Wun42 reports that RCTs evaluating ET propose a variety of mechanisms to explain why exercise might be effective for persons with chronic low back pain. While biomedical mechanisms (e.g., improvements in muscle strength or motor control) are proposed most often, their relationship with positive clinical outcomes is disputed and an increasing number of trials now focus on neurophysiological (e.g., changing pain processing) and psychosocial (e.g., decreasing kinesiophobia) mechanisms.42,43 However, this review concludes that proposed working mechanisms are only effectively evaluated seldomly and emphasizes that investigating mediating effects of different mechanisms is warranted.
Central sensitization has recently been recognized as a potential neurophysiological mechanism underlying multiple chronic pain disorders including chronic low back pain.44 So far, the primary modality to manage symptoms of central sensitization has been pharmacotherapy,44 though results are unsatisfactory.10 Hence, Nijs10 emphasized need for revised treatment plans, preferably of a multimodal approach. Herein, explaining the role of the central nervous system and brain in processing nociceptive information to the patient (i.e., ‘‘pain neurophysiology education’) is brought forward.45 This approach is supported by high patient satisfaction, and has proven to be effective in a variety of chronic pain disorders.44 In addition, exercise is also explicitly displayed as a therapeutic modality with high potential. Exercise might reconceptualize pain-related fear by reintroducing movement previously perceived as a threat, thereby impacting long-term central pain processing.46 As exercise is already advised as a primary treatment strategy for persons with CNSLBP, it is essential to know whether it can impact central sensitization in this population.3 At present, exercise has been shown to acutely improve pain sensation through alterations in pain processing.46,47 But, only a limited amount of studies has evaluated impact of long-term structured exercise programs. In this respect, the current study shows the ability of HIT to decrease CSI scores at six-month follow-up by 12% and even up to 20% in the subsample of persons with baseline scores above the clinically relevant cut-off score.48 This supports the effectiveness of exercise, specifically HIT, to improve symptoms of central sensitization in persons with CNSLBP.
It is also noteworthy that most guidelines for treating symptoms of central sensitization advise exercise therapy targeted towards improving cognitions. However, HIT did not consist of any of the classical components to target this outcome (e.g., integrating pain education or using an exposure-in-vivo design16). Therefore, the authors hypothesize that characteristics of HIT as a modality in itself might support improvement of psychological outcomes (e.g., through promoting self-efficacy and enjoyment of exercise49). Indeed, comparable HIT programs have been found to improve cognitive and mental health in adolescents, have positive effects on depression, anxiety, and resilience in adults with low physical activity levels, and increase faith in own body, elevate mood, and support taking charge of own health in persons with spondyloarthritis.50–52 Furthermore, research in healthy persons found HIT to have important physiological effects. First, it affects central opioidergic mechanisms and is involved in pain, the internal reward system, and emotional processing, more than a MIT program.24 Second, high intensity exercise seems to provide a more elaborate anti-inflammatory response on the body,53 which might support decreasing systemic inflammation in chronic pain.54 Possibly, HIT thus works through a combination of above-mentioned physiological and psychological mechanisms to improve symptoms. While we argue that a decrease of 20% in CSI scores is a substantial improvement, lack of minimal clinically important difference scores for CSI in longitudinal designs makes it difficult to evaluate exact magnitude of this result. This should be of imperative interest for future research.
Regarding the relationship of symptoms of central sensitization with therapy outcomes in CNSLBP, Tanaka12 evaluated predictive value of CSI in management of multiple musculoskeletal disorders and found a relation with pain-related disability. While our study showed equal correlations and potential value of correcting for kinesiophobia, final explanatory value of the regression model was low. It's unclear whether this is due to sole inclusion of persons with CNSLBP (in contrast to multiple pathologies12) or exercise modality (which was indistinctively displayed elsewhere12). Also, profiles of persons with symptoms of central sensitization might also need to be elaborated on with combinations of other psychosocial outcomes. In acute low back pain for example, central sensitization was only found to be a predictor in combination with depression, catastrophizing, and/or sleep.55 Future studies may try to perform more in-depth analyses as to get a better idea of most relevant mediators, confounders, and colliders. However, these evaluations were outside the scope of the current study.
Regarding importance of perceived stress in relation to therapeutic management, a recent review by Buscemi17 displayed evidence supporting the etiologic role of perceived stress and life stressors in development and maintenance of chronic spinal pain. To add to this, two prospective cohort studies showed that psychological stress increases odds of developing low back pain, as well as it becoming chronic, among working adults.56,57 While exercise can improve perceived stress in healthy adults,58 studies related to changes in perceived stress after rehabilitation for persons with musculoskeletal disorders are sparse. Berlowitz59 showed that physical therapy consisting of aerobic exercise showed greater reductions in PSS scores compared with an educational program. This effect was stronger among participants with elevated perceived stress pre-intervention. Other studies have also evaluated effects of yoga exercises on perceived stress in low back pain, showing positive effects.59–61 Nevertheless, all these studies fail to give clear information regarding used exercise protocols and exercise modalities, limiting conclusions that can be drawn regarding their impact.
In the current study, perceived stress improved. However, from a clinical perspective, changes were only small and potentially not clinically relevant, as the MCID is 11 points.62 We hypothesize that this is due to low mean baseline stress score present in the current sample (a score of <14 already relates to low perceived stress level) which might have limited longitudinal changes. Future research should evaluate whether persons with higher baseline perceived stress scores and a longer duration of pain (as this remained as a confounder in the regression model) show different outcomes.
This study has some methodological limitations. First, it consists of a post-hoc exploratory analysis. Therefore, future studies will have to corroborate differences found between depicted groups (i.e., high-CSI/low-CSI) in prospective designs. However, the authors argue that subgrouping based on CSI was justified within the study goal as previous literature has demonstrated the importance of evaluating groups that can be constructed through using CSI cut-off scores.35 Second, no active comparator control group (e.g., usual care or another exercise therapy intervention) was included. While the larger project included one moderate intensity group, a between-group analysis would have been underpowered. Hence, the current study cannot evaluate whether results of HIT differ from more commonly used exercise therapy protocols. However, to our knowledge there is no data available yet on effects of HIT on the researched outcome measures and such a comparison was not the research question of this study. Second, CSI and PSS scores were not evaluated directly after finalizing the HIT program. Although current analyses show effects at six-month follow-up, these effects might thus already be apparent at short-term. Third, the sample with clinically relevant CSI scores was small (n = 14). It would have been interesting to evaluate whether the relationship between baseline/changes in CSI and outcomes of therapy success was higher in the high-CSI group only, however the sample did not allow that analysis due to power limitations. Nonetheless, distribution of both groups with 33% of participants showing clinically relevant CSI scores, greatly resembles expected proportions as displayed in previous literature. Last, low magnitude of impact of symptoms of central sensitization might be due to using a questionnaire instead of psychophysical assessments such as quantitative sensory testing to measure pressure pain thresholds and/or conditioned pain modulation, and blood analyses or brain activation analyses for stress responses. However, most of these assessments still lack a gold standard. CSI has been developed as an indirect easy to administer tool. While CSI does show overlap with other constructs and is prone to false positives, it is less time consuming and more feasible in practice. Therefore, as an indirect tool it can help identify patients whose presenting symptoms are related to central sensitization.36
ConclusionsHIT improves symptoms of central sensitization in persons with CNSLBP. Positive effects are more pronounced in persons with clinically relevant CSI scores. HIT also affects perceived stress. As both outcome measures are only slightly at most related with changes in pain intensity and functional disability, their impact on overall therapy success in persons with CNSLBP seems limited.